Changes in mammary infection status in dairy cows during the dry period using dry cow therapy approaches on three farms
Abstract
This case study evaluated the mammary infection status of dairy cows during the dry periods and explored the associated problems in their quarters with dry cow therapy (DCT). This study assessed intramammary infections, antibiotic efficacy, and antimicrobial resistance of pathogens in 464-quarter milk samples from 59 dairy cows during the dry periods after applying blanket DCT, non-DCT, and selective DCT approaches on three farms. The recovery rates of intramammary infections were 95% (19/20 quarters) with blanket DCT on farm A, 70% (14/20) with non-DCT on farm B, and 19% (4/21) with selective DCT on farm C. Analysis of mammary infections in cows with DCT revealed that mammary infections were controlled by blanket DCT, well controlled by non-DCT, and substantial problems remained in selective DCT. Lower intramammary infection prevalence in the quarters at postpartum appeared to be associated with higher recovery of mammary infections, fewer new infections, and lower uncured mammary infections within the herds. Antibacterial resistance in 14 coagulase-negative staphylococci isolated to six antimicrobial drugs was suggested to be linked to antibiotic use on the farm. Follow-up studies on the quarter-based infection status with DCT will assist in improving mastitis control in cows during the dry period.
1 INTRODUCTION
Mastitis, a widespread disease among dairy cows, leads to substantial economic losses worldwide [International Dairy Federation (International Dairy Federation (IDF), 1999)]. Traditionally, antibiotics have been the primary approach to treating bovine mastitis; however, concerns regarding drug residues and the increasing antimicrobial resistance of mastitis-causing pathogens have prompted the development of nation-specific regulations and general encouragement to reduce the prophylactic use of antimicrobials (World Health Organization (WHO), 2015).
Dry cow therapy (DCT), an antibacterial therapy for mammary infections in dairy cows during the dry period, has been practiced for years. For several decades, the National Mastitis Council (NMC) has recommended a blanket DCT approach, which involves the antimicrobial treatment of all cow quarters, regardless of their infection status, in an effort to control intramammary infections during the dry period. Selective DCT focuses on only treating the infected quarters or cows with antimicrobials for treatment and mastitis prevention (Berry & Hillerton, 2002; Kabera et al., 2021; Niemi et al., 2022). In cases where non-DCT is employed, antimicrobials are strictly limited to herds that produce organic dairy products (Ruegg, 2009). In Finland, Vilar et al. (2018) reported that 78% of dairy farms used selective DCT, 13% applied a blanket DCT approach, and 9% did not use any DCT. Dairy farmers and owners have various herd-related factors, such as size, management style, hygiene practices, and decision-making processes. In addition, the methods and criteria for dry cow management differ across dairy farmers and countries (Kabera et al., 2021; Winder et al., 2020; Zecconi et al., 2019).
In studies on the evaluation of DCT in dairy cows, the efficacy of DCT, epidemiology of pathogens, and risk factors for mammary infections during the dry period with blanket and selective DCT approaches have been well investigated (Kabera et al., 2021; Niemi et al., 2022; Winder et al., 2020). However, changes in mammary infection status in the individual quarters of dry cows with selective DCT and non-DCT approaches have not been fully evaluated on farms. Quarter-based changes in mammary infections during the dry and postpartum periods may help explore the mammary infection problems underlying dry cow management and improve them in association with DCT and non-DCT approaches on farms for the prevention of mammary infections and promote milk quality control in the country.
This case study was conducted to assess the intramammary infection status in the quarters of dairy cows during the dry and postpartum periods within the herds and identify the underlying problems of dry cow management when blanket DCT, non-DCT, and selective DCT approaches were applied in three dairy farms. In addition, the antimicrobial resistance of mastitis-causing pathogens isolated from the cow quarters in relation to the antibiotics used on the dairy farms was examined.
2 MATERIALS AND METHODS
2.1 Farms and cows
Fifty-nine lactating cows were assessed from three dairy farms (A, B, and C) during their dry and postpartum periods to assess their mammary infection status. Three farms were selected based on their participation in the dairy herd improvement (DHI) program, implementation of good dairy practices on the farm, and regular farm visits by university veterinarians. The selection criteria for the cows included factors such as herd size, housing style, annual milk yield, bulk somatic cell (SC) counts, and type of DCT administered (blanket DCT, non-DCT, or selective DCT).
On farm A, located in Eniwa, Hokkaido, Japan, 30 out of 95 clinically healthy dairy cows during the dry and immediate postpartum periods were assessed. The average annual milk production per cow was 12,000 kg, and the mean monthly bulk milk SC counts ranged from 90 to 210 × 103 cells/mL. The farm used a total mixed ratio (TMR) feeding system, free- and tie-stall housing with straw bedding, and a milking parlor system (Herringborne, 10 × 2). A blanket DCT approach using antibiotics in dry cows was performed.
At farm B, a university farm located in Ebetsu, Hokkaido, Japan, 20 out of 72 clinically healthy cows were selected for inclusion. The farm used a TMR feeding system, free-stall housing with straw bedding, and a milking parlor system (Herringborne, 10). The average annual milk production per cow was 9,400 kg, and the monthly bulk milk SC counts ranged from 110 to 230 × 103 cells/mL. Farm B did not use a DCT approach, which was approved by the experimental animal ethics committee (RGU-VH20C20).
At farm C, located in Saijyo, Ehime Prefecture, Japan, 9 out of 86 clinically healthy cows were selected. The farm used a TMR feeding system, free-stall housing with water beds, and voluntary milking systems (VMS, DeLaval). The average annual milk production per cow was 10,000 kg, and the monthly bulk milk SC counts ranged from 120 to 250 × 103 cells/mL, farm C implemented a selective DCT approach in 21 quarters of 9 cows as a unit of the herd. The selective DCT approach was applied to selected quarters based on the criteria of SC counts > 300 × 103 cells/mL of monthly DHI records in cows in the late lactation period with episodes of clinical mastitis in mid- to late-lactation.
The dry cows were accommodated in separate barns, and approximately 1 week before their expected calving date, they were relocated to maternity barns with straw beds.
2.2 Antibiotics for DCT
Antibiotics for dry cows (Cepravin dry cow, 250 mg cephalonium, MSD Animal Health, Tokyo, Japan) were used for DCT. For infusing the antibiotics into cow mammary quarters at the drying-off, the teat orifice was wiped with 70% alcohol, and one product, a tube containing antibiotics, was administered to each cow mammary quarter by skilled farm staff under the guidance of veterinarians. In farm A, this was performed as part of the blanket DCT, whereas in farm C, selective DCT was administered to all four cow quarters. No other drugs, including disinfectants, were given to these cows during the dry-off and postpartum periods.
2.3 Collection of quarter milk
Milk samples were obtained from cows' mammary quarters for bacteriological culture and SC counts before antibiotic infusion into the quarters. In the cows close to the dry period, approximately 50–60 days before the expected calving date, quarter milk samples were collected at dry-off, and milk secretions from postpartum cows were collected within 3 days after parturition by university veterinarians in farms A and B and by the farm veterinarian in farm C. The teats were first dipped in a pre-dip solution for 30 s and then wiped with a paper towel. The fore-stripping milk from the quarters was discarded 2–3 times, the teat orifice was sanitized using 70% alcohol, and the milk was then collected into sterile 15-mL tubes (Sumilon PP, Sumitomo Bakelite Co. Ltd., Tokyo, Japan). Approximately 3–5 ml of quarter foremilk samples were collected aseptically in a sterile culture tube (Eiken Co., Tokyo, Japan) for bacteriological tests. These tubes were immediately placed on ice in a container and stored at −20°C until analysis.
2.4 Bacteriological culture analysis
Aseptically collected milk samples (10 μl) were evenly spread on tryptic soy blood agar plates supplemented with 5% sheep blood (Nissui, Tokyo, Japan). These plates were then aerobically incubated at 37°C for a period ranging between 24 and 48 h. The identification of mastitis-causing pathogens that grew on the blood agar plates followed the procedure outlined by the National Mastitis Council (NMC) (2004). This identification process was based on characteristics such as colony morphology, hemolytic patterns observed on the blood agar, Gram staining, and additional biochemical tests. Quarter milk was categorized as having a bacterial infection if it yielded >300 cfu/ml (Nagahata et al., 2020). The culture results were used to distinguish between mammary secretions from cow quarters with and without mammary infections. The bacteriological culture results were assessed for each quarter milk sample at two-time points: at dry-off and within three days after parturition. Standard microbiological procedures (National Mastitis Council (NMC), 2004) were employed to culture the milk samples and identify bacterial growth. The identified pathogens included coagulase-negative staphylococci (CNS), Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), environmental streptococci (Str. uberis and Str. dysgalactiae), and Corynebacterium bovis (C. bovis).
2.5 Determination of SC counts
The SC counts in the milk samples were determined using an SC counter (ADAM, NanoEnTek, Soul, Korea) and an assay kit (NanoEnTek, VRS-KO2, Gyeonggi-do, Korea). The SC count results are expressed as SC count/mL.
2.6 Minimum inhibitory concentration of isolated mastitis-causing pathogens
The major mastitis-causing pathogens were isolated from quarter milk samples taken after postpartum, specifically, those exhibiting >300 cfu/ml. Minimum inhibitory concentrations (MICs) were determined using the broth dilution method, in accordance with the guidelines established by the Committee for Clinical and Laboratory Standards Institute (CLSI) (MIC Interpretive Standards) and the National Veterinary Assay Laboratory (National Veterinary Assay Laboratory (NVAL), 2013). Antibiotic susceptibility and resistance were evaluated based on the MIC breakpoints, and results were interpreted using the MIC Interpretive Standards (CLSI, USA) (CLSI M7-A8, M100-S21, 2011), National Veterinary Assay Laboratory (NVAL) (2013), and the European committee on antimicrobial susceptibility testing (2022). The antibacterial resistance of isolates from the quarters of postpartum cows of the dairy farms was assessed for six antibacterial drugs: tyrosin (TS), lincomycin (LCM), oxytetracycline (OTC), kanamycin (KM), ampicillin (ABPC), and cefazolin (CEZ).
2.7 Statistical analysis
To assess differences in the intramammary infection rates in dairy cow quarters, positive rates of mastitis-causing pathogens in quarter milk samples collected from the dairy cows were analyzed using the χ2-test and Fisher's exact test. P-values of < 0.05 were considered statistically significant.
3 RESULTS
3.1 Intramammary infection status in quarter milk from cows during the dry and postpartum periods
This study assessed the bacteriological status of 464 mammary quarter milk samples collected from 59 cows during the dry-off and immediate postpartum periods on three dairy farms. Among the mastitis-causing pathogens identified in the quarter milk samples at drying-off, 67.2% (43/64) were CNS, 28.1% (18/64) were En. Str., and 3.1% (2/64) were S. aureus. In the samples obtained from cows immediately postpartum, the pathogens identified were 60.7% (34/56) CNS, 17.9% (10/56) En. Str., 10.7% (6/56) C. bovis, and 7.1% (4/56) S. aureus (data not shown).
During the dry period, the overall incidence of mammary infections in the mammary quarters differed among the farms, with infection rates of 17.1%, 25%, and 58.3% in farms A, B, and C, respectively (data not shown). Mammary infections in the quarters from cows immediately postpartum at farms A, B, and C had rates of 12.1%, 20%, and 74.3%, respectively, and the mammary infection rate in farm C was statistically different (P < 0.05) than those in farms A and B (data not shown).
3.2 Changes in mammary infection status in the quarters from cows during the dry and postpartum periods with blanket DCT, non-DCT, and selective DCT approaches on three dairy farms
The proportions of mammary quarters from cows with mammary infections that changed from positive (+) at drying-off to negative (−) at postpartum, thus indicating bacteriological cure (+ to −), were as follows: 95% (19/20 quarters from 12 cows) for blanket DCT in farm A; 70% (14/20 quarters from 13 cows) for non-DCT in farm B; and 19% (4/21 quarters from 9 cows) for selective DCT in farm C (Table 1). The bacteriological cure rate of the infected quarters in cows from farm C was significantly lower (P < 0.05) than those from farms A and B.
| Farm | ||||||
|---|---|---|---|---|---|---|
| Pathogen† | A | B | C | |||
| Dry-off | Postpartum | Dry-off | Postpartum | Dry-off | Postpartum | |
| + | 17.1% (20/117) |
−: 95.0% (19/20)l +: 5.0% (1/20)o |
25% (20/80) |
−: 70.0% (14/20)n +: 30.0% (6/20)q |
58.3% (21/36) |
−: 19.0% (4/21)m +: 76.2% (16/21)p |
| ー | 82.9% (97/117) |
−: 87.5% (84/96) +: 12.5% (12/96)r |
75% (60/80) |
−: 83.3% (50/60) +: 16.6% (10/60)t |
41.7% (15/36) |
−: 33.3% (5/15) +: 60.0% (9/15)s |
- † Pathogen +: >300 cfu/ml; Pathogen −: no pathogen or <300 cfu/ml.
- Numbers in brackets show the number of positive quarters of the total tested quarters.
- P < 0.05, l-m, n-m, o-p, q-p, r-s, t-s
The proportions of quarters with mammary infections that changed from negative (−) at dry-off to positive (+) at postpartum, indicating new mammary infections (− to +), were as follows: 12.5% (12/96 quarters from 27 cows) in farm A; 16.6% (10/60 quarters from 20 cows) for non-DCT in farm B; and 60% (9/15 quarters from 6 cows) for selective DCT in farm C (Table 1). The mammary infection rate was significantly higher (P < 0.05) in farm C than in farms A and B.
The rates of uncured mammary infections, characterized by the persistence or reoccurrence of mammary infections in cows from the drying-off to postpartum (+ to +), were as follows: 5% (1/20 quarters from 12 cows) for blanket DCT in farm A; 30% (6/20 quarters from 5 cows) for non-DCT in farm B; and 76.2% (16/21 quarters from 7 cows) for selective DCT in farm C. The rate of uncured mammary infections in farm C was significantly higher (P < 0.05) than those in farms A and B.
The differences in intramammary infection status, recovery rates, and new and uncured mammary infections in the mammary quarters of cows at the dry-off and immediate postpartum periods were characterized on three dairy farms with dry cow management approaches (Figure 1).
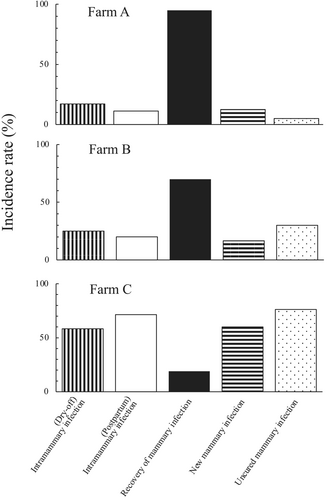
3.3 Monitoring of the infection status of mammary glands in cows during the immediate postpartum and 1-month postpartum periods
To assess the prevalence of mammary infections in the herds, quarters exhibiting the presence of mastitis-causing pathogens (>300 cfu/ml) and elevated SC counts (>1,010 × 103 cells/mL, in colostrum within 5 days postpartum, McDonald & Anderson, 1981) in the mammary secretions from cows were evaluated (Table 2). In this analysis, 3.4% (4/117) of quarters in farm A, 7.5% (6/80) of quarters in farm B, and 8.3% (3/36) of quarters in farm C were identified as cases of subclinical mastitis in the mammary glands. The primary pathogens isolated from the quarters with subclinical mastitis included C. bovis, E. coli, and CNS in farm A; S. aureus, En Str., and CNS in farm B; and CNS and En Str. in farm C.
| Farm | Mammary infection† (quarter) | Cow‡ | Pathogens§ | SC counts (× 103 cells/mL)¶ range |
|---|---|---|---|---|
| A | 3.4% (4/117) | 4 | C. bovis [2], E. coli [1], CNS [1] | 2,247–5,025 |
| B | 7.5% (6/80) | 6 | S. aureus [3], En. Str.[2], CNS [1] | 4,103–31,170 |
| C | 8.3% (3/36) | 3 | CNS [2], En. Str. [1] | 2,916–39,180 |
- C. bovis, Corynebacterium bovis; E. coli: Escherichia coli; CNS, coagulase-negative staphylococci; S. aureus, Staphylococcus aureus;
- En. Str.; environmental streptococci.
- † %: Positive quarters per total tested quarters.
- ‡ Number of cows.
- § >300 cfu/ml. Number in brackets indicates the number of isolates.
- ¶ SC counts > 1,010 × 103 cells/mL, McDonald and Anderson (1981) was used as normal SC values in postpartum dairy cows within three days after parturition.
This study observed the incidence of clinical mastitis in the quarters of cows that required either systemic or local antibiotic therapy for 1 month postpartum. In farm A, seven cases of clinical mastitis in quarters were found, affecting four cows. These cases were attributed to infections caused by E. coli and CNS. In contrast, no cases of clinical mastitis were detected on farms B and C during the assessed period.
3.4 Antibacterial resistance of CNS isolated from cow quarters in the postpartum period
The study evaluated the antimicrobial resistance of CNS isolated from cow quarters during the postpartum period (Table 3). The MICs of the antimicrobial drugs were evaluated to determine the antimicrobial resistance of the isolated CNS to TS, LCM, OTC, KM, ABPC, and CEZ. On farm A, 25% (1/4) of CNS showed resistance to both KM (64 μg/ml) and OTC (16 μg/ml). On farm C, 20% (1/5) of CNS exhibited resistance to TS (>128 μg/ml), LCM (>128 μg/ml), and ABPC (4 μg/ml). No antibacterial resistance was observed in the isolates from cows on farm B.
| Antibiotics | TS | LCM | OTC | KM | ABPC | CEZ | |
|---|---|---|---|---|---|---|---|
| Breakpoint (μg/mL)1 | 16a | 36b | 12c | 64d | 0.5e | 32d | |
| Farm | A (4)2 | 0.5–2 | 0.125–16 | 0.25–16 | 2–64 | 0.125–0.5 | 0.5–8 |
| [1/4]3 | [1/4]3 | ||||||
| B (5) | 1–2 | 0.2–16 | 0.5–4 | 0.25–4 | 0.125–0.25 | 0.5–8 | |
| C (5) | 0.5ー > 128 | 0.5ー > 128 | 0.25–4 | 1–4 | 0.125–4 | 0.25–1 | |
| [1/5]3 | [1/5]3 | [1/5]3 |
- TS, tylosin; LCM: lincomycin; OTC, oxytetracycline; KM, kanamycin; ABPC, ampicillin; CEZ, cefazolin.
- 1 Figures in brackets show the breakpoint values of antibacterial resistance.
- 2 Numbers in brackets indicate the isolates used.
- 3 Number of isolates exceeding the breakpoint values. Positive case per tested samples.
- a Swine reference value (Pringle et al., 2012).
- b Mirajkar et al. (2016).
- c National Veterinary Assay Laboratory (NVAL) (2013).
- d Breakpoints eliminated from Clinical & Laboratory Standards Institute (CLSI) document M100 since 2010 (Reflects M100-Ed31, March 2021).
- e Minimum inhibitory concentration (MIC) interpretive standards CLSI M7 - A8, M100 - S21 (2011).
4 DISCUSSION
The mean prevalence of mastitis-causing pathogens in cow mammary quarters during the dry and postpartum periods varied across the three farms. During the dry period, this prevalence ranged from 17.1% to 58.3%, whereas during the postpartum period, it ranged from 12.1% to 74.3% (data not shown). This wide variation in mammary infections was observed at the quarter and cow levels within these three farms. On farm C, where the intramammary infection rate in the quarters from cows at drying-off was high compared with those on farms A and B, and where the mammary infections appeared to be effectively controlled, these differences are considered to be linked to variations in cow management practices and environmental hygiene on the farms. Similar trends have been observed on farms that employ automatic milking systems, as previously reported by Dohmen et al. (2010) and Hovinen and Pyörälä (2011).
CNS was the most commonly identified mastitis-causing pathogen in the mammary secretions of dairy cows, and this finding is consistent with previous reports (Pyörälä & Taponen, 2009). The presence of CNS and En. Str. as mastitis-causing pathogens in cow quarters during both the dry and postpartum periods emphasizes the importance of implementing mastitis control measures with a specific focus on environmental controls within the farms.
Bacteriological cure, defined as the successful treatment of an infected mammary quarter that tested negative for a previously identified pathogen at the drying-off period after DCT, varies in effectiveness depending on factors such as the cow's age, lactation number, SC count, host immune response, specific pathogens, and antibiotic choice (Sol et al., 1997; Barkema, Van Der Ploeg, et al., 1999). In the three dairy farms included in this study, the recovery rates of intramammary infections, which were determined to be negative for pathogens in quarter milk that had tested positive for pathogens at the drying-off after receiving blanket DCT, non-DCT, and selective DCT, indicated that on farm A, DCT with antimicrobials was effective, as evidenced by the high recovery rates of mammary infections and low incidence of new infections (Figure 1).
On farm B, the recovery rates for mammary infections and rates of new infections in cows receiving non-DCT were favorably controlled and similar to those of cows receiving blanket DCT on farm A. This suggests that proper dry cow management in non-DCT settings can contribute to its effectiveness. The specific factors responsible for the relatively high recovery rates of mammary infections on farm B are not fully understood; however, they may be related to the interactions between minor mastitis-causing pathogens, such as CNS, and the cows' mammary defense mechanisms under conditions of a lower frequency of exposure of the cow quarters to pathogens on the farm. These interactions could play an important role in enhancing spontaneous recovery and phagocytic elimination of pathogens (Nagahata et al., 2020; Sordillo et al., 1997; Trevisi & Minuti, 2018). Simojoki et al. (2011) reported a spontaneous elimination rate of 31.3% for CNS with an experimentally induced intramammary infection. The relatively low SC counts in the herds and dry cow management practices implemented on the farm may have contributed to maintaining mammary health in dry cows.
On farm C, the findings related to the overall intramammary infections, recovery rates, and new infection rates indicate the presence of potential issues within the farm's management practices. The major pathogens causing mammary infections in the cow mammary quarters detected at postpartum were CNS, Str. spp., C. bovis, and S. aureus. This suggests that diverse mastitis-causing pathogens were prevalent and that the cow quarters could be exposed to these microorganisms within the herd. The lower recovery rate is likely associated with the presence of chronic mammary infections that did not respond adequately to treatment within the herd. Regarding the high rates of new intramammary infections within the herd, it was assumed that the quarters were exposed to environmental pathogens during the dry and immediate postpartum periods. In a review of published data comparing blanket and selective DCT approaches for the elimination and prevention of mammary infections during the dry period, Kabera et al. (2021) suggested that the selective DCT approach could reduce the use of antimicrobials during the dry period without negatively affecting udder health, particularly when internal teat sealants are used for the quarters. The use of internal teat sealants (bismuth subnitrate) has currently not been approved for quarters during the dry period in the country. In addition, the high rates of new mammary infections in the quarters suggest that the hygiene levels of free-stall housing conditions, particularly those using recycled materials on the bed floor, should be thoroughly assessed to ensure optimal dairy environments and maintain mammary health in cows during the dry period.
In the milk analysis, an SC count of 200 × 103 cells/mL has been used as the threshold value for the selection of the quarters or cows in the selective DCT approach (Cameron et al., 2014; Pantoja et al., 2009; Zecconi et al., 2020). In the selective DCT approach adopted by farm C, SC values of 300 × 103 cells/mL in quarter milk, which are widely used values as an approved level of bulk milk SC counts on dairy farms (Hokkaido Dairy Milk Recording & Testing Association, 2016), were tentatively used for the selection of quarters or cows for the selective DCT approach on the farm. The cure rate of the infected quarters by selective DCT, expressed as the number of bacteriologically cured quarters divided by the total number of treated quarters, is used as a parameter to evaluate the efficacy of selective DCT on the farms. However, the proportion of quarters needed for DCT to the total number of drying quarters is also considered to be critically important for the prevention of mammary infections and milk productivity in cows on the farm. In addition, the threshold levels of quarters or cows used for selective DCT, i.e., infectious status and SC count levels, remain to be established as standard criteria in the country. Further studies are required to develop more practical and reliable criteria for SC count levels for the selection of cows for selective DCT on farms.
Figure 1 illustrates the results characterizing the intramammary infection status of cows during the dry and postpartum periods based on the dry cow management approaches implemented on the three farms. The analysis of intramammary infection status on the farms, recovery rates, and new and uncured infection rates provides a foundation to elucidate the substantial problems and potential improvements in dry cow management approaches on the farms. The quarter-level of measured variables can likely provide valuable insights into the issues faced by dry cow management on the farm.
A small proportion of quarters were identified as having subclinical mastitis, characterized by increased pathogen counts and markedly elevated SC counts in the mammary secretions and without apparent clinical findings in the mammary glands. This finding suggests that mammary infections present in the quarters could potentially lead to clinical mastitis during the early stages of lactation in postpartum cows. Furthermore, during the 1-month follow-up study, clinical mastitis was observed in seven mammary quarters of four cows on farm A, which was attributed to E. coli and CNS infections are required both systemic and local therapy. Barkema et al. (Barkema, Schukken, et al., 1999; Barkema, Van Der Ploeg, et al., 1999) previously reported that the incidence rates of clinical mastitis caused by E. coli and En. Str. were closely related to factors such as housing conditions, milking hygiene, and machine milking practices.
Previous studies have shown that the occurrence of antimicrobial-resistant bacteria is related to selection pressures that result from antimicrobial consumption in cattle (Alexander et al., 2010; Bergman et al., 2009). As for the antibacterial resistance of mastitis-causing pathogens in the herds, on farm A, antibacterial resistance was observed in the CNS against OTC and KM. In contrast, farm C showed a notable increase in the antibacterial resistance of CNS, particularly against TS, LCM, and ABPC. In farm A, a moderate increase in the MIC values of CNS to CEZ was noted, which suggests a tendency toward increased resistance, reaching approximately 8 μg/ml. These distinct patterns of antibacterial resistance in CNS isolated from the farms appear to be influenced by the use of antibiotics for treating infectious diseases within the farm, as previously discussed in other studies (Roesch et al., 2006; Saini et al., 2012).
These findings could assist with the judgment of whether DCT management approaches are properly applied on farms. It also provides possible suggestions to improve the underlying practical and substantial challenges of dry cow management approaches for the prevention of mammary infections and the promotion of mammary health during the dry and postpartum periods on farms.
ACKNOWLEDGMENTS
The authors express their gratitude to F farm in Eniwa, Hokkaido; RGU farm in Ebetsu, Hokkaido; and W farm in Imabari, Ehime Prefecture, for their cooperation in this research. Special thanks to Ayako Eguchi, Ryo Murakami, Kazuki Noda, and Kanako Inomata from the Animal Health Laboratory School of Veterinary Medicine at Rakuno Gakuen University in Hokkaido, Japan, for their assistance with SC counts in the quarter milk. The authors also extend their appreciation to Dr. Masaru Usui from the School of Veterinary Medicine at Rakuno Gakuen University for their valuable guidance on interpreting the MIC breakpoints for microorganisms and to Dr. Daiji Endoh, also from the School of Veterinary Medicine at Rakuno Gakuen University, for assisting with data retrieval.
CONFLICT OF INTEREST STATEMENT
Authors declare no Conflicts of Interest for this article.




