Comparison of nutrient supply from the dam to fetus and placental development in Holstein and Japanese black cows pregnant with similar or different fetus breeds
Abstract
A lower nutrient supply from Holstein (HOL) dams to beef fetuses than HOL fetuses has been demonstrated, but the underlying factors remain unclear. We investigated maternal, umbilical vein, and calf blood glucose and amino acid concentrations at calving, along with placental development at term, in HOL dams with similar fetuses (HOL-HOL, n = 12), F1 crosses (HOL × Japanese Black [JB]; HOL-F1, n = 4), JB fetuses (HOL-JB, n = 7), and JB dams with similar fetuses (JB-JB, n = 11). Calf birth weight, total cotyledonary weight, and surface area were greater in HOL-HOL compared to JB-JB or HOL-JB (P < 0.05), whereas those of HOL-F1 were similar. Blood amino acid concentrations in the umbilical veins and calves were similar among HOL-HOL, HOL-F1, and HOL-JB. Calf blood glucose concentrations were lower in HOL-F1 than HOL-HOL (P < 0.05), despite similar maternal blood glucose levels. HOL-JB exhibited higher maternal, umbilical vein, and calf blood glucose concentrations than JB-JB (P < 0.05). Therefore, the glucose supply to the fetus may be inhibited in HOL-F1 due to maternal-fetal breed differences. Higher maternal blood glucose concentrations in HOL-JB may result in elevated fetal glucose exposure, potentially affecting postnatal growth and metabolism.
1 INTRODUCTION
The global production of beef calves from dairy cows has increased recently (Berry, 2021). In Japan, 43% of the semen used by Holstein (HOL) cows is Japanese Black (JB), a typical Japanese beef breed (Ministry of Agriculture, Forestry, and Fisheries [MAFF], 2024). Furthermore, 11% of all the JB calves produced annually are derived from dairy cows via embryo transfer (MAFF, 2024). Since JB calves are smaller at birth than HOL calves (Isobe et al., 2003; Isogai et al., 1994), they are actively utilized for artificial insemination or embryo transfer into dairy heifers to prevent dystocia. Moreover, JB calves are purchased at higher prices than dairy calves (MAFF, 2024), thus providing a source of income for dairy farmers. The Nutrient Requirements of Dairy Cattle (National Academies of Science, Engineering, and Medicine [NASEM], 2023) provides nutritional guidelines for dairy cows based on their stage of pregnancy. The Japanese Feeding Standard for Dairy Cows (National Agriculture and Food Research Organization [NARO], 2017) indicates that the nutritional requirements for dairy dams pregnant with beef fetuses are slightly higher than those for beef dams pregnant with beef fetuses. However, it does not specify the differences in nutritional requirements between dairy dams pregnant with dairy and beef fetuses (NARO, 2017). Pregnant JB cows are typically managed individually, whereas dairy cows are managed in herds. Consequently, dairy cows are managed without considering fetal breeds, leaving the effects of fetal growth and nutrient supply unclear.
Glucose and amino acids are the main nutrients required for fetal growth (Bell & Ehrhardt, 2002; Nishida, 2009). Glucose transported down a concentration gradient promotes net glucose transport toward the fetus when the maternal glucose concentration exceeds that of the fetus (Barry & Anthony, 2008; Novakovic et al., 2013). Additionally, it is influenced by glucose transporters, maternal nutrition, and fetal growth capacity (Bell & Ehrhardt, 2002). Most amino acids are transported along a fetal-maternal concentration gradient through active transport processes (Bell & Ehrhardt, 2002; Lager & Powell, 2012; Regnault et al., 2005). Whereas short-term maternal fasting in pregnant ewes did not notably alter the amino acid supply from the dam to the fetus (Lemons & Schreiner, 1983), long-term maternal nutrient restriction reduced blood amino acid concentrations in both the umbilical veins and fetuses (Kwon et al., 2004).
Previously, we investigated blood glucose and amino acid concentrations in dams, umbilical veins, and calves at calving in HOL dams pregnant with HOL or beef (JB or F1 cross of HOL and JB) fetuses (Kawashima et al., 2021). Blood glucose and amino acid concentrations in the umbilical veins of dams pregnant with beef fetuses and in beef calves were lower than those in the umbilical veins of dams pregnant with HOL fetuses and in HOL calves (Kawashima et al., 2021). Maternal blood glucose and amino acid concentrations were similar regardless of fetal breed (Kawashima et al., 2021), suggesting a weak relationship between maternal nutrient status and nutrient supply to the fetus. Thus, the following two factors may contribute to the reduced nutrient transfer from dairy dams to beef fetuses. First, JB calves have a lighter birth weight than dairy calves, which may result in lower nutritional requirements during pregnancy. Second, breed differences between dams and fetuses could inhibit placental development, thereby preventing the efficient supply of nutrients to the fetus. However, in our previous study, we did not include beef dams pregnant with beef fetuses and did not examine the placenta; therefore, we were unable to elucidate the effect of these factors on nutrient supply to the fetus.
Therefore, this study aimed to identify the factors contributing to decreased nutrient supply in dairy dams pregnant with beef fetuses. This was achieved by examining the blood glucose and amino acid concentrations of dams, umbilical veins, and calves at calving as well as placental development at term in HOL and JB dams pregnant with similar or different fetal breeds. Furthermore, the following analyses were conducted: (i) comparison of HOL and JB dams with similar fetuses, (ii) comparison of HOL dams with HOL, F1, and JB fetuses, and (iii) comparison of JB and HOL dams with JB fetuses.
2 MATERIALS AND METHODS
2.1 Animals, feeding, and management
The experimental procedures performed in this study complied with the Guide for the Care and Use of Agricultural Animals of Obihiro University (approval numbers: #20–147, 21–14, 22–13, 22–91, and 23–35).
The experiments were conducted between September 2020 and July 2023 at the Field Center of Animal Science and Agriculture, Obihiro University of Agriculture and Veterinary Medicine, Japan. We examined 23 HOL dams (all heifers) pregnant with HOL (HOL-HOL, n = 12), F1 crosses (HOL × JB; HOL-F1, n = 4), and JB (HOL-JB, n = 7) fetuses, and 11 JB dams (Heifer, n = 4; Multiparous, n = 7) pregnant with JB fetuses (JB-JB, n = 11). HOL cows were artificially inseminated with HOL or JB semen, or they received a JB embryo transfer. Similarly, JB cows were artificially inseminated with JB semen. Approximately 1 month before the expected calving date, the HOL dams were moved to a free-stall shed with a paddock. They were fed a mixed ration of grass and corn silage, and concentrate at 15:00 h. until calving. The diet provided 11.3 kg/day/head, with 993.7 g/day/head of metabolizable protein and 28.1 Mcal/day/head of metabolizable energy on a dry matter basis. Additional dry cow concentrate rations were offered using a feeding station, depending on the number of days before the expected calving date (day −15 to −11: 0.5 kg/cow/day; day −10 to −6: 1.0 kg/cow/day; day −5 to calving: 1.5 kg/cow/day). They had ad libitum access to hay, minerals, and water. The JB dams were grazed on timothy pasture from 09:00 to 15:00 h daily. Approximately 1 month before the expected calving date, they were individually fed hay (2.0 kg/head) and concentrate (2.0 kg/head) at 08:00 and 16:00 h. This diet provided 440.3 g/day/head of metabolizable protein and 9.33 Mcal/day/head of metabolizable energy on a dry matter basis. They had ad libitum access to minerals and water. The rectal temperatures of HOL and JB dams were recorded daily from 15:00 to 16:00 h for 1 week before the expected calving date. When the rectal temperature decreased by 0.5°C from the previous day, they were moved to individual calving pens and housed until calving. After birth, the calves were cleaned and dried with a towel and their umbilical cords were disinfected. Calves were weighed and housed in individual pens until the colostrum was provided. Gestation length and calving difficulty (scored from 1 = unassisted birth to 5 = surgical treatment or death of the cow, Berger, 1994) were recorded for all dams. The same operator assessed the body condition score (BCS) of all dams 2–3 weeks before the expected calving date. The BCS of HOL dams was as follows: HOL-HOL, 3.69 ± 0.08; HOL-F1, 3.81 ± 0.12; HOL-JB, 3.54 ± 0.04, respectively, using a scale from 1 to 5 in 0.25 intervals (Ferguson et al., 1994). Additionally, the BCS of JB dams was 6.09 ± 0.16, which is within the normal range (BCS of 5–6) according to the 1 to 9-point scoring system (Wagyu Registry Association, 2009).
2.2 Sampling and measurement of blood parameters
Blood samples were collected from dams 2–3 weeks before the expected calving (at 07:30 h) and immediately after calving (within 1 h) by caudal venipuncture. Blood samples were collected from the umbilical vein at calving according to the procedure described by Kawashima et al. (2021). In addition, blood samples were collected from calves via the jugular vein immediately after birth (within 1 h), before the first colostrum feeding. Non-heparinized, silicone-coated 9-mL tubes (Venoject, Autosep, Gel + Clot. Act., VP-AS109K; Terumo Corporation, Tokyo, Japan) were used for glucose measurements, and 5 mL tubes containing ethylenediaminetetraacetic acid (Venoject II, VP-NA050K; Terumo Corporation, Tokyo, Japan) were used for amino acid measurements. Blood samples were coagulated in an incubator at 38°C for 10 min to obtain the serum. All tubes were then centrifuged at 2328×g for 15 min at 4°C, and serum and plasma samples were stored at −30 and −80°C, respectively, until further analysis. Serum glucose concentrations were obtained using an automated clinical chemistry analyzer (TBA120FR; Toshiba Medical Systems Co., Ltd., Tochigi, Japan), and plasma amino acid concentrations were obtained using ultra-performance liquid chromatography-mass spectrometry at the NTDS Cooperation (Hokkaido, Japan).
2.3 PLACENTAL COLLECTION AND MEASUREMENT
We collected complete placentas that were spontaneously expelled from the dams within 12 h of calving. One HOL-JB sample could not be collected and was excluded from analysis. The entire placenta was weighed immediately after expulsion. The cotyledons were then individually separated from the intercotyledonary membrane by cutting them with scissors. Cotyledons weighing less than 2.0 g were not included in further analyses (Mashimo et al., 2023) because they could not be completely removed from the chorion and gelatinized amniotic fluid. Individual cotyledons were counted, weighed, flattened on a grid sheet, and photographed for surface area analysis using an image analysis software (ImageJ; https://imagej.net/software/fiji/). Intercotyledonary membrane weight was measured after the umbilical cords were cut. Total cotyledonary weight and surface area were calculated as the sum of all cotyledon weights and surface areas, respectively. The time interval between calving and placental expulsion was also recorded.
2.4 Statistical analysis
We were unable to collect one blood sample from the calves in JB-JB because they required veterinary treatment immediately after birth owing to dystocia. Furthermore, six blood samples were not collected from the umbilical vein (HOL-F1, n = 3; HOL-JB, n = 1; JB-JB, n = 2) because the umbilical cord was cut during the calving process. The sample size of the umbilical vein in HOL-F1 (n = 1) was insufficient and was therefore excluded from the statistical analysis.
All statistical analyses were performed using the SigmaPlot® 14.5 (Systat Software, Inc., San Jose, CA, USA). The normality of the data was tested using the Shapiro–Wilks test. Data were compared between the following breeds: HOL-HOL vs. JB-JB, HOL-HOL vs. HOL-F1 or HOL-JB, and JB-JB vs. HOL-JB. Differences in gestation length, calving difficulty, calf birth weight, placental parameters, serum glucose, and plasma amino acid concentrations were analyzed using the Student's t-test or the Mann–Whitney U test. Calf sex ratios were compared using Fisher's exact test. To examine the distribution of individual cotyledon weights and surface areas in the groups, all data were categorized into four stages by 25% in order of heaviest or largest (first stage: 76–100%; second stage: 51–75%; third stage: 26–50%; fourth stage: 1–25%; Table 1) based on the quartile (Mashimo et al., 2023). Differences in the percentage of each stage were analyzed using Student's t-test or the Mann–Whitney U test. The effect of parity in JB-JB was observed only on the plasma concentrations of total essential amino acids (P = 0.035), branched-chain amino acids (P = 0.019), and glucogenic and ketogenic amino acids (P = 0.037) of dams immediately after calving. However, no effects were noted on other parameters (refer to Table S1-S4 and Figure S1). All data are presented as the mean ± standard error of the mean (SEM), and a P-value of < 0.05 was considered significantly different.
| Stage | Percentage (%) | Cotyledonary weight (g) | Cotyledonary surface area (cm2) |
|---|---|---|---|
| First | 76–100 | 27.28–107.71 | 59.07–251.71 |
| Second | 51–75 | 16.64–27.27 | 38.31–59.06 |
| Third | 26–50 | 9.13–16.63 | 23.78–38.30 |
| Fourth | 1–25 | 2.00–9.12 | 4.82–23.77 |
- All cotyledonary data were categorized from first to fourth stage by 25% based on quartile.
- † Cotyledon weight less than 2.0 g was excluded from the investigation (Mashimo et al., 2023).
3 RESULTS AND DISCUSSION
3.1 Comparison of similar breeds between dam and fetus in HOL and JB cows
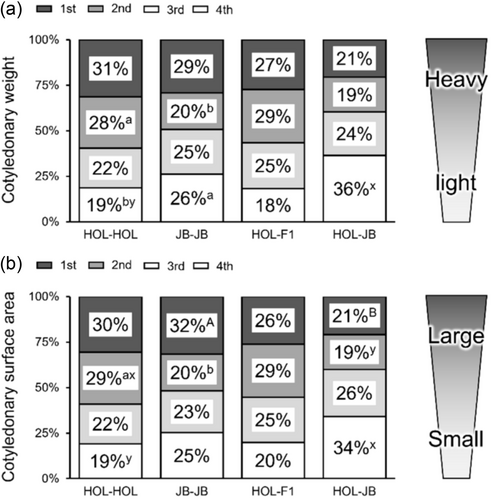
Gestation length was longer in JB-JB than in HOL-HOL (P < 0.001; Table 2). Calves in the JB-JB group had a lower birth weight than HOL-HOL calves (P = 0.004), whereas the sex of the calves (P = 0.414) and calving difficulty (P = 0.196) were similar (Table 2). Total placental weight (P = 0.032), total cotyledonary weight (P < 0.001), total cotyledonary surface area (P < 0.001), and the number of cotyledons (P = 0.006) were greater in the HOL-HOL compared to JB-JB (Table 3). Regarding individual cotyledons, the percentages of the second stage of cotyledonary weight and surface area were lower (P = 0.016; P = 0.005), and the fourth stage of cotyledonary weight (P = 0.049) was higher in JB-JB than in HOL-HOL (Figure 1).
| Breed | (i) HOL-HOL | (ii) JB-JB | (iii) HOL-F1 | (iv) HOL-JB | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number | 12 | 11 | 4 | 7 | (i) Vs. (ii) | (i) Vs. (iii) | (i) Vs. (iv) | (ii) vs. (iv) |
| Gestation length (day) | 277.3 ± 1.3 | 291.3 ± 1.1 | 283.3 ± 1.3 | 285.6 ± 1.2 | <0.001 | 0.023 | <0.001 | 0.003 |
| Calving difficulty† | 1.83 ± 0.21 | 2.55 ± 0.39 | 1.00 ± 0.00 | 2.00 ± 0.49 | 0.196 | 0.033 | 1.000 | 0.320 |
| Calf birth weight (kg) | 41.0 ± 1.1 | 35.5 ± 1.3 | 40.0 ± 1.7 | 36.3 ± 2.2 | 0.004 | 0.646 | 0.047 | 0.724 |
| Sex of the calves (male/female) | 5/7 | 7/4 | 0/4 | 3/4 | 0.414 | 0.245 | 1.000 | 0.630 |
- Values are presented as means ± standard error of the means. †Score 1, unassisted birth (natural, without human assistance); score 2, easy calving with human assistance; score 3, difficult calving with few humans; score 4, dystocia (requiring much more force than normal); and score 5, surgical treatment or death of cow (Berger, 1994).
- Abbreviations: HOL-HOL, Holstein dam pregnant with Holstein fetus; HOL-F1, Holstein dam pregnant with F1 cross (HOL × JB) fetus; HOL-JB, Holstein dam pregnant with Japanese Black fetus; JB-JB, Japanese Black dam pregnant with Japanese Black fetus.
| Breed | (i) HOL-HOL | (ii) JB-JB | (iii) HOL-F1 | (iv) HOL-JB | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number | 12 | 11 | 4 | 6 | (i) Vs. (ii) | (i) Vs. (iii) | (i) Vs. (iv) | (ii) vs. (iv) |
| Duration of placenta expulsion (h)† | 4.61 ± 0.33 | 4.30 ± 0.48 | 4.38 ± 1.19 | 3.63 ± 0.42 | 0.593 | 0.789 | 0.096 | 0.372 |
| Total placental weight (kg)‡ | 5.48 ± 0.34 | 4.57 ± 0.19 | 4.93 ± 0.48 | 4.82 ± 0.72 | 0.032 | 0.411 | 0.353 | 0.668 |
| Inter-cotyledonary membrane weight (kg) | 2.66 ± 0.25 | 2.48 ± 0.14 | 2.21 ± 0.36 | 2.81 ± 0.55 | 0.552 | 0.369 | 0.963 | 0.457 |
| Total cotyledonary weight (kg)§ | 2.50 ± 0.11 | 1.77 ± 0.08 | 2.25 ± 0.24 | 1.68 ± 0.20 | <0.001 | 0.299 | 0.001 | 0.618 |
| Total cotyledonary surface area (m2)§ | 0.56 ± 0.02 | 0.41 ± 0.01 | 0.49 ± 0.03 | 0.39 ± 0.04 | <0.001 | 0.113 | 0.001 | 0.594 |
| Number of cotyledons (n)§ | 120.8 ± 10.8 | 82.7 ± 5.6 | 108.3 ± 12.4 | 104.3 ± 14.1 | 0.006 | 0.548 | 0.383 | 0.109 |
- Values are presented as means ± standard error of the means.
- Abbreviations: HOL-HOL, Holstein dam pregnant with Holstein fetus; HOL-F1, Holstein dam pregnant with F1 cross (HOL × JB) fetus; HOL-JB, Holstein dam pregnant with Japanese Black fetus; JB-JB, Japanese Black dam pregnant with Japanese Black fetus.
- † Interval from calving to placental expulsion.
- ‡ Weight that was spontaneously expelled within 12 h of calving.
- § Cotyledon less than 2.0 g were excluded from the analysis (Mashimo et al., 2023).
Two to three weeks before expected calving, the serum glucose concentrations of dams in JB-JB were lower than those in HOL-HOL (P = 0.019); plasma amino acid concentrations were similar (Table 4). Immediately after calving, the plasma total essential (P < 0.001), branched-chain (P = 0.001), ketogenic (P < 0.001), and glucogenic and ketogenic amino acid (P < 0.001) concentrations of JB-JB dams were higher than those of HOL-HOL dams (Table 5). The serum glucose (P < 0.001; P < 0.001) and plasma total (P = 0.015; P = 0.016), total essential (P = 0.021; P = 0.010), and total non-essential (P = 0.016; P = 0.023), branched-chain (P = 0.023; P = 0.006), glucogenic (P = 0.013; P = 0.017), and ketogenic amino acid (P = 0.018; P = 0.012) concentrations in JB-JB were lower than those in HOL-HOL from umbilical veins and calves, respectively (Table 5).
| Breed | (i) HOL-HOL | (ii) JB-JB | (iii) HOL-F1 | (iv) HOL-JB | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number | 12 | 11 | 4 | 7 | (i) Vs. (ii) | (i) Vs. (iii) | (i) Vs. (iv) | (ii) vs. (iv) |
| Glucose (mg/dL) | 72.3 ± 1.3 | 66.7 ± 2.1 | 69.3 ± 1.7 | 74.8 ± 2.5 | 0.019 | 0.390 | 0.177 | 0.396 |
| Total amino acid (μmol/L) | 224.9 ± 4.6 | 226.4 ± 9.9 | 269.6 ± 23.3 | 274.7 ± 11.8 | 0.893 | 0.005 | 0.027 | 0.069 |
| Total essential amino acid (μmol/L) | 101.3 ± 3.2 | 106.0 ± 5.1 | 130.9 ± 11.3 | 125.1 ± 8.5 | 0.601 | 0.006 | 0.006 | 0.038 |
| Total non-essential amino acid (μmol/L) | 123.6 ± 2.6 | 120.3 ± 5.9 | 138.7 ± 12.2 | 149.6 ± 7.6 | 0.604 | <0.001 | 0.138 | 0.148 |
| Branched-chain amino acid (μmol/L) | 55.2 ± 1.8 | 60.8 ± 2.9 | 73.0 ± 6.4 | 70.9 ± 5.5 | 0.176 | 0.003 | 0.005 | 0.066 |
| Glucogenic amino acid (μmol/L) | 170.0 ± 3.3 | 167.3 ± 7.3 | 201.8 ± 18.7 | 210.0 ± 9.5 | 0.737 | <0.001 | 0.044 | 0.064 |
| Ketogenic amino acid (μmol/L) | 24.6 ± 0.9 | 27.1 ± 1.3 | 32.53.0 | 28.7 ± 2.4 | 0.133 | 0.063 | 0.006 | 0.079 |
| Glucogenic and ketogenic amino acid (μmol/L) | 29.2 ± 0.8 | 31.1 ± 1.7 | 34.3 ± 2.1 | 35.0 ± 2.4 | 0.424 | 0.010 | 0.017 | 0.252 |
- Values are presented as means ± standard error of the means.
- Abbreviations: HOL-HOL, Holstein dam pregnant with Holstein fetus; HOL-F1, Holstein dam pregnant with F1 cross (HOL × JB) fetus; HOL-JB, Holstein dam pregnant with Japanese Black fetus; JB-JB, Japanese Black dam pregnant with Japanese Black fetus.
| Breed | (i) HOL-HOL | (ii) JB-JB | (iii) HOL-F1 | (iv) HOL-JB | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number of dam, umbilical vein, calf | 12, 12, 12 | 11, 9, 10 | 4, 1, 4 | 7, 6, 7 | (i) Vs. (ii) | (i) Vs. (iii) | (i) Vs. (iv) | (ii) vs. (iv) |
| Glucose (mg/dL) | ||||||||
| Dam | 108.6 ± 3.6 | 104.8 ± 5.6 | 120.0 ± 10.0 | 125.4 ± 4.6 | 0.574 | 0.194 | 0.011 | 0.020 |
| Umbilical vein | 73.6 ± 5.1 | 42.2 ± 5.6 | 63.0 | 65.5 ± 10.9 | <0.001 | ― | 0.448 | 0.025 |
| Calf | 138.0 ± 15.2 | 60.0 ± 7.3 | 65.5 ± 7.6 | 112.4 ± 16.7 | <0.001 | 0.018 | 0.295 | 0.006 |
| Total amino acid (μmol/L) | ||||||||
| Dam | 169.7 ± 6.7 | 173.3 ± 6.4 | 182.7 ± 9.1 | 188.9 ± 13.6 | 0.705 | 0.331 | 0.172 | 0.260 |
| Umbilical vein | 418.1 ± 36.1 | 299.6 ± 16.9 | 382.8 | 411.0 ± 79.4 | 0.015 | ― | 0.126 | 0.574 |
| Calf | 379.2 ± 38.6 | 261.5 ± 15.8 | 309.1 ± 59.7 | 325.8 ± 67.8 | 0.016 | 0.368 | 0.128 | 0.845 |
| Total essential amino acid (μmol/L) | ||||||||
| Dam | 55.4 ± 2.4 | 73.7 ± 4.2 | 61.0 ± 2.6 | 60.5 ± 3.5 | <0.001 | 0.238 | 0.232 | 0.043 |
| Umbilical vein | 134.8 ± 11.6 | 98.5 ± 5.9 | 127.7 | 126.2 ± 26.1 | 0.021 | ― | 0.239 | 0.303 |
| Calf | 114.0 ± 12.0 | 73.9 ± 5.0 | 86.3 ± 19.8 | 91.2 ± 19.4 | 0.010 | 0.263 | 0.076 | 0.770 |
| Total non-essential amino acid (μmol/L) | ||||||||
| Dam | 114.3 ± 4.7 | 99.6 ± 5.6 | 121.7 ± 11.5 | 128.4 ± 10.5 | 0.056 | 0.484 | 0.177 | 0.018 |
| Umbilical vein | 283.4 ± 25.1 | 201.0 ± 12.5 | 255.0 | 284.8 ± 53.8 | 0.016 | ― | 0.099 | 0.708 |
| Calf | 265.2 ± 26.9 | 187.6 ± 12.3 | 222.8 ± 40.5 | 234.6 ± 48.7 | 0.023 | 0.431 | 0.237 | 0.922 |
| Branched-chain amino acid (μmol/L) | ||||||||
| Dam | 31.9 ± 1.6 | 43.7 ± 2.9 | 34.8 ± 2.0 | 35.0 ± 2.6 | 0.001 | 0.363 | 0.242 | 0.041 |
| Umbilical vein | 64.2 ± 5.2 | 47.8 ± 3.2 | 57.6 | 63.5 ± 15.0 | 0.023 | ― | 0.349 | 0.289 |
| Calf | 55.9 ± 5.4 | 36.3 ± 2.6 | 44.6 ± 3.1 | 46.5 ± 11.1 | 0.006 | 0.328 | 0.128 | 0.770 |
| Glucogenic amino acid (μmol/L) | ||||||||
| Dam | 140.4 ± 5.6 | 133.6 ± 5.5 | 150.7 ± 10.9 | 158.1 ± 12.5 | 0.405 | 0.388 | 0.157 | 0.059 |
| Umbilical vein | 348.7 ± 29.8 | 248.6 ± 14.2 | 313.9 | 346.6 ± 66.5 | 0.013 | ― | 0.640 | 0.099 |
| Calf | 321.6 ± 31.9 | 224.8 ± 13.5 | 265.5 ± 9.3 | 280.0 ± 58.0 | 0.017 | 0.385 | 0.176 | 0.845 |
| Ketogenic amino acid (μmol/L) | ||||||||
| Dam | 13.6 ± 0.7 | 18.2 ± 1.0 | 14.7 ± 0.8 | 14.0 ± 1.0 | <0.001 | 0.422 | 0.779 | 0.011 |
| Umbilical vein | 33.2 ± 4.1 | 20.5 ± 1.4 | 31.3 | 29.2 ± 8.4 | 0.018 | ― | 0.190 | 0.216 |
| Calf | 27.7 ± 4.4 | 13.9 ± 1.3 | 18.7 ± 1.0 | 20.1 ± 6.7 | 0.012 | 0.306 | 0.108 | 0.845 |
| Glucogenic and ketogenic amino acid (μmol/L) | ||||||||
| Dam | 15.1 ± 0.7 | 20.9 ± 1.3 | 16.9 ± 1.1 | 16.3 ± 0.8 | <0.001 | 0.194 | 0.272 | 0.016 |
| Umbilical vein | 34.8 ± 2.5 | 29.7 ± 2.0 | 35.2 | 34.4 ± 5.0 | 0.147 | ― | 0.937 | 0.334 |
| Calf | 28.4 ± 2.4 | 22.0 ± 1.6 | 24.2 ± 1.8 | 24.8 ± 3.5 | 0.053 | 0.396 | 0.405 | 0.445 |
- Values are presented as means ± standard error of the means.
- Abbreviations: HOL-HOL, Holstein dam pregnant with Holstein fetus; HOL-F1, Holstein dam pregnant with F1 cross (HOL × JB) fetus; HOL-JB, Holstein dam pregnant with Japanese Black fetus; JB-JB, Japanese Black dam pregnant with Japanese Black fetus.
Consistent with a previous study (Isobe et al., 2003), JB-JB exhibited a longer gestation period and lower calf birth weight than HOL-HOL. Furthermore, the total cotyledonary size was smaller in JB-JB than in HOL-HOL. Similar results were observed when comparing primiparous cows in JB-JB and HOL-HOL (Table S1-S2). Placental size directly affects the nutrient transport capacity required to support fetal growth (Reynolds, Ferrell, et al., 1990). A positive correlation between placental and calf birth weights has been reported in both dairy and beef cows (Echternkamp, 1993; Zhang et al., 1997; Zhang et al., 1999). Therefore, the difference in placental size between HOL-HOL and JB-JB reflects differences in the nutritional requirements necessary for fetal growth. In addition, JB-JB had fewer cotyledons than HOL-HOL. Several studies have shown no correlation between the number of cotyledons and the birth weight of dairy and beef calves (Echternkamp, 1993; Zhang et al., 1997; Zhang et al., 1999). However, Kornmatitsuk et al. (2003) reported differences in cotyledon numbers between Swedish Red and White cows and Swedish HOL cows. These findings emphasize that the variation in cotyledon number between HOL-HOL and JB-JB may be related more to breed-specific differences than to nutrient transport ability.
HOL-HOL had higher serum glucose concentrations before calving than JB-JB. During late pregnancy, the increasing nutritional demand for fetal growth and lactation leads to elevated blood glucose concentrations due to reduced insulin sensitivity in peripheral tissues (Bell, 1995; Hayirli, 2006). HOL cows are susceptible to strong insulin resistance (Hasegawa et al., 2019; Kawashima et al., 2016), and have lower responsiveness to insulin during the periparturient period than JB cows (Shingu et al., 2002). Thus, prepartum glucose concentrations may reflect the differences in insulin responsiveness between HOL and JB dams. However, the blood glucose concentrations of HOL-HOL and JB-JB were within the typical reference ranges during this period (Hasegawa et al., 2019; Kawashima et al., 2016; Kawashima et al., 2021; Watanabe et al., 2014). Immediately after calving, the plasma amino acid concentrations in HOL-HOL dams were lower than those in JB-JB dams. The concentration of most amino acids gradually decreases as parturition approaches, reaching their lowest levels at calving (Bell et al., 2000; Zhou et al., 2016), particularly when there is a substantial increase in the amino acid demand of the mammary gland at the onset of lactation (Bell et al., 2000). Because HOL cows have much higher milk production than JB cows (Shingu et al., 2002), they require more amino acids and, consequently, could have lower blood amino acid levels immediately after calving.
The present study showed that serum glucose and plasma amino acid concentrations in umbilical veins and calves were lower in JB-JB than in HOL-HOL. There is limited research on the effect of breed differences on nutrient transfer from the dam to fetus, but Ashworth et al. (2011) demonstrated that Scottish Blackface sheep have lighter placental and fetal weights and lower fetal total amino acid concentrations than Suffolk sheep. These findings are consistent with the results of HOL-HOL and JB-JB calves, suggesting that the lower birth weights of JB calves mean fetal nutrient requirements are lower, consequently reducing the nutrient supply to the fetus.
3.2 Comparison of pregnant HOL dams with similar or different fetus breeds
Gestation length was longer in HOL-F1 (P = 0.023) and HOL-JB (P < 0.001) than in HOL-HOL (Table 2). Calving difficulty was higher in HOL-HOL than in HOL-F1 (P = 0.033) and was similar to that in HOL-JB (P = 1.000) (Table 2). The sex ratio of the calves was similar in HOL-HOL and HOL-F1 (P = 0.245) or HOL-JB (P = 1.000) (Table 2). The birth weight of HOL-JB calves was lighter than that of HOL-HOL (P = 0.047), whereas it did not differ between the HOL-HOL and HOL-F1 groups (P = 0.646) (Table 2). No statistical differences in placental parameters were observed between HOL-HOL and HOL-F1; however, HOL-JB had a lighter total cotyledonary weight (P = 0.001) and smaller total cotyledonary surface area (P = 0.001) than HOL-HOL (Table 3). As for individual cotyledons, HOL-JB had a lower percentage of second-stage cotyledonary surface area (P = 0.011) and higher percentages of fourth-stage cotyledonary weight (P = 0.002) and surface area (P = 0.005) than HOL-HOL (Figure 1).
Two to three weeks before the expected calving, serum glucose concentrations were similar between the groups (Table 4). However, plasma total (P = 0.005; P = 0.027), total essential (P = 0.006; P = 0.006), branched-chain (P = 0.003; P = 0.005), glucogenic (P < 0.001; P = 0.044), and glucogenic and ketogenic amino acid (P = 0.010; P = 0.017) concentrations in HOL-F1 and HOL-JB were higher than those in HOL-HOL (Table 4). Similarly, the total non-essential in HOL-F1 (P < 0.001) and the ketogenic amino acid concentration in HOL-JB (P = 0.006) were higher than those in HOL-HOL (Table 4). Immediately after calving, the serum glucose concentration of HOL-JB dams was higher than those of HOL-HOL dams (P = 0.011), although no differences were observed in the plasma amino acid concentrations between HOL-HOL and HOL-F1 or HOL-JB (Table 5). Serum glucose and plasma amino acid concentrations in the umbilical veins were similar in HOL-HOL and HOL-JB groups (Table 5). Although the calf plasma amino acid concentrations were not different between HOL-HOL and HOL-F1 or HOL-JB, serum glucose concentrations in HOL-F1 calves were lower than those of HOL-HOL calves (P = 0.018; Table 5).
In this study, calving difficulty in HOL-JB averaged 2.00 ± 0.49, with three cows scoring 3 or higher, limiting the efficacy of using JB embryos in HOL cows to prevent dystocia. This limitation is likely due to the increasing size of JB calves resulting from recent breeding practices (Inoue et al., 2020). Additionally, HOL dams carrying beef fetuses tended to experience longer gestation periods. Furthermore, calf birth weight, total cotyledonary size, and individual cotyledon size were comparable between HOL-HOL and HOL-F1, indicating similar fetal nutritional requirements and placental development. In contrast, HOL-JB exhibited lighter calf birth weights, smaller total cotyledonary sizes, and a higher proportion of small cotyledons than HOL-HOL. This suggests that JB fetuses may have lower nutritional requirements despite similar maternal breeds, resulting in a reduced placental size.
Calf serum glucose concentrations were lower in the HOL-F1 group than in the HOL-HOL group, whereas maternal glucose levels before and after calving were similar in both groups. Glucose uptake into the placenta and transport to the fetus is mediated by glucose transporters (GLUTs), whose transport capacity is influenced by their abundance, activity, and localization in the placental membranes (Novakovic et al., 2013; Sibley et al., 2005). Although the current study was limited to the investigation of placental development (such as cotyledonary weight and surface area), it is possible that HOL-JB may exhibit reduced glucose transport capacity via GLUTs because of the different breeds of the dam and fetus. Glucose is the main energy substrate for fetal and placental metabolism in all mammals (Bell & Ehrhardt, 2002). In ewes, however, placental glucose is not directly transported to the fetus but is rapidly converted to lactate and fructose (Bell & Ehrhardt, 2002). Fructose is the most abundant hexose sugar in the fetal fluid and blood of ungulate mammals, such as cattle, sheep, and pigs (Kim et al., 2012). Thus, there may be differences in the ratio of placental glucose conversion to lactate and fructose between HOL-HOL and HOL-F1. Therefore, further investigations into placental glucose transport and metabolic mechanisms are warranted to elucidate the factors contributing to the reduced glucose supply to the fetus in HOL-F1. In contrast, no differences in serum glucose concentrations were observed between the HOL-HOL and HOL-JB groups in either the umbilical veins or calves. As explained earlier, maternal glucose levels were higher in HOL-JB, which promotes a maternal-fetal glucose concentration gradient, potentially facilitating fetal glucose uptake, despite the lighter weight of the calves and placenta.
Contrary to previous findings by Kawashima et al. (2021), the plasma amino acid concentrations in the umbilical veins and calves of HOL-F1 and HOL-JB were comparable to those of HOL-HOL. Maternal prepartum blood concentrations of total, total essential, and total non-essential amino acids in HOL-HOL were consistent with previous reports (Kawashima et al., 2021). However, in HOL-F1 and HOL-JB, these concentrations were higher than in HOL-HOL and HOL dams pregnant with beef fetuses in a previous study (Kawashima et al., 2021). Brown et al. (2011) demonstrated that fetal amino acid concentrations were influenced by changes in maternal amino acid concentrations. In addition, amino acid supplementation in pregnant dairy cows upregulates the expression of placental amino acid transporters (Batistel et al., 2017). Although the reason for the higher maternal blood amino acid levels in HOL-F1 and HOL-JB is unclear, it is possible that elevated maternal amino acid concentrations improve amino acid supply to the fetus. However, when comparing HOL-HOL with HOL-BEEF (both HOL-F1 and HOL-JB), plasma total essential amino acid concentrations of calves were lower in HOL-BEEF than in HOL-HOL (HOL-HOL; 114.0 ± 12.0 μmol/L, HOL-BEEF; 89.4 ± 13.7 μmol/L, P = 0.049), whereas no difference in calf birth weight (HOL-HOL; 40.1 ± 1.1 kg, HOL-BEEF; 37.7 ± 1.6 kg, P = 0.091) was found. Therefore, in HOL-F1 and HOL-JB, there may have been a limited supply of some amino acids transported via the placenta to the fetus, even though maternal amino acid concentrations were higher than in HOL-HOL. Additional studies are warranted to investigate the abundance, activity, and localization of placental amino acid transporters to elucidate the mechanisms of placental amino acid transport in HOL dams pregnant with beef fetuses.
3.3 Comparison of JB fetuses from similar or different dam breeds
Gestation length in HOL-JB was shorter than in JB-JB (P = 0.003; Table 2). Calving difficulty (P = 0.320), calf birth weight (P = 0.724), and calf sex ratio (P = 0.630) were similar between groups (Table 2). No statistical differences were observed in total placental weight, inter-cotyledonary membrane weight, total cotyledonary weight, total cotyledonary surface area, or number of cotyledons between the groups (Table 3). However, for individual cotyledons, the percentage of the first-stage cotyledonary surface area was lower in HOL-JB (P = 0.021) than in JB-JB (Figure 1).
Two to three weeks before the expected calving, serum glucose (P = 0.396) concentrations were similar between two groups (Table 4). In contrast, the plasma total essential amino acid concentrations of HOL-JB dams were higher than those of JB-JB dams (P = 0.038; Table 4). Immediately after calving, the serum glucose (P = 0.020) and plasma total non-essential amino acid (P = 0.018) concentrations were higher in HOL-JB than in JB-JB (Table 5). In contrast, plasma total essential (P = 0.043), branched-chain (P = 0.041), ketogenic (P = 0.011), and glucogenic and ketogenic amino acid (P = 0.016) concentrations were lower in HOL-JB dams than in JB-JB dams (Table 5). The serum glucose concentrations in the umbilical veins (P = 0.025) and calves (P = 0.006) were higher in HOL-JB than in JB-JB, although no statistical differences were observed in plasma amino acid concentrations between the groups (Table 5).
Consistent with the findings of Isobe et al. (2003), JB-JB calves had a longer gestation period than HOL-JB. Calf birth weight, total cotyledonary weight, and surface area were similar, regardless of dam breed. These results were similar when comparing primiparous cows in JB-JB and HOL-JB (Table S1-S2). However, HOL-JB had a lower proportion of large cotyledons than did JB-JB. The number of cotyledons in cows is established early in pregnancy (Neto et al., 2009) and remains unchanged during subsequent pregnancies (Laven & Peters, 2001). Rapid fetal growth occurs late in pregnancy, with approximately 90% of birth weight gained during this period (Ferrell, 1989; Redmer et al., 2004; Reynolds & Redmer, 1995). Thus, the bovine placenta cannot respond to increased fetal nutrient demand by increasing the number of cotyledons (Van Eetvelde et al., 2016). Bovine placental growth can continue throughout pregnancy (Reynolds, Millaway, et al., 1990), expanding the cotyledonary surface area in response to fetal nutritional requirements during late pregnancy (Bertolini et al., 2006; Leiser et al., 1997; Van Eetvelde et al., 2016). As mentioned previously, because JB dams have fewer cotyledons than HOL dams, JB-JB needs to enlarge individual cotyledons to meet the increased fetal nutritional demands, possibly resulting in a higher proportion of larger cotyledons than HOL-JB.
Plasma total essential amino acid concentrations were higher before calving but lower immediately after calving in HOL-JB dams than in JB-JB dams. This suggests that HOL dams require more amino acids for lactation as calving approaches. Immediately after calving, plasma non-essential amino acid concentrations were higher in HOL-JB dams than in JB-JB dams. Glycine, one of the most abundant non-essential amino acids, is mobilized from muscle tissue to compensate for amino acid deficiency before calving (Shibano et al., 2003). Blood glycine concentrations immediately after calving increased in HOL-JB (before calving: 32.6 ± 3.9 μmol/L, after calving: 44.6 ± 3.4 μmol/L) but decreased in JB-JB (before calving: 38.7 ± 3.4 μmol/L, after calving: 34.7 ± 4.8 μmol/L), suggesting greater glycine mobilization from muscle tissue in HOL dams. Additionally, serum glucose concentrations immediately after calving were higher in HOL-JB cows than in JB-JB cows, which may reflect the differences in insulin sensitivity between HOL and JB dams (Shingu et al., 2002).
Amino acid concentrations in the umbilical veins and calves did not differ between two groups, suggesting that the amino acid requirements of JB fetuses are similar, regardless of the dam breed. Conversely, serum glucose concentrations in both the umbilical veins and calves were higher in HOL-JB than in JB-JB group. As mentioned above, dams in the HOL-JB had higher glucose concentrations, which facilitated the maternal-fetal concentration gradient, potentially enhancing glucose supply to the fetus. However, despite a higher glucose supply to the fetus in the HOL-JB group, the calf birth weights remained similar between the groups. The intrauterine nutritional environment can alter offspring growth and development, including organ function, tissue development, and metabolism (Hoffman et al., 2017). These effects persist throughout postnatal life (Du et al., 2010; Nathanielsz et al., 2007; Shasa et al., 2015). Therefore, further research is required to investigate how exposure to elevated glucose levels in HOL-JB fetuses affects postnatal calf development and glucose metabolism.
In conclusion, HOL dams pregnant with F1 cross fetuses may experience inhibited glucose supply to the fetus despite comparable placental development and maternal glucose concentrations. Furthermore, HOL dams with JB fetuses had higher maternal blood glucose levels than JB dams with JB fetuses, suggesting that the fetuses may be exposed to high glucose concentrations, potentially affecting postnatal growth and metabolism. Further investigation of the placental transport function and postnatal offspring performance is warranted to elucidate the effects on nutrient transfer and fetal growth when dairy dams are pregnant with beef fetuses.
ACKNOWLEDGMENTS
The authors thank Hanon Ohban, Sayaka Ito, and Tomono Katagiri (Obihiro University, Japan) for supporting our research, and Yusuke Sugimoto (Ajinomoto Co., Inc.) for technical support with the amino acid analysis. This study was supported by the Kuribayashi Scholarship, Academic Foundation, and JSPS KAKENHI (grant number: JP22H02489).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The authors agree to share the data and other artifacts supporting the results in the paper by archiving them in an appropriate public repository.




