L-Citrulline: A novel hypothermic amino acid promoting thermotolerance in heat-exposed chickens
Abstract
With global warming becoming of increasing concern, poultry farms are experiencing a concomitant increase in heat stress. Chickens are very sensitive to high ambient temperature (HT), so the development of novel nutrients that will help deal with the challenge posed by heat stress is vital. We revealed that L-citrulline (L-Cit) can reduce body temperature in chickens. Orally administered L-Cit solution has been found to provide heat tolerance in chickens and to result in reduced food intake. Heat exposure and oral administration of L-Cit led to increased levels of plasma insulin, whereas heat stress led to a decline in plasma thyroxine. Dietary administration of L-Cit was also shown to be effective to reduce heat stress in broiler chickens. Moreover, L-Cit was found to be metabolized in the liver within 1 h of its administration, and in L-Cit-treated broiler chicks, the Cit-Arginine cycle and the Krebs cycle were found to be active. L-Cit has not yet been approved for inclusion in the poultry diet, so it is important to find alternative sources of L-Cit. Taken together, these findings suggest that L-Cit may serve as an important novel nutrient with the ability to produce heat tolerance in chickens under HT.
1 INTRODUCTION
One of the most significant greenhouse gases (i.e., gases that trap heat) is carbon dioxide (CO2). It is produced by the extraction and burning of such fossil fuels as coal, oil, and natural gas, as well as resulting from wildfires and natural processes such as volcanic eruptions. Since the 18th century, when the modern industry began, human activities have raised the amount of CO2 in the atmosphere by 50%. In other words, compared with its value in 1750, there is now 150% more CO2 in the atmosphere. This increase is greater than the increase that occurred naturally at the end of the last ice age 20,000 years ago. Between 2002 and 2022, the amount of CO2 in Earth's atmosphere (i.e., 8–12 km above ground level) has risen from 365 parts per million (ppm) to over 400 ppm. This increase has resulted in global warming, with the atmosphere being up to 1.5°C hotter than pre-industrial levels (IPCC, AR6, Hennessy et al., 2022).
The high ambient temperature (HT) induced by global warming is leading to chickens experiencing heat stress. Poultry farmers in tropical and subtropical regions in particular are facing serious problems in this regard because heat stress has a negative effect on poultry production. Heat stress occurs when the amount of heat generated by the animals is higher than the amount of heat dissipated in their immediate environment (Renaudeau et al., 2012). Chickens are especially prone to heat stress in HT conditions because they do not have sweat glands, their body is covered in feathers, and they have a high metabolic rate (Bartlett & Smith, 2003; Chowdhury, 2019; Soleimani et al., 2010; Yahav & Hurwitz, 1996). When they are affected by heat stress, both their food intake and their body weight (BW) gain decrease, negatively impacting production and increasing mortality (Azad et al., 2010; Daghir, 2008; Lin et al., 2006). Absorption of arginine (Arg), an essential amino acid in chickens, has been shown by Balnave and Oliva (1991) to significantly decrease with exposure of chickens to heat stress. Dietary supplementation of certain essential amino acids has been found in several studies to help alleviate the problems caused by heat stress in birds (Brake et al., 1998; Daghir et al., 2003; Dai et al., 2012; Mendes et al., 1997; Rose & Uddin, 1997; Willemsen et al., 2011). Increased levels of some amino acids could be usefully administered to heat stress-affected chickens in their diet. It is therefore crucial to gain a better understanding of how to implement such a dietary strategy, as well as to understand the role played by amino acids in heat-stressed birds' metabolism.
An increase in plasma citrulline (Cit) occurred in layer chicks within a short time (15 or 30 min) following exposure to HT at 35°C (Ito et al., 2014). However, plasma Cit was found to decrease when layer chicks were exposed to prolonged HT at 35°C for 48 h (Chowdhury et al., 2014). Using these findings, we conducted a further study in which we administered Cit orally to layer chicks. Although it increased in the chicks' plasma after short-term heat stress (15 or 30 min at 35°C) and decreased as a result of long-term heat stress (24 or 48 h at 35°C), we found that with oral administration, it acted hypothermically in the chicks (Chowdhury et al., 2015, 2017). In the past, Cit attracted relatively little interest, most likely because it has no tRNA and is not used for protein synthesis, though it can be present in proteins as it is a target for posttranslational modifications (Curis et al., 2005). Recent studies, on the other hand, have emphasized the importance of this amino acid both in cellular metabolism and in affording thermotolerance in chickens. A summary of this amino acid's metabolic and thermoregulatory processes would therefore be useful. For brevity's sake, this paper will be limited to the metabolism and thermoregulatory role of Cit in chickens, with only brief references to mammals where appropriate.
2 CITRULLINE AND ITS METABOLISM
2.1 Physical and chemical properties
Cit (CAS: 372–75–8) is a colorless solid at ambient temperature and pressure with a melting point of 222°C. It is an α-amino acid with an asymmetric carbon and therefore presents two enantiomers. Like most amino acids, the L form is its natural form. Cit (C6H13N3O3) is an α-amino acid with a molar mass of 175 g mol−1. Its polar lateral chain makes it relatively soluble in water in low doses, though at higher doses, it becomes insoluble. Because it can form peptide bonds in biological systems, this amino acid can be present in proteins, but this must always result from a posttranslational modification of the protein (Curis et al., 2005) as it has no known codon in the genetic table.
2.2 Cit synthesis
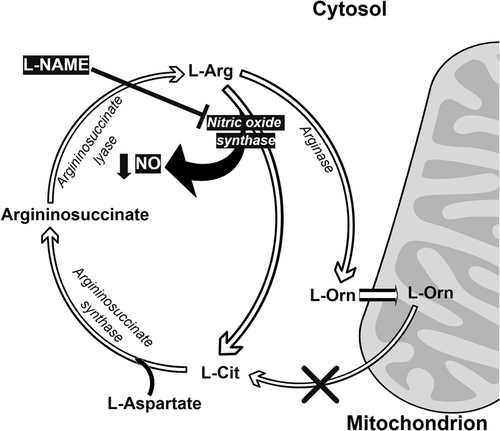
Nitric oxide (NO) synthesis occurs in all tissues and is carried out by NO synthase (NOS) enzymes. The three different NOS families differ in their level of expression and in their localization: nNOS mainly occurs in neural cells, whereas iNOS is in macrophages and eNOS is in endothelial cells. These enzymes all have the same method of synthesizing NO from arginine (Arg) with the release of Cit, following the reaction shown in Figure 1 (Chowdhury et al., 2017). Chickens lack the enzyme carbamoyl phosphate synthetase, which means they are unable to synthesize L-Cit from L-ornithine (L-Orn) (Tamir & Ratner, 1963). In this process, Cit is catabolized with aspartate and ATP by a cytosolic enzyme, arginosuccinate synthase (ASS), to produce arginosuccinate, pyrophosphate, and AMP. Husson et al. (2003) have shown that ASS is almost ubiquitous in mammals, with higher levels being found in the liver and the kidney and the lowest levels occurring in the intestine (in adults). In the liver, gene expression is modulated by nutritional status and by hormones, with glucocorticoids, glucagon, and glutamine increasing ASS levels and insulin, growth hormone, and oleic acid decreasing them.
2.3 Cit transport
No Cit-specific transporter has yet been found in any cell type, although various cell types can take up or release Cit, and several studies have shown that Cit can be transported by the usual generic amino acid transporters (Curis et al., 2005). A Cit transport system exists for all cells in the nervous system (microglial cells, astrocytes, and neurons) (Schmidlin et al., 2000). Because it is not sodium- dependent, it may use the L amino acid carrier system for long-chain amino acids (Schmidlin et al., 2000). Thermoregulation is controlled by the brain (Romanovsky, 2007), and it has recently been reported that in broiler chickens, orally administered L-Cit can enter the brain (Chowdhury et al., 2022). However, the mechanism for transporting L-Cit through the blood–brain barrier in chickens is still unknown.
2.4 Cit metabolism
The free Cit can be catabolized into any of the three metabolic pathways that are present at different levels. The first of these pathways is Arg biosynthesis, which involves Cit exchanges at the level of the whole body; the second is the NO cycle, which can involve the recycling of Cit locally; and the third pathway is the complete urea cycle, which takes place in the liver and only exists in mammals; it is not present in birds. Dietary Cit is absorbed by the intestine. Some studies have shown that when it is present luminally, Cit accumulates in enterocytes, suggesting that a transporter is involved (Vadgama & Evered, 1992). Most of this absorption seems to occur between the middle and lower parts of the ileum (Vadgama & Evered, 1992). However, Cit is not an essential amino acid (if the definition of “essential” is extended to non-protein amino acids), and most of the circulating Cit is derived from the conversion of glutamine in the enterocytes (Wu, 1998).
Cit is readily converted into Arg in a half urea cycle through the action of ASS and argininosuccinate lyase (ASL), and it can therefore be used as a precursor of NO. Because the brain neurons producing NO are not able to reconvert Cit into Arg as they do not express the ASS and ASL enzymes, Cit is released from the neurons and taken up by surrounding neural cells, where it is reconverted to Arg. The newly formed Arg is then released, to be taken up by neurons to form more NO (Wiesinger, 2001), and so on. However, if this cycle is not controlled, there could be excessive production of NO, which could damage the surrounding cells. One means of control is through the inhibition of ASS by NO (Hao et al., 2004). Cit has been detected as one of the intermediaries of the urea cycle in hepatocytes. However, this Cit pool is very labile, with no Cit being released into general circulation, as all of the synthesized Cit is converted into argininosuccinate by cytoplasmic ASS (Windmueller & Spaeth, 1981). It has been shown that hepatocytes involved in the urea cycle cannot take up Cit from portal circulation and that there is only limited uptake from arterial circulation (Windmueller & Spaeth, 1981). Cit metabolism in the liver is thus strictly compartmentalized and is not connected to any of the other metabolic pathways involving Cit.
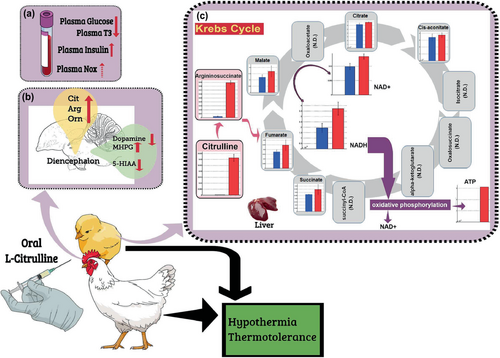
A metabolomic study aimed at revealing the characteristics of liver metabolites of chicks that had been orally administered L-Cit was recently carried out to shed more light on the metabolism of L-Cit (Chowdhury, Ouchi, Haraguchi, & Bungo, 2021). In this study, capillary electrophoresis–time-of-flight mass spectrometry (CE-TOFMS) and liquid chromatography–time-of-flight mass spectrometry (LC-TOFMS) were carried out on liver samples following the oral administration of L-Cit. In total, 361 liver metabolites were identified. Although only a small number of samples were used for each group, principal component analysis and heatmap patterns were used to confirm that the metabolites could be distinguished from each other by their composition. Of the 361 compounds detected in the liver, 41 of them, including amino acids related to the Cit-Arg cycle, argininosuccinic acid, Arg, Orn, and Cit, as well as gamma-aminobutyric acid, glycine, histidine, and nicotinamide adenine dinucleotide (NAD), occurred in abundance in L-Cit-treated livers. In contrast, 24 of the compounds containing fatty acids, amino acids, and cyclic adenosine monophosphate were found in much smaller numbers in the L-Cit-treated group. These data imply that the Cit-Arg cycle and the Krebs cycle metabolism are active in L-Cit-treated broiler liver (Figure 2), but there is a lower activity in fatty acid metabolism.
 ), decrease (
), decrease ( ), or a tendency to increase (
), or a tendency to increase ( ). T3, 3,5,30-triiodothyronine; Nox, total NO2 and NO3; Cit, citrulline; Arg, arginine; Orn, ornithine; MHPG, 3-methoxy-4-hydroxyphenylglycol; 5-HIAA, 5-hydroxyindoleacetic acid; NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide hydrogen.
). T3, 3,5,30-triiodothyronine; Nox, total NO2 and NO3; Cit, citrulline; Arg, arginine; Orn, ornithine; MHPG, 3-methoxy-4-hydroxyphenylglycol; 5-HIAA, 5-hydroxyindoleacetic acid; NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide hydrogen.3 THERMOREGULATION IN BIRDS
Birds have a thermoregulatory system that enables a relatively constant body temperature to be maintained over a wide range of environmental temperatures. This system consists of (1) a sensory part, which detects changes in the environment (via thermo-, osmo-, and baro-receptors); (2) an integrating part—namely, the thermoregulatory center in the preoptic anterior hypothalamus (PO/AH), where temperature-sensitive neurons monitor changes in local temperature and information about the temperature received from peripheral thermoreceptors. The set point of body temperature is then defended by mechanisms enabling the production or loss of heat depending on the thermal status of the bird; and (3) a command part, comprising neurological and endocrine signals that lead to mechanisms for body temperature control further downstream, such as shivering thermogenesis (ST), non-shivering thermogenesis (NST), sensible and latent heat loss (including respiratory and cutaneous heat loss), peripheral vasoconstriction or vasodilation, and behavioral changes (such as wing dropping, basking, huddling, and shade/cover seeking).
Potential mechanisms for enhancing the capacity for thermogenesis include increasing muscle mass (see Milbergue et al., 2018), increasing the mass-specific aerobic enzyme capacity of muscle tissue (Bicudo et al., 2002; Liknes & Swanson, 1996; and potentially also of the liver, see Liu et al., 2006), and increasing the mitochondrial density and/or respiration rate in order not only to provide fuel for aerobic metabolism but also possibly to increase NST (Collin et al., 2003; Dridi et al., 2004). It was established in the late 1950s that the most important hormones controlling thermogenesis are thyroid hormones (THs, triiodothyronine, T3, and thyroxine, T4) (e.g., Decuypere et al., 2005; Klandorf et al., 1981; Mellen & Wentworth, 1958, 1962). There is experimental evidence for “obligatory thermogenesis”—another term for THs' modulation of thermoregulation within the thermoneutral zone (TNZ). Among such experiments are those which made birds hypothyroid (i.e., lacking THs) using drugs blocking TH release, and those in which thyroid glands were removed (thyroidectomy), both of which found that heat production was reduced in poultry (e.g., Mellen & Wentworth, 1962). On the other hand, experimental administration of THs was found to stimulate thermogenesis (Arieli & Berman, 1979). The injection of corticotropin-releasing hormone (CRH) in neonatal chicks was also found to induce thermogenesis, as well as increase body temperature (Elhussiny, Tran, et al., 2022; Tachibana et al., 2004). The injection of CRH in chicken embryos also led to an increase in thyroid-stimulating hormone (TSH) and in the concentration of circulating T4 and T3 (Meeuwis et al., 1989), potentially via CRH receptors, expressed in the thyrotrophs (De Groef et al., 2005). This suggests that there are interactions between CRH and THs that affect thermoregulation. Interestingly, the hypothalamus-pituitary-thyroid (HPT) axis became less responsive to CRH in adult chickens (Geris et al., 2003). It is likely that this was because the interaction with the peripheral TH deiodinase enzymes was altered (Darras et al., 2006), demonstrating that such interactions between hormones involved in thermoregulation may vary across ontogenetic stages. It has also been reported that the thermoregulatory response to thyrotropin-releasing hormone is dependent upon stimulation of the hypothalamus-pituitary–adrenal axis rather than the HPT axis and, as a result, may differ across neonatal stages (Takahashi et al., 2005). However, it has recently been found that exposure of 4-week-old broilers (average BW: 1.8 kg) to an HT resulted in increased plasma T3 and decreased T4 (Chowdhury, Ouchi, Han, et al., 2021), which indicates that in market-age broilers, the HPT axis may have an active role in thermoregulation.
4 L-CIT IN THERMOREGULATION
It is well documented that not only are amino acids constituents of protein, but they are also important regulators of physiological and behavioral processes, including stress responses (Asechi et al., 2006; Erwan et al., 2014; Hamasu, Haraguchi, et al., 2009a; Hamasu, Shigemi, et al., 2009b; Kurata et al., 2011; Kurauchi et al., 2010). Although few studies have focused on the altered free amino acid concentrations found in the brain of chickens exposed to heat stress, Ito et al. (2014) found that levels of several free essential and nonessential amino acids had increased in the brain, plasma, and muscle of layer chicks exposed to short-term heat stress (15–30 min at 35°C). However, they did also find the levels of some other amino acids to be significantly reduced. It was further found that body temperature was decreased with orally administered L-Cit, but not L-Arg or L-Orn, under thermoneutral temperature (i.e., control) conditions (CT; Chowdhury et al., 2015). Orally administered L-Cit also lowered the body temperature in layer chicks exposed to heat (Chowdhury et al., 2017). Taken together, these findings indicate that L-Cit has a physiological function to reduce body temperature.
It has been proposed that thermoregulation through cutaneous dissipation (Mathai et al., 1997) is an important physiological function of NO (Szabo, 1996), which is produced when L-Arg is converted to L-Cit by NOS (Palmer et al., 1987). Hence, it is possible that NO has a hypothermic function in chicks. Nevertheless, our findings suggest that NO may not be the main factor in the reduction in body temperature and thermotolerance that results from the oral administration of L-Cit, which was confirmed by NOS inhibitor, NG-nitro-L-arginine methyl ester hydrochloride (Figure 1; Chowdhury et al., 2017). However, Uyanga, Zhao, et al. (2022) showed that when L-Cit is injected centrally, but not intraperitoneally, there is a reduction in body temperature and an increase in hypothalamic NO concentration and NOS synthesis. Additionally, dietary administration of 1% L-Cit in broilers from 1 to 42 days old caused a lowering of rectal temperature under CT and an increase in NOS isoforms (Uyanga, Zhao, et al., 2022). Thus, L-Cit may or may not change the NO concentration, depending on which subjects are being administered, as well as the means of administration (Figure 2). However, NO is clearly not solely responsible for the reduction in body temperature observed in L-Cit-treated chicks, and it has been suggested that hypothermia resulting from L-Cit administration could be due to the reduction in plasma glucose (Chowdhury et al., 2017; Figure 2).
Orally administered L-Cit can increase the concentration of Cit, Arg, and Orn in the brain under both CT and HT conditions (Chowdhury et al., 2022; Figure 2). Concentrations in the brain of tryptophan and its metabolite serotonin (5-HT) were lower under HT than under CT. Although HT did not alter brain concentrations of tyrosine, there was a reduction in dopamine (DA, a metabolite of tyrosine), whereas methoxyhydroxyphenylglycol (MHPG, a metabolite of DA) increased in concentration under HT compared with CT. Orally administered L-Cit decreased concentrations in the brain of both tryptophan and tyrosine under CT and HT without changing 5-HT; however, DA levels declined under HT. Furthermore, there was an increase in MHPG concentrations. These results suggest that the metabolism of amino acids and DA in the brain can be enhanced by orally administered L-Cit. Metabolic changes in the brain as a result of oral administration of L-Cit may affect the thermoregulatory center in the brain, leading to reduced body temperature and bringing about thermotolerance in heat-exposed broiler chickens. Elhussiny, Nishimura, et al. (2022) recently showed that norepinephrine is responsible for taurine-induced hypothermic effects in neonatal chicks. It is therefore possible that DA metabolism in the brain that is dependent on orally administered L-Cit might contribute to the hypothermic function of L-Cit.
It has also been found that orally administered L-Cit results in increased liver Cit, argininosuccinate, NAD+, and NAD + hydrogen (H) (NADH), which indicates the activation of the Krebs cycle. Furthermore, ATP concentration was found to have increased in one of three liver samples, which suggests that anabolic metabolism might have been stimulated by the oral administration of L-Cit (Figure 2; Chowdhury, Ouchi, Haraguchi, & Bungo, 2021).
5 L-CIT AND THERMOTOLERANCE IN LAYER CHICKS AND BROILER CHICKENS
In an experiment examining the effect of L-Cit on thermotolerance (Chowdhury et al., 2017), chicks received a dual dose of L-Cit (15 mmol/10 mL/kg BW/administration) by oral administration prior to exposure to HT, because a preliminary study found that a single orally administered dose did not provide thermotolerance. After the chicks were placed in the temperature-controlled chambers, rectal temperature was increased in the chicks under HT. Orally administered L-Cit was found to have reduced the rectal temperature in heat-exposed chicks down to the level of the non-heat-exposed control chicks. The finding in this experiment is that HT caused the rectal temperature to increase, which is a common physiological response when animals are exposed to heat stress (Ito et al., 2014, 2015). Interestingly, however, oral administration of 30 mmol of L-Cit was found to ameliorate the increase in rectal temperature under HT. Thus, oral administration of L-Cit can reduce the rectal temperature under heat stress.
In another study (Chowdhury, Ouchi, Han, et al., 2021) involving market-age broilers, one group of broilers received a dual dose of orally administered L-Cit (20 mmol/head/administration; 40 mmol/head in total, using an elastic-plastic needle on a syringe), as a preliminary study had found that a single dose of orally administered L-Cit (20 mmol/head) was not sufficient to provide thermotolerance. They were then exposed to an HT (30 ± 1°C) chamber. After the first oral administration of L-Cit, the broilers were kept at room temperature for 60 min. This was followed by a second oral administration, after which the broilers were kept at room temperature for another 30 min before being placed in a temperature-controlled chamber, where they were exposed to HT for 120 min. The control group was administered two doses of 10 mL of 0.25% methylcellulose solution in the same manner as the treatment group before being placed in a temperature-controlled chamber under CT (22 ± 1°C) for 120 min. Rectal temperature was measured at 0, 60, and 120 min after subjects were placed in the temperature-controlled chambers. Orally administered L-Cit was found to cause a reduction in food intake. In chicks under CT and under HT, orally administered L-Cit caused a reduction in body temperature. HT resulted in an increased rectal temperature in broilers. There was a significant interaction between HT and time, whereby the difference in rectal temperature between the HT broilers and the CT broilers was more pronounced between 0 and 60 min and 0 and 120 min. Periods of reduced food availability have been found to cause hypothermia in various endotherms (Hohtola et al., 1991; Hoshino, 1996; Penas & Benito, 1986; Phillips et al., 1991; Sakurada et al., 2000). Therefore, during the entire 210 min of the experiment, we also analyzed the total food intake in the L-Cit-treated broilers. Interestingly, we found that orally administered L-Cit caused both food intake and plasma T3 to be suppressed, whereas plasma insulin increased (Figure 2; Chowdhury, Ouchi, Han, et al., 2021). These findings clearly indicate that L-Cit can suppress overall metabolic functions and heat production, and this could be very helpful for poultry farmers with broilers that need to adapt to heat stress because feed withdrawal is currently a common practice in poultry farms experiencing HT conditions during the summer.
Another study (Uyanga, Zhao, et al., 2022) investigated whether dietary supplementation with L-Cit (so that it provides 1% of the basal diet) could ameliorate the effects of acute heat stress on broilers, as well as help with redox balance and inflammatory responses. Ross 308 broilers (288 chicks) were subjected to one of two environments: CT (24°C) or HT (35°C) for 5 h. They were fed one of two diets: control or L-Cit. The results showed that heat stress caused the ear, rectal, and core body temperatures of broilers to increase and also led to a higher respiratory rate. There was an intermittent time effect on the rectal and core body temperature readings, but L-Cit supplementation lowered the mean core body temperature more than the control diet did. Antioxidant assays showed that superoxide dismutase increased during heat stress, and supplementation with L-Cit promoted the production of catalase. In addition, during heat stress, L-Cit induced more glutathione peroxidase activity than the control diet did. Heat stress upregulated hypothalamic heat shock protein (HSP)-90, but L-Cit downregulated heat shock factor (HSF)-1 and expression of HSP-60 mRNA. Under CT, HSF-3 mRNA expression was downregulated by L-Cit. Taken together, these data indicate that under both CT and HT, L-Cit influenced the antioxidant-defense heat-shock response and that L-Cit may have a direct and/or indirect role in the central regulation of thermotolerance. During HT, the mRNA expression of peroxisome proliferator-activated receptor γ coactivator 1-α, avian uncoupling protein, cytochrome C oxidase subunit 3, and ATP synthase F1 subunit beta (ATP5β) in the breast muscle were all down-regulated. However, supplementation with L-Cit resulted in the upregulation of the mitochondrial transcription factor A, and during HT, L-Cit increased ATP5β expression to a similar level to that of CT-housed broilers. It has thus been demonstrated that dietary L-Cit can promote muscle ATP generation during heat stress in broilers (Uyanga, Zhao, et al., 2022). It has also been reported that oral administration of L-Cit caused an increase in the liver of both NADH and NAD+, which are electron carriers and may increase oxidative phosphorylation activity in the mitochondria (Chowdhury, Ouchi, Haraguchi, & Bungo, 2021). Although ATP was not detected in all the groups, it did increase in one sample in the L-Cit group (Chowdhury, Ouchi, Haraguchi, & Bungo, 2021). These results indicate that ATP production may be enhanced by orally administered L-Cit, and thus, an anabolic activity could be promoted, which might help in lowering the body temperature, because catabolic metabolism under HT increases body temperature (Chowdhury et al., 2012; Maeda et al., 2017; Shim et al., 2006).
6 ALTERNATIVE SOURCES OF SYNTHETIC L-CITRULLINE IN THERMOREGULATION AND THERMOTOLERANCE
Consumption of crystallized L-Cit has not yet been approved (Food and Agricultural Materials Inspection Center, Japan), and the fact that it cannot be used in poultry feed is a matter of concern. Studies related to the hypothermic functions of non-crystallized L-Cit might therefore provide useful information. Erwan et al. (2014) reported that orally administered D-aspartate (D-Asp), which is also known to be hypothermic, lowered the body temperature of chicks under both CT and HT. Further, administering live bacteria into an animal's digestive tract has long been reported to produce beneficial effects in terms of the growth and performance of the host. Oral administration of a medium containing live bacteria-producing D-Asp has also been found to reduce body temperature in chicks (Do et al., 2017). Therefore, a medium containing live bacteria producing L-Cit was used to examine its effect on the regulation of body temperature under CT. Tran et al. (2019) showed that the medium containing live bacteria producing L-Cit significantly reduced both rectal and surface temperatures in chicks under CT. There was a significant interaction between treatment and time in the reduction of rectal and surface temperatures, with a pronounced reduction in body temperature occurring 60 min after the medium was administered. Chronic oral administration of the medium was found to significantly reduce both rectal and surface temperature of chicks. However, when the equimolar amount of L-Cit alone was administered, it was unable to reduce body temperature (Tran et al., 2019). Oral administration of a medium containing D-Asp-producing live bacteria has been found to reduce body temperature, as well as downregulating avian uncoupling protein (avUCP) and upregulating avian adenine nucleotide translocator (avANT) in the skeletal muscle of chicks treated with the medium (Do et al., 2017). Therefore, it is possible that avUCP or avANT might have been involved in the reduction of body temperature brought about by the L-Cit-producing live bacteria.
Because watermelon is a natural source of L-Cit, a study was carried out to examine the effect of watermelon waste (i.e., watermelon rind [WR]) on the body temperature and plasma free amino acids of chicks (Nguyen et al., 2020). Fourteen-day-old chicks were given acute oral administration of WR extract (WRE) (2 mL) under CT. Fifteen-day-old chicks were orally administered 1.6 mL of either WRE, a low dose of L-Cit (7.5 mmol/10 mL), or a high dose of L-Cit (15 mmol/10 mL) under CT. In both experiments, the rectal temperature and the plasma free amino acids were analyzed. In another experiment, following dual oral administration of (1.6 mL) WRE or L-Cit (15 mmol/10 mL), 15-day-old chicks were exposed to HT (35 ± 1°C, 2 h) to monitor changes in rectal temperature. Acute oral administration of WRE was found to significantly reduce the rectal temperature under CT. The degree of this reduction by WRE was similar to that brought about by the administration of a high dose of L-Cit. Moreover, as a result of the oral administration of WRE, rectal temperature was significantly low at HT. Oral administration of 2 mL of WRE, but not 1.6 mL, increased plasma Cit concentrations in comparison with the control group. In conclusion, WRE was found to induce immediate hypothermia not only under CT after a single administration but also under HT conditions following dual oral administration. A particular dose of WRE can increase the concentration of Cit in plasma. By regulating plasma free amino acids, WRE could play a role in reducing body temperature in chicks.
7 FUTURE PERSPECTIVES
Because Arg is provided in poultry diet as it is an essential amino acid, which chickens cannot produce sufficiently, and it can synthesize Cit as a byproduct of NO production. Therefore, Cit has not been considered to add in the poultry diet. However, recent studies showed that Cit administration can minimize heat stress in chickens (Chowdhury et al., 2017; Chowdhury, Ouchi, Han, et al., 2021; Uyanga, Liu, et al., 2022; Uyanga, Zhao, et al., 2022). Moreover, a concern with dietary Arg supplementation is its potential antagonism with basic amino acids, including Arg, lysine, and histidine, whereas Cit is a neutral amino acid and does not share the same transporters with basic amino acids (Wu & Morris, 1998). It was further reported that oral administration of L-Cit significantly increased plasma and brain Arg, Orn, and Cit (Chowdhury et al., 2022). Therefore, Cit administration may be helpful in chickens to minimize heat stress and to increase plasma Arg. However, some feeding trial with dietary supplementation or oral administration of L-Cit in layer and broiler chickens under farming condition is needed to examine the required dose to minimize summer heat stress.
8 CONCLUSIONS
Heat stress is a major concern in poultry production owing to increased global warming because it affects the amino acid metabolic activity in chickens. One particular amino acid—namely, L-Cit—appears to be useful as a biomarker of heat stress because its concentration is affected by heat stress. When administered orally or supplemented in the diet, this amino acid has been found to afford thermotolerance in chickens. Alternative sources of synthetic L-Cit, such as live bacteria that produce L-Cit, and WRE, could also be used in chickens to minimize body temperature increment under heat stress.
ACKNOWLEDGMENTS
The author was awarded the 2022 Society Award of the Japanese Society of Animal Science for his paper, “Elucidation of the mechanism of heat stress in chickens and research on improvement of heat tolerance.” I would like to express my sincere appreciation to M. Furuse and T. Bungo for their wholehearted support of my study on the subject of heat stress. I am also grateful to all the students who contributed to our original work on L-Cit in chickens. My sincere thanks go to T. Bungo and M.Z. Elhussiny for their careful reading of the manuscript. I acknowledge M.Z. Elhussiny for his generous assistance in the writing of this article. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science [grant numbers JP15K07694, JP18K19721, and JP19H03110 to VSC as well as JP17KT0077 and JP21H02344 to TB] and by grants from the Kieikai 2014, 2015 and the Ito Zaidan 2022 Research Foundation, Japan to VSC.
CONFLICT OF INTEREST STATEMENT
The author declares that he has no conflict of interest.




