MiR-222 inhibits apoptosis in porcine follicular granulosa cells by targeting the THBS1 gene
Abstract
Apoptosis of granulosa cells affects follicular atresia and reproduction and is regulated by miRNAs and the expression of certain genes. For the present study, we investigated the regulatory relationship between microRNA-222 (miR-222) and THBS1 in porcine follicular granulosa cells (pGCs) and its effects on apoptosis to provide empirical data for developing methods to improve pig fecundity. Results revealed that miR-222 promotes the proliferation of pGCs. MiRNA mimics and luciferase reporter assays revealed that miR-222 functions as an anti-apoptotic factor in pGCs. MiR-222 mimics in pGCs result in the upregulation of the anti-apoptotic BCL-2 gene, down-regulation of the proapoptotic caspase-3 gene, and inhibition of apoptosis. MiR-222 inhibitors reduced BCL-2 and had no significant effect on caspase-3. MiR-222 mimics promoted estrogen levels. Inhibition of THBS1 inhibited pGC apoptosis. Transfection of THBS1-siRNA reduced the proapoptotic BAX gene. MiR-222 can directly target the 3′-untranslated region of the THBS1 gene. MiR-222 mimics suppressed THBS1 mRNA and proteins, but these were upregulated by the miR-222 inhibitor. Transfection of THBS1-siRNA resulted in the inhibition of the miR-222 inhibitor, which suggests that miR-222 inhibits pGC apoptosis by targeting THBS1. These findings suggest that miR-222 and THBS1 play important roles in follicular atresia, ovarian development, and female reproduction.
1 INTRODUCTION
Physiological processes in the ovary, such as follicular development, ovulation and, corpus luteum formation, are dependent on adequate angiogenesis, and any event that disrupts the normal angiogenic process may result in ovarian dysfunction and may lead to infertility (Greenaway et al., 2007). Porcine follicular granulosa cells (pGCs) are important in follicles, and apoptosis of pGCs is the main cause of follicular atresia. In cells, miRNAs regulate a variety of important physiological and pathological processes, including cell proliferation (Han, Yin, & Zhang, 2018), differentiation (Wu et al., 2019), apoptosis (Yao, Pan, Du, Zhang, & Li, 2018), and hormone biosynthesis and secretion (Song et al., 2018). Previous studies have indicated that miR-222 is an important regulatory factor for cancer development (Galardi et al., 2007; Gong et al., 2018; Gu et al., 2018; Guo et al., 2018; Huang, Ouyang, Wei, & Yu, 2018; Iida et al., 2018; le Sage et al., 2007; Liu et al., 2018). In the bovine ovaries, the expression level of miR-222 was found to be low in preovulatory follicles and high in corpus luteum (Özdemir & Çomaklı, 2018). MiR-222 was also found to be expressed in the human follicular fluid and promoted estradiol secretion in the KGN cells (Sang et al., 2013). Thrombospondin-1 (THBS1) is a large extracellular matrix-associated protein that is an anti-angiogenic and is important for cell development and differentiation, and for normal follicular development (McGray, Gingerich, Petrik, & LaMarre, 2011). This complex can inhibit follicular angiogenesis and directly induce granulosa cell apoptosis in a dose-dependent manner (Garside, Harlow, Hillier, Fraser, & Thomas, 2010).
Several previous studies have identified a number of miRNAs that regulate the THBS1 gene in different types of cells, e.g., miR-487b, the miR-17-92 cluster, miR-194, miR-18 and miR-19, miR-27b, and miR-let-7d (Asama et al., 2019; Dogar, Semplicio, Guennewig, & Hall, 2014; Farberov & Meidan, 2018; Feng et al., 2015; Miao et al., 2018; Sundaram et al., 2011; van Almen et al., 2011). In a recent study, a quantitative proteomic analysis of PK-15 cells that were transfected with miR-222 mimics and infected with transmissible gastroenteritis virus (TGEV) indicated that THBS1 was regulated by miR-222 (Zhao et al., 2019). However, there are few reports of miR-222 in pGCs. The role of THBS1 in pGCs apoptosis and its regulation by miR-222 are currently unclear. For the present study, we examined the effects of aberrant expression of miR-222 and THBS1 on the proliferation and apoptosis of pGCs and elucidated the regulatory relationship between miR-222 and THBS1. The empirical data reported herein can provide a reference for developing reproductive regulations for the raising of pigs and improving their fecundity.
2 MATERIALS AND METHODS
2.1 Cell culture and transfection
Fresh porcine ovaries were obtained from a commercial slaughter house and transported back to the laboratory within 1 hr and pGCs were collected from their follicles (3–6 mm diameter). The cells were cultured at 37°C and 5% CO2 in DMEM/F-12 medium (Gibco) containing 10% fetal bovine serum (FBS) (Gibco), 100 units/ml penicillin, and 100 mg/ml streptomycin (Gibco). 293T cells were incubated at 37°C and 5% CO2 in DMEM containing 10% FBS. MiR-222 mimics, mimics NC, miR-222 inhibitor, inhibitor NC, THBS1-siRNA, and NC-siRNA were purchased from GenePharma (GenePharma, Shanghai, China) (Table 1). These small oligonucleotides were transfected into the cells at different final concentrations using Lipofectamine 3,000 reagent (Invitrogen, Waltham, MA). All experiments were performed in triplicate.
| Name | Sequence (5′-3′) |
|---|---|
| mimics NC |
Sense: UUCUCCGAACGUGUCACGUTT Antisense: ACGUGACACGUUCGGAGAATT |
| miR-222 mimics |
Sense: AGCUACAUCUGGCUACUGGGUCUC Antisense: GACCCAGUAGCCAGAUGUAGCUUU |
| inhibitor NC | CAGUACUUUUGUGUAGUACAA |
| miR-222 Inhibitor | GAGACCCAGUAGCCAGAUGUAGCU |
| siTHBS1 |
Sense: CCCUGAGGCAAAUGAAGAATT Antisense: UUCUUCAUUUGCCUCAGGGTT |
| NC siRNA |
Sense: UUCUCCGAACGUGUCACGUTT Antisense: ACGUGACACGUUCGGAGAATT |
2.2 Quantitative real-time PCR
Total RNA of pGCs was extracted using TRIzol and the RT reactions were performed using SYBR Premix Ex Taq (TaKaRa, Osaka, Japan) and a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) following the manufacturer's instructions. The PCR reaction (20 μl volume) comprised 1 μl cDNA, 1.5 μM of each primer, 10 μl 2x SYBR Green PCR Master Mix (TaKaRa, Osaka, Japan), and water up to the total volume. Thermal cycling conditions were 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The relative expression level was calculated using the 2−ΔΔCt method. The GAPDH gene used for endogenous internal controls for mRNA expression. The primers for qRT-PCR are listed in Table 2.
| Name | Primer | Sequence (5′-3′) |
|---|---|---|
| THBS1 |
Forward Reverse |
CAACTCAGGGCAGGAAGACT GGGCTGGGTTGTAATGGAAT |
| BCL-2 |
Forward Reverse |
GCGACTTTGCCGAGATGTC CCGAACTCAAAGAAGGCCAC |
| Bax |
Forward Reverse |
AGCTGCAGAGGATGATCGC CCAGTTGAAGTTGCCGTCAG |
| Caspase-3 |
Forward Reverse |
TAACCCGAGTAAGAATGT ATACCAGTTGAGGCAGAC |
2.3 Western blotting
pGCs were collected 72 hr after transfection, and whole cell lysates were prepared in RIPA buffer (50 mM Tris HCl, pH 8, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% Triton X-100, and proteinase inhibitors) (Solarbio, Beijing, China). Protein concentration was measured by the BCA method (Pierce, Shanghai, China). The proteins were separated by 10% SDS gel and transferred onto PVDF membranes. The membranes were blocked with 5% skimmed milk and then incubated at 48°C overnight with a diluted (1:1,000) monoclonal anti-THBS1 antibody (Abcam) or anti-GAPDH antibody (1:3,000) as an internal control, followed by a secondary antibody (1:5,000) for 1–2 hr at room temperature. After incubation with antibodies according to the manufacturer's instructions, an ECL kit (Fermantas) was used to visualize the protein bands. Densitometric analysis was performed to quantify the signal intensity.
2.4 Methyl thiazolyl tetrazolium assay
For methyl thiazolyl tetrazolium (MTT) assays, pGCs were seeded into 96-well plates and transfected with miR-222 mimics, mimics NC, miR-222 inhibitor, and inhibitor NC. Cell proliferation was assessed at 48 and 72 hr post-transfection.
2.5 Apoptosis assay
The level of apoptosis was detected using anti-annexin V FITC/PI staining and flow cytometry analysis. After cells were transfected and incubated for 48 hr, cells were digested with trypsin and resuspended in 500 μl of binding buffer solution containing 5 μl annexin V-fluorescein isothiocyanate and 10 μl propidium iodide (BestBio, Shanghai, China). Stained cells were counted using a FACS Calibur™ flow cytometry instrument (BD Biosciences, NJ).
2.6 Plasmid construction and dual-luciferase assay
THBS1 was predicted in silico as a target of miR-222. To find the binding sites of miR-222 on the THBS1 gene, the GP-miRGLO dual-luciferase miRNA target expression vector containing the wild-type THBS1 3′-UTR was constructed. Meanwhile, the GP-miRGLO reporter vector containing the wild-type and mutational-type miR-222 promoters was also constructed. Mutant plasmids were constructed using a MutanBEST Kit (TaKaRa Osaka, Japan) according to the manufacturer's instructions. 293T cells were transfected with the constructed plasmids and miR-222 or NC mimics. After transfection for 24 hr or 48 hr, 293T cells were collected and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions.
2.7 Enzyme-linked immunosorbent assay
After 48 hr of transfection, the pGCs culture medium, containing 10% FBS, was collected by centrifugation at 2000×g for 20 min. Estrogen concentrations were measured using porcine estrogen ELISA kits (Ji Yin Mei, Wuhan, China) according to the manufacturer's instructions.
2.8 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics v20.0 (SPSS Inc., Chicago, IL). A two-tailed Student t-test and ANOVA were used to evaluate the statistical significance of the observed differences. p < 0.05 and p < 0.01 were considered as significant and highly significant, respectively.
3 RESULTS
3.1 MiR-222 and the proliferation/inhibition of apoptosis in pGCs
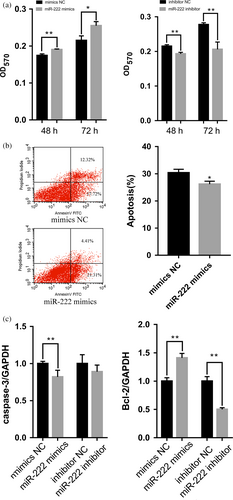
To examine the functions of miR-222 in pGCs, miR-222 mimics and inhibitors were transfected into pGCs cultured in vitro. An MTT assay revealed that pGC proliferation increased significantly after transfection with miR-222 mimics (p < 0.05) and reduced significantly after transfection with miR-222 inhibitors (p < 0.01) (Figure 1a). Apoptosis rate was significantly higher in pGCs transfected with miR-222 mimics than those transfected with NC mimics (p < 0.05) (Figure 1b). In addition, the overexpression of miR-222 significantly increased the expression level of the antiapoptotic BCL-2 gene (p < 0.01) and reduced the expression level of the proapoptotic caspase-3 gene (p < 0.01). MiR-222 inhibitor significantly reduced the expression level of the BCL-2 gene (p < 0.01), but had no significant effect on the expression level of the caspase-3 gene (Figure 1c). These results indicated that miR-222 is an anti-apoptotic factor and increases cell numbers of pGCs in vitro.
3.2 MiR-222 and estrogen secretion in pGCs
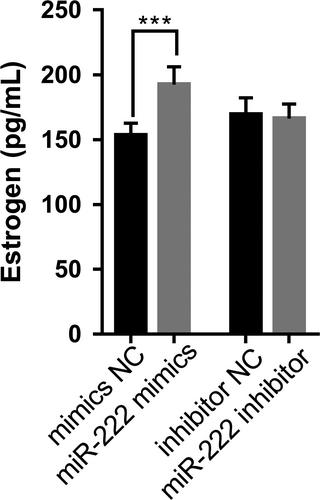
To examine the effect of miR-222 on estrogen secretion in pGCs, miR-222 mimics and inhibitors were transfected into pGCs cultured in vitro. The enzyme-linked immunosorbent assay revealed that estrogen levels in pGCs were upregulated significantly after transfection with miR-222 mimics (p < 0.001) (Figure 2). However, there was no significant change in estrogen level in pGCs after transfection with miR-222 inhibitor (p > 0.05) (Figure 2).
3.3 Inhibition of the THBS1 gene and inhibition of apoptosis in pGCs
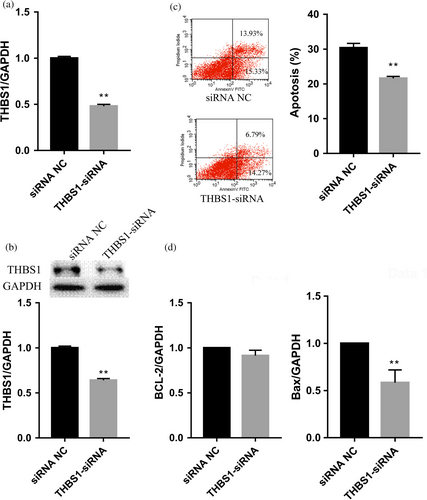
To explore the effect of the THBS1 gene on apoptosis in pGCs, the THBS1 gene was knocked down by RNA interference in pGCs cultured in vitro. The qRT-PCR and western blot analyses confirmed that the expression levels of THBS1 mRNA and proteins were significantly lower in pGCs transfected with THBS1-siRNA than those transfected with NC-siRNA (p < 0.01; Figure 3a,b). A flow cytometry analysis showed that the apoptosis rate of the THBS1-siRNA group was significantly lower than that of the NC-siRNA group (p < 0.01) (Figure 3c). Transfection of THBS1-siRNA significantly reduced the expression level of the proapoptotic BAX gene (p < 0.01) but had no significant effect on the expression level of the antiapoptotic BCL-2 gene (Figure 3d). The results indicate that inhibition of THBS1 expression inhibits apoptosis in pGCs.
3.4 The THBS1 gene as a target of miR-222
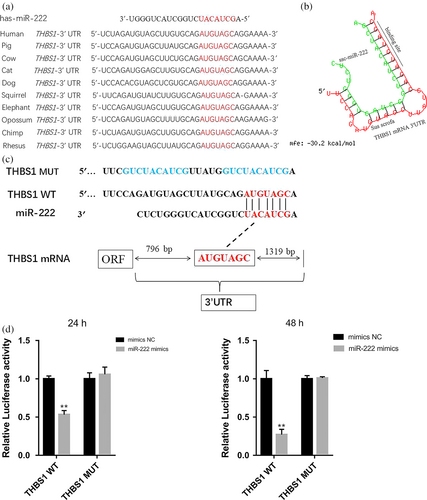
MiR-222 is better studied for ovarian cancer (Amini-Farsani, Sangtarash, Shamsara, & Teimori, 2018; Li, Feng, Coukos, & Zhang, 2011; Sun et al., 2013), but not for pig ovaries. Two bioinformatics tools (TargetScan and miRBase) were used to search the common candidate miRNAs targeting THBS1. Furthermore, porcine miR-222 shares a common seed region (GCUACAU) with other vertebrates. Alignment of the miR-222 seed sequence with the 3′-UTR of THBS1 from pigs and other vertebrates exhibited complementary pairing (Figure 4a). The data suggest that miR-222 is highly conserved in vertebrates. The potential binding sites for miR-222 were detected in the 3′-UTR of pig THBS1 mRNA at positions 797–803 nt using RNAhybrid software (Figure 4b). To determine whether miR-222 can regulate THBS1 gene, the putative miR-222 target sites in the porcine THBS1 3′-UTR were cloned downstream of the luciferase gene in the pmirGLO dual-luciferase reporter vector to generate pmirGLO-THBS1-3′-UTR (Figure 4c). 293T cells were transiently cotransfected with the reporter plasmid and miR-222 or NC mimics and were detected at 24 hr and 48 hr. Overexpression of exogenous miR-222 repressed the activity of the luciferase reporter fused to the THBS1 3′-UTR (p < 0.05) (Figure 4d). However, luciferase activity was not significantly altered when the cells were cotransfected with the miR-222 mimics and a THBS1 reporter 3′-UTR construct containing a mutation in the miR-222 binding site (p > 0.05) (Figure 4d). These results suggest that miR-222 can regulate the porcine THBS1 gene by binding to conserved sites in its 3′-UTR.
3.5 MiR-222 regulation of the expression of THBS1 in pGCs
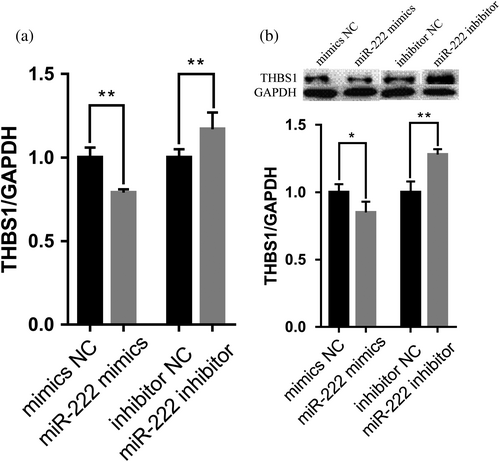
The qRT-PCR results showed that THBS1 mRNA expression level significantly decreased following the overexpression of miR-222 and increased after miR-222 inhibitor transfection (p < 0.01) (Figure 5a). The western blot analysis showed that THBS1 protein expression level significantly decreased following the overexpression of miR-222 (p < 0.01) and increased after miR-222 inhibitor transfection (p < 0.05) (Figure 5b).
3.6 MiR-222 inhibition of apoptosis in pGCs through targeting THBS1
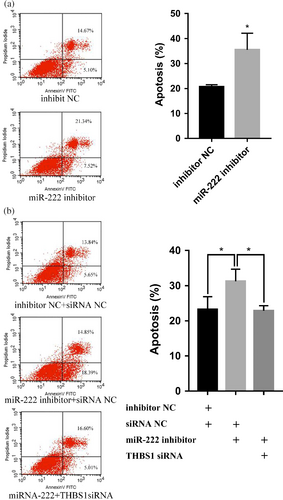
To further investigate whether the effects of miR-222 on pGCs apoptosis were mediated through THBS1, pGCs were co-transfected with a miR-222 inhibitor and THBS1-siRNA. The results revealed that miR-222 inhibitor can increase pGC apoptosis (p < 0.01; Figure 6a), but that knockdown of THBS1 can significantly suppress the promotion of pGC apoptosis by miR-222 inhibitors (p < 0.01; Figure 6b). These data indicate that miR-222 inhibits the apoptosis of pGCs by targeting THBS1.
4 DISCUSSION
In mammalian ovaries, only a few follicles ovulate during ovarian follicular development and more than 99% of the follicles undergo degenerative atresia (Liu et al., 2014). The apoptosis of pGCs is closely related to follicular atresia because pGCs play a key role in follicular development and atresia. MiRNAs are also important for ovarian function and pGC apoptosis (Cao et al., 2015; Liu et al., 2014; Yao et al., 2018). Recent studies have shown that miR-222 can regulate cell proliferation, migration, invasion, or apoptosis in liver cancer (Liu et al., 2018), pancreatic cancer (Li et al., 2018), fibroblasts in hypertrophic scars (Zhang, Zhang, Zhang, Hong, & Lin, 2017), gastric cancer (Tan, Tang, Bi, Li, & Jia, 2018) and epithelial ovarian cancer (Sun et al., 2013). However, studies on miR-222 in pGCs have not been reported. Here, miR-222 was identified as a key regulator of pGC apoptosis in mammals. We found that miR-222 could promote the cell numbers and inhibit the apoptosis of pGCs, which is consistent with the observed functions of miR-222 in other cells. The overexpression of miR-222 increases the expression level of the antiapoptotic BCL-2 gene and reduces the expression level of the proapoptotic caspase-3 gene, whereas the miR-222 inhibitor reduced the expression level of BCL-2. These results indicated that miR-222 increased granulosa cell numbers are likely to be attributed to the anti-apoptotic roles of it.
MiR-222 inhibits the expression of the KIT receptor in mammalian undifferentiated spermatogonia (Yang, Racicot, Kaucher, Oatley, & Oatley, 2013). Previous studies have reported that miR-222 plays a role in oocyte maturation and fertilization in cows (Gilchrist, Tscherner, Nalpathamkalam, Merico, & LaMarre, 2016) and during the follicular development of the cow ovary (Özdemir & Çomaklı, 2018) or breast cancer (Petrovic et al., 2016). MiR-222 is involved in the regulation of ER-α expression. Moreover, estrogen signaling plays a critical role in the development of the female reproductive system, and the generation of a primordial follicle may be dependent on both estrogen and ER-α signaling pathways (Juengel, Heath, Quirke, & McNatty, 2006; Juengel et al., 2002). Estrogen is predominantly synthesized and secreted from the granulosa cells of growing preovulatory follicles (Özdemir & Çomaklı, 2018). Studies have shown that miR-222 played important roles in the steroidogenesis pathway and significantly increased estradiol production in goat granulosa cells (Gao et al., 2018; Sang et al., 2013). In our study, we observed that miR-222 promotes estrogen in pGCs indicating that miR-222 is important in the development of the porcine reproductive system. The promoting effects of miR-222 on estrogen of pGCs might be attributed to the increased cell numbers due to the anti-apoptotic effects of miR-222.
In the ovary, follicle occurrence, ovulation, and corpus luteum formation are dependent on adequate angiogenesis. THBS1 is an important stromal cell protein that inhibits angiogenesis and is associated with tumorigenesis (Osz, Ross, & Petrik, 2014). This protein affects bovine luteal function and was inhibited by luteinizing signals (LH and insulin) (Farberov & Meidan, 2014, 2016). RNA interference knockdown of THBS1 can reduce TNFα-induced apoptosis (Greenaway et al., 2007). The inhibition of THBS1 receptor CD36 can reduce follicular apoptosis and promote the proliferation of pGCs in mice (Osz et al., 2014) The role of THBS1 in follicular function or atresia has been previously studied using various animal models and was found to be upregulated during follicular atresia (Garside et al., 2010; Greenaway, Gentry, Feige, LaMarre, & Petrik, 2005; Petrik, Gentry, Feige, & LaMarre, 2002; Thomas, Wilson, Silvestri, & Fraser, 2008). Anti-inhibin alpha subunit treatment in pGCs cultured in vitro can downregulate THBS1 (Cai et al., 2015). THBS1 mediates IGF-I-induced pGCs steroidogenesis and proliferation during follicular development (Grado-Ahuir et al., 2009). However, the function of the THBS1 gene in pGCs is not fully understood. In our study, it was found that interference with THBS1 can inhibit the apoptosis of pGCs and significantly downregulate the mRNA expression of apoptosis-related Bax gene. These results suggest that THBS1 may be a proapoptotic factor in pGCs. The PI3/Akt signaling pathway may be an important mediator of the effects of THBS1 in the ovary (Osz et al., 2014). This information can be used as a reference for future research.
Based on these findings, we further identified THBS1 as the target of miR-222. The results of the bioinformatic analysis and luciferase assay demonstrate that miR-222 directly binds to the 3′UTR of THBS1. In a recent study, quantitative proteomic analysis of miR-222-mimic-transfected and TGEV-infected PK-15 cells revealed that THBS1 is a target for miR-222 (Zhao et al., 2019). This result is consistent with those of the present study. In addition, we investigated how this regulatory function affects the apoptosis of pGCs. MiR-222 inhibitor was added to increase the apoptotic rate of pGCs, but after siRNA interfered with the expression of THBS1, the action of the miR-222 inhibitor was inhibited. These results may further confirm the direct effects of THBS1 on miR-222 regulation of pGCs apoptosis.
In conclusion, we demonstrated that miR-222 controls estrogen levels and acts as an anti-apoptotic factor to control pGCs apoptosis by targeting THBS1 in the porcine ovary. These findings suggest that miR-222 and THBS1 play important roles in follicular atresia, ovarian development, and female reproduction. Further research building on our findings may provide new insights into the mechanisms of pGC apoptosis and follicular atresia.
ACKNOWLEDGMENTS
This research was supported by grants from the National Sci-Tech Support Plan of China (2015BAD03B01), the National Natural Science Foundation of China (Nos. 31572377 and 31402037), the Anhui Provincial Sci-Tech Major Project (Nos. 17030701061 and 17030701008), the National Key Research and Development Program of China (No. 2017YFD0600805), the Anhui Provincial Natural Science Foundation (1508085QC52), and the Anhui Provincial Sci-Tech plan (Nos. 1704a07020085 and 1704a07020090).




