Method for evaluating milk production in mouse mammary gland
Abstract
Lactation is a characteristic physiological function of mammals and is important for nourishing infants and the dairy industry; however, the molecular mechanisms underlying the function remain to be elucidated. A technique to directly evaluate the quantity and quality of milk in mice is necessary for the study of the lactation mechanism in vivo. By measuring the changes in milk amount after different durations of milk accumulation (0–24 h) using a ductal cannulation technique and oxytocin supplementation, we estimated the milk production rate at a single mammary gland level. In addition, collected milk was available to assess milk quality, including creamatocrit, osmolarity, and concentrations of ions, lactose, and total protein. Moreover, as a proof of principle, the effects of intraductal administration of a hypertonic solution to the abdominal mammary gland were examined. This stimulation increased milk amount, possibly by osmosis, compared with the contralateral control gland. These results demonstrated that this method is useful for examining the lactation ability and mechanisms in vivo. Studies using this method will contribute to the further understanding of lactation mechanisms in mammals.
1 INTRODUCTION
Milk is an exocrine fluid produced in the mammary gland of postpartum female mammals. It contains water and nutrients, including carbohydrates, proteins, fats, and electrolytes (Shennan & Peaker, 2000; Truchet & Honvo-Houéto, 2017). The successful milk production in the mammary glands is crucial for livestock animals in two aspects: the nurturing of juvenile animals and milk yield in dairy farms. Although the mechanism of milk production has been extensively investigated, the molecular basis for milk production, particularly electrolytes and water transport, remains partially unclear (Neville et al., 2020). To gain insight into the molecular mechanisms underlying milk production, research using mice, which are small, convenient experimental mammals that can be genetically engineered with ease, is indispensable. Evaluating milk production in mice has traditionally been achieved indirectly by measuring milk intake in the offspring with methods such as weighing pups before and after suckling (weigh-suckle-weigh method) (Jara-Almonte & White, 1972; McDonald & Nielsen, 2006) or measuring the transport of water labeled with isotopes (Knight et al., 1986; Stacey et al., 1995). Although these methods provide valuable data to evaluate maternal lactation, they are susceptible to the factors attributed to the pups. For example, the amount of milk exceeding the intake by the pups cannot be measured. Moreover, these methods are not suitable for accessing the function of a single mammary gland. Methods for mechanical milking from mouse mammary glands have already been established using a combination of aspiration, massage, and oxytocin administration (DePeters & Hovey, 2009; Görs et al., 2009; Muranishi et al., 2016). These are useful to collect milk samples from each mammary gland with little insult to the mice, but not necessarily appropriate for assessing milk quantity, because the amount of collected milk is likely affected by protocol and proficiency. In addition, daily milk yield measured by mechanical milking is less compared with that measured by the weigh-suckle-weigh method (Jara-Almonte & White, 1972). Therefore, more direct and sensitive methods for evaluating milk production in mouse mammary glands at a single gland level are necessary to further examine mechanisms of milk production. We have previously reported a technique for mammary ductal cannulation to monitor the oxytocin-induced milk ejection from a mammary gland (Kamikawa & Seko, 2020). However, this method had limitations as we could not assess the milk production rate because of the lack of precise control of the milk accumulation period and the stimuli from the pups. Additionally, obtaining pure milk was challenging because the inserted cannula and connected tube were filled with a solution for milk ejection detection (Kamikawa & Seko, 2020). In the present study, we addressed these issues as follows. First, we minimized the interindividual difference in suckling stimuli to tested mammary glands by unifying the number of pups and available nipples. Second, the stored milk at the onset of the milk accumulation period was removed by suckling from fasted pups. Third, pure milk was directly collected through a mammary ductal cannula that did not contain the miscible solution inside. Thereafter, we accurately estimated the milk production rate and measured milk quality at a single mammary gland level. Using this method, the effects of intraductal administration of a hypertonic ionic solution on milk production were determined. Thus, we demonstrate that the improved method is applicable for measuring the milk production rate and studying the mechanisms underlying lactation, which is an important physiological function of mammary glands, in vivo.
2 MATERIALS AND METHODS
2.1 Ethical approval
The experimental procedures and animal care were performed in accordance with the Regulations on Management and Operation of Animal Experiments at Obihiro University of Agriculture and Veterinary Medicine and were approved by the University's Animal Care and Use Committee (no. 21-49, 22-25).
2.2 Measurement of stored milk in mammary gland of lactating mice
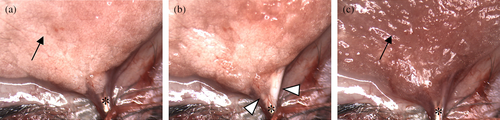
C57BL/6J Jms Slc female mice (Nihon SLC, Shizuoka, Japan) on Day 15 of lactation were used for the assays. The number of pups was adjusted to five and the nipples of the cervical and inguinal mammary glands were sealed with tissue adhesive (Aron Alpha A Sankyo; Daiichi Sankyo, Tokyo, Japan) within 5 days after parturition. These preparations were performed in anticipation of the equal development of abdominal mammary glands for the assay and reducing the variation among individuals. Fasted pups that were separated for 2 h from their dam beforehand were reunited with the dam for 2 h to reduce milk volume in the mammary glands before milk accumulation (McDonald & Nielsen, 2006). Milk accumulation was initiated by reseparation of the pups. At the end of milk accumulation at 0, 1, 3, 6, and 24 h, the mice were anesthetized with medetomidine hydrochloride (0.3 mg/kg), midazolam (4.0 mg/kg), and butorphanol tartrate (5.0 mg/kg). Anesthetized mice were placed in the supine position on a heater at 35°C and the midline of the abdominal skin was incised. The principal ducts beneath the nipples of the abdominal mammary glands were separated from the surrounding interstitial tissue and a cannula made from a 27G needle was inserted from a small incision of the duct (Kamikawa & Seko, 2020). The cannula was connected with a syringe via a silicon tube. When an abdominal mammary gland was occasionally involuted, it was excluded from the assay. Milk was aspirated with the syringe after the intraperitoneal administrations of oxytocin (250 pmol/mice, twice, Peptide Institute, Inc., Osaka, Japan), which is sufficient to eject almost all of the milk in the mammary glands (Kamikawa & Seko, 2020). Figure 1 shows the typical appearance of this procedure. Before milk ejection, the mammary gland exhibited a reddish milky white color. After oxytocin stimulation, milk was ejected and accumulated in the principal ducts. Successful milk collection can be confirmed by the smooth removal of intraductal milk by aspiration, accompanied by a shift in tissue color to deep reddish-brown (Figure 1). After milk collection, the mice were sacrificed by cervical dislocation without ever gaining consciousness. The total weight of the cannula and syringe before the examination was subtracted from the weight of the cannula, syringe, and collected milk after the examination to determine the amount of collected milk. When milk was collected from two-sided abdominal glands and the amount was measured separately, the average value was recorded as the milk amount from one abdominal mammary gland per mouse.
2.3 Assay of milk quality
The milk collected from mice in the 6-h accumulation group was used for the assay. A portion of the collected milk was immediately centrifuged in a glass capillary at 15,000 ×g for 15 min at room temperature to measure the percentage of cream fraction (creamatocrit). The skimmed milk fraction was recovered from the capillary and its osmolarity was measured using a vapor pressure osmometer (Wescor, UT, USA). The remaining milk was centrifuged at 4000 ×g for 5 min and the skimmed milk was stored at −80°C. The skimmed milk was diluted 1:1 with pure water and its ionic concentration and total protein (TP) were measured by ion selective electrometry and colorimetric method, respectively (TBA-120FR; Canon Medical Systems, Tochigi, Japan). The lactose concentration was measured using a commercial kit (BioAssay Systems, CA, USA) after deproteinizing 10 μL of skimmed milk by adding 60 μL of 1N HCl, followed by centrifugation at 12,000 ×g for 5 min at 4°C, and subsequent neutralization of the 30 μL of the supernatant with 30 μL of 1N NaOH.
The milk density was determined by weighing 10 μL of normal milk collected from four dams on Days 10–18 of lactation after a 4-h accumulation. In this experiment, the matching of the number of pups and the fasting procedure for pups before milk accumulation were omitted.
2.4 Investigation of the effect of the intraductal administration of a hypertonic solution
Prior to intraductal treatment, the milk inside the mammary glands on Days 15–17 of lactation was removed by the suckling of five starved pups as described above. After anesthetizing with medetomidine hydrochloride, midazolam, and butorphanol tartrate, 50 μL of hypertonic solution (5x phosphate-buffered saline [PBS] containing [in mM] 684.5 NaCl, 13.4 KCl, 7.35 KH2PO4, 40.5 Na2HPO4; 1432 mmol/kg in our measurement) was intraductally injected to either side of the abdominal mammary glands through the nipple with a 31G needle, whereas an isotonic solution (PBS containing [in mM] 136.9 NaCl, 2.68 KCl, 1.47 KH2PO4, 8.10 Na2HPO4; 286 mmol/kg in our measurement) was administered to the other side as a control. The surgical procedure for ductal cannulation was initiated approximately 20 min after intraductal treatment. The stored milk was collected 1 h after the intraductal treatment and the collected milk was analyzed as described above.
2.5 Statistics
Data are expressed as means ± standard deviations (SD). Analysis of variance (ANOVA) was used for the comparison of mean values among three or more groups. A paired t-test was used for the comparison of mean values between two samples obtained from the same mouse. A value of p < 0.05 was considered as statistically significant.
3 RESULTS AND DISCUSSION
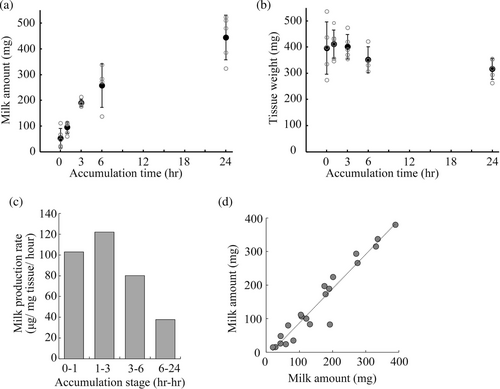
To assess the milk production rate, we collected milk from the mammary glands over different milk accumulation time periods. Successful milk collection was confirmed in all groups, suggesting almost all stored milk was equally collected by this procedure despite differences in milk accumulation time. For mice in the 0-h accumulation group, 52.3 ± 38.0 mg of milk was collected from the abdominal mammary gland. After 1, 3, 6, and 24 h of milk accumulation, 94.5 ± 22.3, 189.7 ± 14.0, 256.8 ± 84.7, and 444.0 ± 86.6 mg of milk was collected, respectively (Figure 2a). No significant difference was seen in the average weight of the gland after milk collection (Figure 2b; p > 0.05 by ANOVA). Moreover, the amount of milk collected by this method was more compared with the mechanical milking method (DePeters & Hovey, 2009; Görs et al., 2009; Muranishi et al., 2016). Therefore, this method is more effective for evaluating the amount of stored milk in a mammary gland compared with previous methods. Considering the tissue weight of the tested mammary glands, the milk production rates were calculated to be 102.9, 122.1, 80.1, and 37.8 μg/mg tissue/hour during the period of 0–1, 1–3, 3–6, and 6–24 h of milk accumulation, respectively (Figure 2c). These results suggest that milk production reaches its maximum rate between 1 and 3 h and then tapers. This result is consistent with a previous study reporting molecular changes in the early stages after forced weaning (Uejyo et al., 2015). In that study, expression of lactation-related genes was downregulated 3 h after forced weaning. In addition, signal transducer and activator of transcription (STAT) 5, a key molecule for milk production, was inactivated and STAT3, a key molecule for mammary gland involution, was activated at 6 h after forced weaning.
This method also had the advantage that milk quality is concomitantly assessable with milk quantity. The creamatocrit of milk and the osmolarity and concentrations of Na+, K+, Cl−, lactose, and TP in skimmed milk were measured using milk samples obtained after 6 h of milk accumulation. The mean values of the measurements are listed in Table 1.
| Osm (mmol/kg) | Cream (%) | Na+ (mM) | K+ (mM) | Cl− (mM) | TP (g/dL) | Lactose (mM) |
|---|---|---|---|---|---|---|
| 344 ± 9 | 23.6 ± 3.3 | 21.0 ± 1.4 | 67.0 ± 2.6 | 34.5 ± 5.9 | 11.9 ± 0.2 | 161.9 ± 3.6 |
- Abbreviations: Cream, creamatocrit; Osm, osmolarity; TP, total protein.
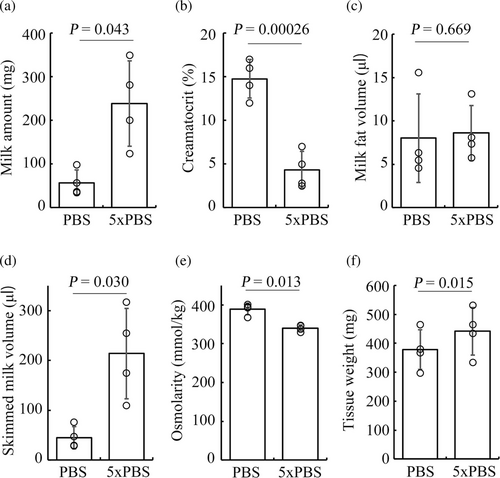
The amount of milk obtained from a one-sided mammary gland was closely matched with that from the contralateral gland, regardless of the duration of milk accumulation (Figure 2d). Therefore, it became possible to evaluate the effect of a particular treatment on milk production by comparing the stored milk volume between the treated mammary gland and the contralateral control mammary gland. To confirm this, we determined the effect of the intraductal administration of a hypertonic ionic solution on milk production. The injection of a hypertonic solution facilitated milk production with less of a cream fraction than the control (Figure 3a,b). The milk volume was estimated from the normal milk density value. Given that the density was 1.07 ± 0.01, the estimated volume of skimmed milk from the gland with hypertonic stimulation was significantly higher than that of isotonic stimulation, whereas the cream volume was almost identical (Figure 3c,d). The osmolarity of the skimmed milk with hypertonic stimulation was lower compared with that by isotonic stimulation in contrast to the osmolarity of the injected solutions (Figure 3e). The mammary gland stimulated with the hypertonic solution exhibited an increase in tissue weight (Figure 3f). This might be related to an edematous change due to ions that leaked into the interstitial space. These results suggest that the intraductal ionic hyperosmolarity increases the amount of milk by inducing transepithelial osmosis, and clearly show that this method is useful for evaluating the effects of a particular treatment on milk production at the single gland level.
In conclusion, we have proposed a novel method for assessing milk production at a single mammary gland level in mice. While we should pay attention to the effects of surgical procedures on the obtained results, the direct milk collection enables us to quantitatively measure the amount and contents of milk. By combining pharmacological tools, gene recombination techniques, molecular expression analyses such as immunohistochemical techniques, and/or histological examinations, this method will facilitate the study of the molecular mechanisms involved in lactation in vivo. It is expected that further understanding the mechanisms of lactation using experimental animals will support the production of livestock and increase milk yield in dairy animals.
AUTHOR CONTRIBUTIONS
Akihiro Kamikawa conceived the project, designed the experiments, and wrote the manuscript. Hibiki Sakai and Akihiro Kamikawa performed experiments and analyzed the data.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI (grant number JP22K06037). The authors would like to thank K. Tagawa and A. Uchiyama for their assistance in conducting this study. The authors would like to thank Enago (www.enago.jp) for the English language review. This work was supported by the Station for management of common equipment and the Veterinary Medical Center of Obihiro University of Agriculture and Veterinary Medicine.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.




