Protective effects of sodium humate on the intestinal barrier damage of Salmonella Typhimurium-challenged broilers
Abstract
Salmonella Typhimurium (S. Typhimurium) infections can lead to severe intestinal damage and reduce growth performance in broilers. Thus, this study examined the potential mitigating impact of sodium humate (HNa) on intestinal barrier damage resulting from S. Typhimurium infection in broilers. A total of 320 1-day-old Arbor Acres broilers were randomly assigned into 5 treatments with 8 replicates. On d 22–24, broilers in the CON group were challenged with 1 ml of PBS, while broilers in the other groups were challenged with 1 ml of 3 × 109 CFU/ml S. Typhimurium, daily. Dietary administration with 4 g/kg of HNa increased (P < 0.05) the final body weight, jejunal secretory immunoglobulin A (sIgA), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), and catalase (CAT) levels as compared with the MOD group broilers. Furthermore, HNa alleviated intestinal barrier damage by increasing villus height (VH), upregulating protein expression of Occludin, Claudin-1, and zonula occludens-1 (ZO-1), inhibiting toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) signaling pathway activation, and decreasing the secretion of inflammatory cytokines (P < 0.05). Collectively, the present study showed that HNa mitigated intestinal barrier damage induced by S. Typhimurium infection in broilers.
1 INTRODUCTION
Salmonella, a major zoonotic food-borne pathogen, can cause serious public health and food safety concerns (Lawrence et al., 2022). The World Health Organization (WHO) reported that 350,000 people die each year from Salmonella infection worldwide, posing a serious public health threat and causing considerable economic losses (Thompson et al., 2018). In addition, the European Food Safety Authority (EFSA) reports that the consumption of poultry products is one of the main causes of public health problems due to Salmonella infections (European Food Safety et al., 2017). Salmonella Typhimurium (S. Typhimurium) is a Salmonella pathovar that causes severe intestinal injury and poor growth performance in young broilers (Bourassa et al., 2018; Peng et al., 2022). On top of that, adult broilers infected with S. Typhimurium are typically asymptomatic, they have the potential to transmit the pathogen to humans via poultry products (Chen, Zhang, et al., 2021). Thus, effective prevention and control of S. Typhimurium infections in broilers has been a research hotspot.
The crucial role of the intact intestinal barrier in defending against pathogen invasion has been widely reported (Chiang et al., 2022; Foley et al., 2021). However, there is evidence that Salmonella infection can increase intestinal permeability and induce intestinal dysfunction by reducing the expression of tight junctions among epithelial cells and intensifying inflammatory responses in the intestine (Baumler & Sperandio, 2016; Devlin et al., 2022). Therefore, maintaining intestinal homeostasis is essential to maintaining body health. To enhance growth performance and mitigate morbidity, antibiotics have historically been employed in broiler production as a means of preventing and controlling salmonellosis (Gut et al., 2018). However, some countries or institutions have implemented restrictions or stringent prohibitions on the utilization of antibiotics in poultry production as a result of concerns regarding bacterial resistance and adverse public health consequences (Abudabos et al., 2019; Yang et al., 2015). Taken together, natural products possessing antimicrobial and anti-inflammatory properties, as well as enhancing intestinal barrier function, have received more and more attention.
Humic acids (HAs), generated through the decomposition and conversion of organic matter within peat, are a class of macromolecular organic acids composed of aromatics. As reported, HAs have good physiological activity, adsorption, and complexation and have been widely used in agriculture, medicine, and healthcare fields (Murbach et al., 2020). Sodium humate (HNa) is the salt of HAs, containing hydroxyl, carboxyl, and methoxy functional groups. HNa is rich in vitamins, alkaloids, and other biologically active substances contributing to its pharmacological effects such as antibacterial, anti-inflammatory, antioxidant, hemostatic, antidiarrheal, and immunoregulation, in addition to possessing a strong capacity for ion exchange and adsorption. In traditional Chinese medicine, HNa is mainly used for hemostasis and antidiarrhea (Ji et al., 2016). Studies have shown that HNa enhances the growth performance of broilers and piglets (Dominguez-Negrete et al., 2019; Kaevska et al., 2016). In addition, our prior study has indicated the antibacterial properties of HNa against enterotoxigenic Escherichia coli (ETEC) and S. Typhimurium (Wang et al., 2023; Wang, He, et al., 2022). Our further study showed that HNa relieved S. Typhimurium infection-induced intestinal injury via modulating intestinal microbiota and intestinal mucosal immunity in mice (Wang et al., 2023). Similarly, an earlier study found that supplementation with HNa reduced Escherichia coli (E. coli) colonization in the jejunum of piglets (Wang et al., 2020). Furthermore, HNa has been extensively proven to possess bacteriostatic, immunoregulatory, and antioxidant properties in piglets, mice, and European seabass (Brandts et al., 2021; Ji et al., 2016; Macri et al., 2015). To date, the safeguarding influence of HNa against S. Typhimurium-induced intestinal inflammation in broilers has been not reported, and its protective mechanism remains unclear. Therefore, the study aimed to evaluate the protective effects of HNa on growth performance, intestinal morphology, intestinal inflammation, antioxidant capacity, and intestinal barrier function in S. Typhimurium-infected broilers.
2 MATERIALS AND METHODS
2.1 Preparation of materials
HNa (purity, 75%) was provided by the Institute of Coal Chemistry, Chinese Academy of Sciences (Shan xi, China). It consists of 75% humic acid (dry basis), 20.52% burning residue (dry basis), 14.22% water (air dry basis), and 4.48% water soluble substances (dry basis) according to the analysis report of the product. S. Typhimurium was provided by the Harbin Veterinary Research Institute, CAAS. S. Typhimurium was incubated at 37°C for 12 h in Luria-Bertani broth and then centrifuged at 8,000 rpm for 10 min. Phosphate-buffered saline (PBS) was used to wash bacteria three times. Finally, PBS was used to adjust the S. Typhimurium concentration to 3 × 109 CFU/ml.
2.2 Animals and experimental design
The experiment was conducted using a total of 320 male one-day-old Arbor Acres broiler chicks that possessed similar initial body weight (41.63 ± 0.64 g) following the protocols of Animal Care approved by the Shandong Agricultural University (approval ref no. SDAUA-2022-100). The broilers were randomly assigned into five treatment groups with eight replicates of eight birds each: CON group (basal diet), MOD group (basal diet + S. Typhimurium infection), L-HNa group (basal diet extra 2 g/kg of HNa + S. Typhimurium infection), M-HNa group (basal diet extra 4 g/kg of HNa + S. Typhimurium infection), H-HNa group (basal diet extra 6 g/kg of HNa + S. Typhimurium infection). The trials lasted for 24 d. The basal diets were formulated to meet the bird's nutritional requirements according to NY/T 33–2004 (People's Republic of China Agricultural Industry Standard Chicken Feeding Standard; Table 1). On d 22–24, the CON group broilers were orally challenged with 1 ml of PBS, while the MOD, L-HNa, M-HNa, and H-HNa groups broilers were orally challenged with 1 ml of 3 × 109 CFU/ml S. Typhimurium, daily. The experimental design was referred to Marcq et al. (2011). In the whole experiment, water and food were freely available to all broilers. The temperature was gradually reduced from 35 to 22°C by 0.5°C per day, and the relative humidity was maintained at 65%. Artificial light (10–20 lx) was provided in a 23 h light/1-h dark program throughout the entire experimental period. Weekly recordings of broiler body weight (BW) and feed intake were used to calculate the average daily feed intake (ADFI), average daily gain (ADG), as well as feed/gain ratio (F/G).
| Ingredient | % | Nutrient levels | % |
|---|---|---|---|
| Corn | 55.60 | Metabolizable energy, MJ/kg | 12.10 |
| Expanded soybean meal | 29.00 | Crude protein | 21.50 |
| Cottonseed meal | 2.50 | Calcium | 0.96 |
| Wheat flour | 4.00 | Total phosphorus | 0.66 |
| Hydrolyzed feather meal | 1.50 | Lysine | 1.45 |
| Soybean oil | 2.00 | Methionine | 0.54 |
| Dicalcium phosphate | 0.90 | Threonine | 0.91 |
| Limestone | 1.50 | ||
| Bentonite | 1.00 | ||
| Premixb | 2.00 | ||
| Total | 100.00 |
- a The experimental diet was the same basal diet supplemented with 0, 2, 4, and 6 g of HNa/kg of the basal diet. All nutrient levels were analyzed values, except metabolizable energy.
- b Supplied per kilogram of diet: vitamin A, 11,500 IU; cholecalciferol, 3,500 IU; vitamin E, 30 mg; vitamin K3, 5 mg; thiamin, 3.38 mg; riboflavin, 9.0 mg; pyridoxine, 8.96 mg; vitamin B12, 0.025 mg; choline chloride, 800 mg; calcium pantothenate, 13 mg; niacin, 45 mg; biotin, 0.15 mg; folic acid, 1.20 mg; Mn, 60 mg; Fe, 66.5 mg; Zn, 88 mg; Cu, 8.8 mg; I, 0.70 mg; Se, 0.288 mg.
2.3 Sample collection
On day 25, eight broilers per group were selected for sampling. Fresh feces were collected from broilers in each group for S. Typhimurium counts. Feces were plated on SS agar plates after tenfold dilutions with PBS and incubated at 37°C for 24 h. All counts were performed in triplicate. Blood samples were obtained from the wing vein and subsequently transferred into tubes to extract serum samples. After blood sampling, the broilers were sacrificed by cervical dislocation. Subsequently, three chickens per group were randomly screened and approximately 1 cm of jejunum was excised and immersed in a 4% paraformaldehyde solution for histomorphometric analysis. The remaining jejunal samples were rapidly frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
2.4 Histomorphological analysis of jejunum
After being fixed in 4% paraformaldehyde, the jejunal tissue was washed, dehydrated in graded ethanol, and then transparentized with xylol and embedded in paraffin. Thereafter, a 5 μm thickness section of each sample was deparaffinized in xylene, graded, rehydrated, and stained with hematoxylin–eosin (H&E). Jejunum tissue damage was assessed by light microscopy (Olympus, Tokyo, Japan). The villus height (VH) and crypt depth (CD) of the jejunum were measured using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA), subsequently, calculating the ratio of villus height to crypt depth.
2.5 Determination of inflammatory cytokines, immunoglobulins, and antioxidant capacity
Blood samples were obtained from the wing vein and subsequently transferred into vacuum tubes for collection of serum samples. The collected serum was used to detect the levels of interleukin-1β (IL-1β), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), immunoglobulin M (IgM), and immunoglobulin G (IgG). ELISA kits were used according to the manufacturer's instructions (Jingmei Biotechnology, Jiangsu, China).
The jejunal tissues were homogenized in 0.9% saline at a ratio of 1:9 (w/v), and then centrifuged at 5000 rpm for 10 min at 4°C. The supernatants of the homogenates were used to detect the concentration of jejunal secretory immunoglobulin A (sIgA), malondialdehyde (MDA), and the activities of jejunal total antioxidant capacity (T-AOC), catalase (CAT), and total superoxide dismutase (T-SOD). Assay kits were used according to the manufacturer's instructions (Nanjing Jiancheng Co., Ltd, Nanjing, China). The results were expressed as activities of antioxidant enzymes and concentration of MDA per mg of protein in the jejunal tissues of broilers. Additionally, the serum levels of diamine oxidase (DAO) and D-Lactate (D-lac) were also detected using the same assay kits. Detailed assay protocols refer to our previous studies (Fan et al., 2024; Yu et al., 2022).
2.6 Total RNA extraction and quantitative real-time PCR
The Eastep Super Total RNA Extraction Kit (Promega Biotech Co, Ltd, Beijing, China) was used to isolate the total RNA from the jejunum of broilers. The purity and concentration of RNA were assessed using a microspectrophotometer (NanoDrop Products, Wilmington, DE, USA), while the integrity of the RNA was evaluated through 2.0% agarose gel electrophoresis. The RNA was extracted and subsequently subjected to reverse transcription to generate complementary DNA, employing the PrimeScript™ RT reagent Kit and gDNA Eraser Kit (Takara Biomedical Technology, Beijing, China). qRT-PCR procedure was performed to determine the gene expression using ChamQ SYBR® qPCR Master Mix Kit (Vazyme Biotechnology, Nanjing, China) and the Roche 480 System (Roche, Switzerland). In Table 2, primer sequences were presented and β-actin was used as an internal reference. Target gene abundance was calculated using the 2−ΔΔCt method.
| Gene | GeneBank ID | Primer sequences (5′ → 3′) | Product size (bp) |
|---|---|---|---|
| IL-1β | NM_204524.2 | F:TTTTTGAGCCCGTCACCTTC | 111 |
| R:AGCACTTCTGGTTGATGTCG | |||
| TNF-α | HQ739087.1 | F:GAACCCTCCGCAGTACTCAG | 116 |
| R:AACTCATCTGAACTGGGCGG | |||
| IL-8 | DQ393272.2 | F:CCTCCTCCTGGTTTCAGCTG | 136 |
| R:TGGCGTCAGCTTCACATCTT | |||
| IL-10 | NM_012854.2 | F:CAGACCAGCACCAGTCATCA | 96 |
| R:TCCCGTTCTCATCCATCTTCTC | |||
| Occludin | NM_205128.1 | F:ATGCACCCACTGAGTGTTGG | 93 |
| R:GAGGTGTGGGCCTTACACAG | |||
| ZO-1 | XM_015278981.2 | F:AGCCCCTTGGTAATGTGTGG | 87 |
| R:TTGGGCGTGACGTATAGCTG | |||
| Claudin-1 | NM_001013611.2 | F:GGTATGGCAACAGAGTGGCT | 91 |
| R:CAGCCAATGAAGAGGGCTGA | |||
| TLR4 | KP410249.1 | F:CGGCTCCGCATCTTGGATAT | 148 |
| R:GGGCTTGGAGTGGCTTGTAT | |||
| IκBα | NM_001001472.2 | F:CAGCACTACACTTGGCCGTA | 101 |
| R:GGAGTAGCCCTGGTAGGTCA | |||
| NF-κB p65 | NM_001012887.2 | F:AAGATCTGGTGGTGTGCCTG | 137 |
| R:AGTGGAACCTTTCGCGGATT | |||
| β-Actin | NM_205518.1 | F:ACCGGACTGTTACCAACACC | 116 |
| R:CCTGAGTCAAGCGCCAAAAG |
- Abbreviations: F, forward; IκBα, inhibitor of NF-κB alpha; IL, interleukin; NF-κB p65, nuclear factor kappa B p65; R, reverse; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; ZO-1, Zonula occludens-1.
2.7 Immunohistochemistry analysis
The paraffin-embedded slides containing jejunum tissue were initially subjected to deparaffinization using dimethyl benzene and subsequent rehydration through a series of graded ethanol solutions. Subsequently, the slides were immersed in a 3% hydrogen peroxide solution and subsequently blocked with 3% bovine serum albumin (BSA) for a duration of 30 min, followed by ZO-1 primary antibodies (1:200, Wanlei Biotechnology, Liaoning, China) incubation overnight at 4°C: Behind, the slides were incubated with horseradish peroxidase-labeled goat antirabbit IgG secondary antibodies at room temperature for a duration of 50 min. The slides were dyed with diaminobenzidine (DAB) substrate color liquid and then counterstained with a hematoxylin-stain solution. Each slide was taken images with a microscope (Nikon DS-U3, Nikon, Tokyo, Japan) and analyzed integral optical density (IOD) with Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA).
2.8 Western blot analysis
Frozen jejunal tissue samples were homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer containing proteinase inhibitors for obtaining total protein. Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, Shanghai, China) was used to obtain protein for the detection of the protein expression of TLR4 (19,811–1-AP; Proteintech Group, Inc., Wuhan, China), IκBα (10,268–1-AP; Proteintech), NF-κB p65 (10,745–1-AP; Proteintech), Occludin (DF7504; Affinity Biosciences, Jiangsu, China), Claudin-1 (AF0127; Affinity), ZO-1 (DF2250; Affinity), β-actin (20,536–1-AP; Proteintech), and Lamin B (66,095–1-Ig; Proteintech). Extracted protein was denatured at 95°C, electrophoresed on SDS-PAGE gels, and then transferred to polyvinylidene difluoride (PVDF) membranes. After blocking the membranes with 5% skimmed milk, the PVDF membrane was subjected to an overnight incubation at 4°C with the primary antibody, followed by a 1 h incubation at room temperature with HRP-conjugated secondary antibody (1:10,000, Biosharp, BL003A). ECL chemiluminescence reagents (Nanjing Vazyme Biotech Co., Ltd) and Imager-Bio-Rad instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used to detect the target protein expression. The integrated density (ID) of the protein bands was analyzed using Image J software. Data are presented as target protein (ID)/β-actin (ID) or Lamin B.
2.9 Statistical analysis
All of the data were presented as mean ± standard errors of the mean (SEM). The statistical differences were analyzed by one-way ANOVA (SPSS 22, Inc., IBM, Chicago, USA) followed by Tukey's HSD test. Statistical significance was indicated as P < 0.05.
3 RESULTS
3.1 Growth performance
As shown in Table 3, S. Typhimurium infection led to a severe decline in the final BW and ADG at days 22 to 24 as compared with the CON group (P < 0.05). In addition, broilers in the L-HNa and H-HNa groups had lower ADG and higher F/G at days 22–24 as compared with the CON group (P < 0.05). HNa administration at 4 g/kg improved the final BW and ADG (days 22–24) of S. Typhimurium-infected broilers as compared with the MOD group (P < 0.05). No remarkable difference was found in initial BW, ADG (days 1–21 and 1–24), F/G (days 1–21 and 1–24), and ADFI (days 1–21, 22–24, and 1–24) among groups (P > 0.05).
| Items | Groups | P-value | ||||
|---|---|---|---|---|---|---|
| CON | MOD | L-HNa | M-HNa | H-HNa | ||
| Initial BW, g | 42.50 ± 1.96 | 42.15 ± 1.16 | 42.65 ± 1.93 | 42.34 ± 1.59 | 42.06 ± 1.09 | 0.925 |
| Final BW, g | 978.16 ± 14.69a | 933.32 ± 29.03b | 960.13 ± 25.99ab | 976.51 ± 18.84a | 958.90 ± 13.41ab | 0.046 |
| Pre-infect (1–21 days) | ||||||
| ADFI, g/day | 46.87 ± 3.11 | 46.92 ± 1.96 | 47.73 ± 3.17 | 47.83 ± 3.48 | 47.26 ± 2.86 | 0.536 |
| ADG, g/day | 39.87 ± 1.09 | 39.85 ± 1.17 | 40.62 ± 1.98 | 40.77 ± 1.05 | 40.65 ± 1.71 | 0.397 |
| F/G, g/g | 1.17 ± 0.08 | 1.18 ± 0.04 | 1.17 ± 0.05 | 1.17 ± 0.09 | 1.16 ± 0.05 | 0.734 |
| Post-infect (22–24 days) | ||||||
| ADFI, g/day | 56.13 ± 3.63 | 50.17 ± 6.58 | 52.23 ± 8.73 | 55.58 ± 6.91 | 52.51 ± 4.01 | 0.178 |
| ADG, g/day | 46.08 ± 5.59a | 30.53 ± 9.89b | 35.03 ± 6.95b | 39.55 ± 6.29ab | 34.56 ± 5.59b | 0.001 |
| F/G, g/g | 1.22 ± 0.20c | 1.64 ± 0.61a | 1.49 ± 0.48ab | 1.40 ± 0.32bc | 1.52 ± 0.37a | 0.004 |
| Overall (1–24 days) | ||||||
| ADFI, g/day | 51.50 ± 1.47 | 48.55 ± 2.79 | 49.98 ± 4.39 | 51.71 ± 2.01 | 49.88 ± 2.75 | 0.205 |
| ADG, g/day | 40.59 ± 1.59 | 37.45 ± 1.60 | 39.10 ± 1.89 | 40.22 ± 1.78 | 38.94 ± 1.56 | 0.236 |
| F/G, g/g | 1.27 ± 0.13 | 1.29 ± 0.19 | 1.28 ± 0.12 | 1.28 ± 0.16 | 1.28 ± 0.17 | 0.742 |
- Note: The values in the same with different letters (a, b) mean significantly different (P < 0.05).
- Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, feed/gain.
- a Data are represented as mean ± SEM.
3.2 Intestinal morphology
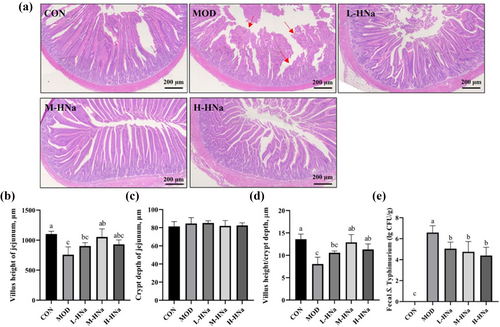
As shown in Figure 1a, S. Typhimurium infection damages the structures of the broilers jejunum and causes breakage and shortening of the intestinal villi. In accordance with the histological findings, the infection of S. Typhimurium significantly decreased the VH and VH/CD in the jejunal region when compared to the CON group (P < 0.05). Importantly, HNa administration alleviated intestinal damage as evidenced by increased VH and VH/CD (P < 0.05). Moreover, the CD was not altered among groups (P > 0.05). In addition, the number of S. Typhimurium in the feces of MOD group broilers was significantly higher than that in the CON group (P > 0.05). However, the administration of HNa reduced the number of fecal S. Typhimurium compared with the MOD group (P < 0.05).
3.3 Inflammatory cytokines
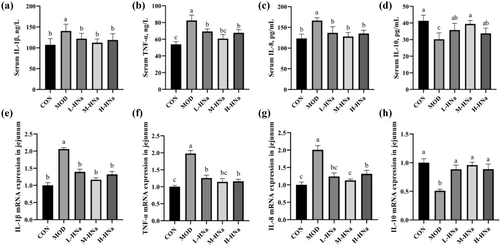
As shown in Figure 2a–d, S. Typhimurium infection significantly increased the concentrations of serum IL-1β, TNF-α, and IL-8 and decreased the concentration of serum IL-10 as compared with the CON group (P < 0.05). As expected, administration of different concentrations of HNa reduced the concentrations of serum inflammatory cytokines (P < 0.05). Similarly, S. Typhimurium infection significantly up-regulated the mRNA levels of IL-1β, TNF-α, and IL-8 and down-regulated the mRNA expression of IL-10 in the jejunum, while pretreatment with different concentrations of HNa reversed that (Figure 2e–h).
3.4 Intestinal immune function and antioxidant capacity
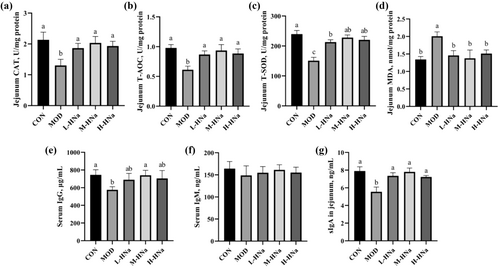
To investigate whether HNa pretreatment could improve the antioxidant capacity, we determined the activity of antioxidant enzymes and the concentration of MDA in the jejunum. In comparison to the CON group, after S. Typhimurium infection, the activities of CAT, T-AOC, and T-SOD significantly decreased, and the concentration of MDA significantly increased (Figure 3a–d; P < 0.05). Compared with the MOD group, administration of different concentrations of HNa increased the activities of CAT, T-AOC, and T-SOD and decreased the concentration of MDA (P < 0.05). In addition, the immunomodulatory effect of HNa was also detected. As shown in Figure 3e–g, the results indicate that HNa supplementation increased IgG concentration in serum and sIgA concentration in the jejunum (P < 0.05). However, serum concentration of IgM did not differ among groups (P > 0.05).
3.5 Relative protein expression of TLR4/NF-κB signaling pathway
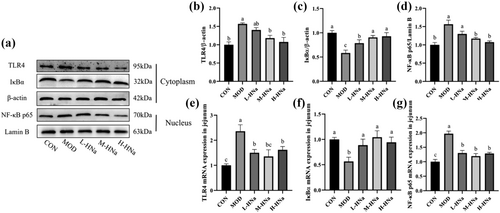
To investigate the protective effect of HNa in response to intestinal damage in S. typhimurium-infected broilers, we examined the mRNA and protein expression of TLR4/NF-κB signaling pathway in the jejunum. As shown in Figure 4, in comparison to the CON group, the infection of S. Typhimurium led to a notable elevation in the mRNA expression levels of TLR4 and NF-κB p65, while concurrently causing a significant reduction in IκBα (P < 0.05). Similarly, the result of western blot also showed that S. Typhimurium infection increased the protein expression of TLR4 and NF-κB p65 but decreased IκBα in the jejunum of broilers as compared with the CON group (P < 0.05). Importantly, administration of HNa inhibited the activation of TLR4/NF-κB signaling pathway caused by S. Typhimurium infection (P < 0.05).
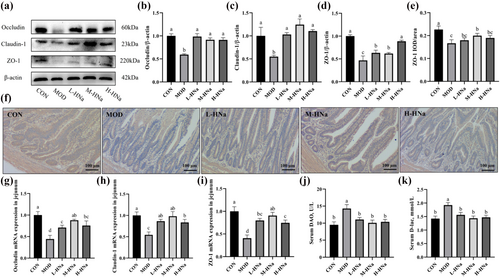
3.6 Intestinal barrier integrity
As shown in Figure 5g–i, in comparison with the CON group, broilers in the MOD group exhibited lower levels of mRNA expression for Occludin, Claudin-1, and ZO-1 in the jejunum (P < 0.05). Besides, the results of the western blot and IHC also confirmed that S. Typhimurium infection significantly decreased the Occludin, Claudin-1, and ZO-1 protein expression in the jejunum (Figure A-F; P < 0.05). As expected, administration of HNa attenuated the damage to the intestinal barrier integrity caused by S. Typhimurium infection (P < 0.05). We further detected the indicators of intestinal permeability. According to the results, S. Typhimurium infection increased the activity of DAO and concentration of D-lac in the serum when compared to the CON group (Figure 5j–k; P < 0.05). Importantly, administration of different concentrations of HNa decreased the levels of DAO and D-lac in the serum (P < 0.05). Taken together, pretreatment with HNa improves intestinal barrier damage caused by S. Typhimurium infection in broilers.
4 DISCUSSION
Salmonella is an important pathogenic bacterium capable of infecting humans and animals, characterized by intestinal damage and diarrhea (Raffatellu et al., 2009). The transmission of Salmonella through the consumption of contaminated food is widely acknowledged (Devlin et al., 2022). Thus, the control of Salmonella infection in broilers can substantially mitigate the public health hazard associated with Salmonella. Studies have confirmed that Salmonella infections can cause severe intestinal injury and reduced growth performance in broilers (Chen, Zhang, et al., 2021). Prior research has confirmed that HNa possesses antibacterial properties and confers protective effects on intestinal health in mice and piglets (Wang et al., 2008; Wang, He, et al., 2022). The present study examined the effects of HNa on growth performance, intestinal morphology, intestinal immune function, antioxidant capacity, intestinal inflammation signaling pathway, and intestinal barrier integrity in broilers infected with S. Typhimurium. Here, we found that broilers infected with S. Typhimurium presented with reluctance to move, lethargy, and low water and feed intake. In agreement with previous reports (Choi et al., 2022), this study found that S. Typhimurium challenge decreased the final BW of broilers. Importantly, our study revealed that the administration of 4 g/kg HNa improved the final BW and ADG (days 22–24) of S. Typhimurium-challenged broilers. Similarly, our prior investigation reported that pretreatment with HNa effectively mitigated the decline in BW of mice infected with S. Typhimurium (Wang et al., 2023). Additionally, the growth-enhancing impact of HNa has been documented in piglets (Kaevska et al., 2016). These results indicate that HNa may have growth-promoting effects in animals. Interestingly, the results of this study indicated that broilers in the M-HNa group had better growth performance than broilers in the H-HNa group. Referring to previous relevant studies, we found that the effects of natural products, minerals, or trace elements on animals are not exactly dose-dependent. In addition, due to the lack of relevant studies on the use of HNa in broilers, the exact reasons for this require further research. Importantly, the present study showed that the administration of different concentrations of HNa alleviated the intestinal barrier damage of S. Typhimurium-challenged broilers.
The importance of intestinal health is widely recognized as it greatly influences growth performance and overall health status in animals. Our previous study found that S. Typhimurium infection induced severe intestinal injury in mice, as evidenced by the increased intestinal lesion score, serious intestinal inflammation response, disordered intestinal microbiota, and intestinal barrier dysfunction (Wang et al., 2023). Here, our study revealed that infection with S. Typhimurium resulted in structural damage to the jejunum of broilers and the breakage, shortening, and shedding of the jejunum villi. The villus-crypt tissue structure of the jejunum contributes to the maintenance of intestinal health. Studies have confirmed that the decreased VH and increased CD serve as indicators of intestinal damage (Deng et al., 2023). Many studies have also shown that S. Typhimurium has detrimental effects on the intestinal structure manifested as decreased VH and increased CD in broilers (Chen, Zhang, et al., 2021; Choi et al., 2022; Peng et al., 2022). Consistent with this, the present study found that S. Typhimurium-infected broilers had significantly lower VH and VH/CD than the CON group. Importantly, dietary supplementation with 4 g/kg of HNa effectively mitigated the reduction in VH caused by S. Typhimurium infection. Furthermore, the results of H&E stain showed that HNa administration at 2 g/kg and 6 g/kg also restored jejunal morphology. Intestinal permeability is a widely recognized metric used to assess the functionality of the intestinal barrier (Yu et al., 2022). Serum levels of DAO and D-lac are the main indicators of intestinal mechanical barrier integrity. To further investigate the protective effects of HNa on intestinal barrier integrity, we assessed the serum levels of DAO and D-lac. The results showed that dietary supplementation with HNa significantly decreased the levels of serum DAO and D-lac, which indicated that HNa can effectively preserve the intestinal barrier integrity in S. Typhimurium-infected broilers. Similarly, previous research provided evidence for the protective impact of HNa on the intestinal barrier integrity in mice and piglets infected with pathogenic bacteria (Maguey-Gonzalez et al., 2018; Trckova et al., 2017). The above studies further confirmed that HNa could protect the intestinal barrier integrity.
Numerous studies have demonstrated that pathogenic microorganism infections could elicit intestinal oxidative stress (Baumler & Sperandio, 2016; Croxen & Finlay, 2010). It was reported that the intestinal tissues of mice infected with E. coli exhibited dramatically elevated levels of MDA, and decreased activities of SOD and CAT (Wang, He, et al., 2022). The antioxidant enzymes CAT, T-AOC, and T-SOD are widely known to play an essential role in catalyzing superoxide anion and hydrogen peroxide (H2O2), respectively, to form non-toxic compounds (Wang, Liu, et al., 2022). The level of lipid peroxidation can be reflected by MDA. Previous research substantiated the antioxidant property of HNa (Wang et al., 2020). Similarly, the current study found dramatically decreased activities of antioxidant enzymes (CAT, T-AOC, and T-SOD), while increased MDA concentration in the intestine of broilers infected with S. Typhimurium. As expected, HNa administration significantly increased the intestinal antioxidant enzyme activities of S. Typhimurium-infected broilers. In addition, Wang et al. (2008) confirmed that the administration of HNa increased antioxidant enzyme activity in weaned piglets. Our recent study has demonstrated that pretreatment with HNa increased the activities of antioxidant enzymes in the intestinal of mice (Wang et al., 2023). Furthermore, HNa was shown to possess immunomodulatory effects in addition to antioxidant properties (Brandts et al., 2021). Thus, we further examined the immunomodulatory effects of HNa in S. typhimurium-infected broilers. As is well known, intestinal mucosal immunity is primarily mediated by secretory IgA (Panda & Colonna, 2019). The present study showed that S. Typhimurium infection significantly reduced the jejunal sIgA concentration in broilers. As expected, dietary supplementation with different concentrations of HNa resulted in a significant elevation of sIgA concentration in the jejunum of broilers, as compared to that in the MOD group. In addition, dietary supplementation with 4 g/kg HNa also increased the serum IgG concentration.
The body's immune system serves as the primary barrier against the pathogens invasion. The connection between innate and adaptive immunity is facilitated by macrophages, which possess a diverse range of functions such as cytokine secretion, phagocytosis, pathogen elimination, and the preservation of immune homeostasis (Franken et al., 2016). Furthermore, toll-like receptors (TLRs), serving as sentinels of innate immunity, have the potential to engage in the recognition and defense mechanisms against invading pathogens. More precisely, toll-like receptors (TLRs) induce the activation of proinflammatory cytokines and chemokines at the transcriptional level, thereby initiating defense mechanisms in response to the recognition of pathogen ligands (Liu & Cao, 2016). Although inflammatory responses are crucial to the host's defense against pathogens, their excessive activation leads to the development of a cytokine storm, subsequently resulting in intestinal damage. Studies have been extensively reported on the involvement of TLR4 and its downstream transcription factor NF-κB in inflammatory responses. A known classical signaling pathway, TLR4/NF-κB, is believed to induce massive inflammation in intestinal injury caused by pathogens infection (Chen, Tan, et al., 2021; Liu et al., 2022). It has been shown that activating the TLR4/NF-κB pathway can promote the release of proinflammatory cytokines (Kovler et al., 2021). Studies indicated that upon stimulation of the intestine by pathogens, the TLR4 was activated, which then activates NF-κB, promoting downstream proinflammatory cytokines secretion. Furthermore, NF-κB protein is responsible for its function and Rel homology domain where IκBα binds. IκBα acts as an inhibitor of NF-κB through the formation of an inactive NF-κB/IκBα complex within the cytoplasm (Sivick et al., 2014; Yu et al., 2022). In this study, the mRNA and protein expression of TLR4 and NF-κB p65 exhibited significant upregulation, whereas IκBα displayed downregulation in the MOD group. Consistently, the inflammatory cytokines such as IL-1β, TNF-α, and IL-8 were significantly increased. IL-10 could inhibit inflammatory signals during instances of intestinal inflammation (Tong et al., 2022; Yu et al., 2022). This study indicated that the administration of HNa increased the mRNA and protein levels of IL-10 in the jejunum of S. Typhimurium-infected broilers. Importantly, dietary supplemented with different concentrations of HNa reduced intestinal inflammation due to S. Typhimurium infection, as evidenced by decreased expression of TLR4, NF-κB p65, and inflammatory cytokines. Our previous study also observed that pretreatment with HNa effectively mitigated the intestinal injury caused by S. Typhimurium infection in mice by suppressing the TLR4/NF-κB signaling pathway and inflammatory cytokines (Wang et al., 2023). Overall, the present study illustrated that HNa administration alleviated intestinal inflammation caused by S. Typhimurium infection.
The intestine plays a crucial role in the maintenance of body's immune system and nutrient absorption (Chiang et al., 2022). The intact intestinal mucosal barrier could protect the intestine against potential damage caused by pathogenic microorganisms (Das et al., 2022). The intestinal barrier integrity relies on the tight junctions (TJs) among intestinal epithelial cells. TJs, assembled from a variety of proteins, are located in the vicinity of the apical epithelial cells between neighboring cells, controlling the permeability of the paracellular transport pathway, and thus being an integral part of the intestinal physical barrier. TJs primarily encompass tight junction proteins like Occludin, Claudin-1, and zonula occludens-1 (ZO-1). Claudins and Occludin are transmembrane proteins that play an important role in the selective permeability of the intestinal epithelium. ZO-1 is the bridge that connects connexins with the actin cytoskeleton. These proteins interact to maintain intestinal barrier function (Foley et al., 2021). The latest study reported that infection caused by pathogenic bacteria can initiate mucosal immune responses, consequently leading to intestinal inflammation and further disrupting the intestinal tight junctions (Jalanka et al., 2023). In addition, pathogenic bacteria infection can disrupt the intestinal tight junctions and intestinal structure. The present results found that S. Typhimurium infection resulted in a notable reduction in the mRNA and protein expression of Occludin, Claudin-1, and ZO-1 in the jejunum of broilers. Furthermore, it can be inferred from the diminished jejunal VH and VH/CD, as well as the elevated serum DAO and D-lac levels, that the S. Typhimurium infection has adversely affected the intestinal barrier integrity. The administration of different concentrations of HNa in the diet appeared a mitigating effect on the intestinal damage induced by S. Typhimurium infection, as evidenced by the increased mRNA and protein expression of Occludin, Claudin-1, and ZO-1. In accordance with that, our recent research revealed that HNa alleviated the intestinal injury caused by S. Typhimurium through the upregulation of intestinal tight junction-associated proteins (Wang et al., 2023; Wang, He, et al., 2022). In conclusion, the alleviative effects of HNa on intestinal inflammation may be related to inhibiting the activation of the intestinal TLR4/NF-κB signaling pathway and increasing the expression of tight junction proteins.
In summary, HNa administration alleviated the intestinal barrier damage induced by S. Typhimurium infection by improving intestinal morphology, inhibiting intestinal inflammation, and increasing the activities of intestinal antioxidative enzymes and intestinal TJ protein expression in broilers. In the present study, dietary supplementation with 4 g/kg of HNa is superior to other dosages in mitigating the intestinal barrier damage of S. Typhimurium-challenged broilers.
ACKNOWLEDGMENTS
This work was supported by the earmarked fund for CARS36.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.




