Relationship among ovarian follicular status, developmental competence of oocytes, and anti-Müllerian hormone levels: A comparative study in Japanese wild boar crossbred gilts and Large White gilts
Abstract
The aim of this study was to investigate the ovarian follicular development, developmental competence of oocytes, and plasma anti-Müllerian hormone (AMH) levels of Japanese wild boar crossbred (wild hybrid) gilts, whose litter size is inferior to that of European breeds. Ovary and plasma samples were collected from two different breeds of gilts (wild hybrid and Large White breeds). The ovaries from the wild hybrid gilts had a lower average numbers of secondary follicles and vesicular follicles in ovarian cross-sections and of good quality oocytes collected from ovarian follicles as compared with those from Large White gilts (p < 0.05). The development rate to the blastocyst stage of good quality oocytes after in vitro maturation, fertilization and culture was also lower (p < 0.05) in wild hybrid gilts than in Large White gilts. Plasma AMH levels with >0.16 ng/ml were detected in 8.3% of the examined wild hybrid gilts and 33% of the Large White gilts. These results indicate that the low reproductive performance of wild hybrid breed may result in part from low numbers of vesicular follicles and good quality oocytes, and low developmental competence of oocytes. Moreover, plasma AMH levels may support low number of vesicular follicles in ovaries of wild hybrid gilts.
1 INTRODUCTION
A major source of economic loss for pork producers is the poor reproductive performance of sows. The litter size, which varies in each breed, is an important factor that affects profitability in the pig industry. Thus, crossbreeding has long been known to be an effective way to improve reproductive performance, including litter size (Rothschild, 1996). Some domestic pigs have been produced by crossing Japanese wild boars with European breeds to establish the branding of pigs in the rural area of Japan. For example, the wild boar × Large White × Duroc breed (wild hybrid) has been established using a quantitative trait loci technique to improve meat quality (Nii et al., 2005, 2006). However, the average litter size (7.7 per sow) of wild hybrid breeds is lower than that (10.6 per sow) of European breeds such as the Large White breed (Canario et al., 2006). Female hybrids that had been repeatedly mated with European wild boars have been reported to have a decreased litter size (Klimienė & Klimas, 2010), but the factors influencing the low litter size of crossbreeds with wild boar have not been investigated well.
Porcine in vitro production (IVP) systems consist of three major steps: in vitro maturation (IVM), in vitro fertilization (IVF), and in vitro culture (IVC) of fertilized embryos. The parameters of each step are indicative of the quality of oocytes for the successful development to piglets (Funahashi, 2003; Kikuchi, 2004; Kikuchi et al., 2002). Therefore, evaluations of the maturation, fertilization and development of oocytes by IVP procedures can be beneficial markers to assess the reproductive performance of sows. However, it has been reported that plasma levels of anti-Müllerian hormone (AMH) are correlated with the number of early vesicular follicles in cows (Rico et al., 2009), goats (Monniaux et al., 2011), and humans (Fanchin et al., 2003). Therefore, AMH is also considered a useful endocrine marker to assess reproductive performance in the fields of obstetrics and gynecology (van Rooij et al., 2002, 2004, 2005). In pigs, it has been demonstrated that AMH appears for the first time during follicular development in the fusiform granulosa cells of recruited primordial follicles and continues to be present in granulosa cells up to the antral stage (Almeida et al., 2018), indicating that plasma AMH levels may be an effective marker of reproductive performance, including litter size (Steel, Athorn, & Grupen, 2018).
In this study, we investigated the reasons for the low reproductive performance of wild hybrid sows by analyzing follicular conditions, including oocyte quality, follicle number, the developmental competence of oocytes, and plasma AMH levels.
2 MATERIALS AND METHODS
2.1 Oocytes preparation and plasma samples
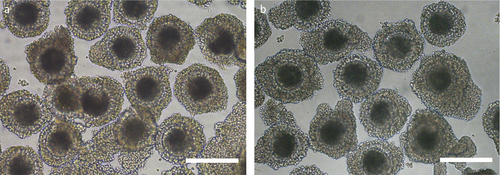
Porcine ovary and blood samples were obtained from approximately 6-month-old Large White gilts and 7-month-old wild hybrid gilts at a local slaughterhouse and were transported within 3 hr to the laboratory in physiological saline at 30°C for the ovaries and on ice for the blood samples. The gilts were categorized into either the follicular phase or luteal phase of the estrous cycle by examining the morphological appearance of their ovarian tissue, in which the donors were categorized as follows: follicular phase, visible follicles were present, and luteal phases, one or more pronounced corpora lutea were present. Only ovaries from gilts in the follicular phase were selected and washed three times with prewarmed physiological saline solution that was supplemented with 100 IU/ml penicillin G potassium (Meiji, Tokyo, Japan) and 0.1 mg/ml streptomycin sulfate (Meiji). The follicles on the ovarian surface were sliced using a surgical blade on a sterilized dish, and the cumulus–oocyte complexes (COCs) were collected under a stereomicroscope. The COCs were graded according to the number of cumulus cell layers: grade A, three or more layers of cumulus cells (Figure 1); grade B, one or two layers of cumulus cells; and grade C, partial cumulus cells or denuded oocytes. Blood samples in heparinized tubes were centrifuged at 1,500 g for 15 min at 4°C. The plasma in each sample was separated and then stored at −80°C until the measurement of AMH.
2.2 IVM and assessment of meiotic competence
Approximately, 50 COCs were separately cultured in 500 μl of maturation medium according to the COC grade. The maturation medium consisted of tissue culture medium 199 with Earle's salts (TCM 199; Invitrogen Co., Carlsbad, CA, USA), was supplemented with 10% (v/v) porcine follicular fluid, 0.6 mM cysteine (Sigma-Aldrich, St. Louis, MO, USA), 50 μM β-mercaptoethanol (Wako Pure Chemical Industries Ltd., Osaka, Japan), 50 μM sodium pyruvate (Sigma-Aldrich), 2 mg/ml D-sorbitol (Wako), 10 IU/ml equine chorionic gonadotropin (Kyoritu Seiyaku, Tokyo, Japan), 10 IU/ml human chorionic gonadotropin (Kyoritu Seiyaku), and 50 μg/ml gentamicin (Sigma-Aldrich), and was covered with mineral oil (Sigma-Aldrich) for 22 hr in 4-well dishes (Nunc A/S, Roskilde, Denmark). Subsequently, the COCs were transferred into the maturation medium without hormone supplementation and cultured for an additional 22 hr. The incubation of the COCs was conducted at 39°C in a humidified incubator containing 5% CO2.
To assess the meiotic status of the oocytes following IVM, some oocytes were denuded, fixed, and permeabilized in Dulbecco's phosphate buffered saline (DPBS; Invitrogen) that was supplemented with 3.7% (w/v) paraformaldehyde and 1% (v/v) Triton X-100 (Sigma-Aldrich) at 25°C for 15 min. Permeabilized oocytes were then placed on glass slides and stained with 1.9 mM bisbenzimide (Hoechst 33342; Sigma-Aldrich) before being mounted with coverslips. After an overnight incubation at 4°C, the oocytes were examined by fluorescence microscopy. Based on their chromatin configuration, they were classified as germinal vesicle, condensed chromatin, metaphase I, or metaphase II. Oocytes with diffusely stained cytoplasmic characteristics of nonviable cells and those in which chromatin was unidentifiable or not visible were classified as degenerated.
2.3 IVF and assessment of fertilization
IVF was performed according to methods described previously (Nguyen et al., 2017). Briefly, frozen-thawed ejaculated spermatozoa that were collected from wild hybrid boar were transferred into 5 ml of porcine fertilization medium (Research Institute for the Functional Peptides Co., Yamagata, Japan) and washed by centrifugation at 500 g for 5 min. The pelleted spermatozoa were resuspended in fertilization medium and adjusted to 5 × 106 cells/ml. Next, approximately 50 COCs were transferred to 500 μl of sperm-containing fertilization medium, covered with mineral oil in 4-well dishes and coincubated for 5 hr at 39°C with 5% CO2 and 5% O2. After coincubation, the inseminated zygotes were denuded of cumulus cells and the attached spermatozoa by mechanical pipetting.
To assess the fertilization of the oocytes, some denuded zygotes were mounted on glass slides and fixed with acetic acid:ethanol (1:3 v/v) for 72 hr. The fixed zygotes were stained with acetic orcein (1% orcein in 45% acetic acid) and examined by phase contrast microscopy. Oocytes containing both female and male pronuclei were considered to be fertilized and were categorized as monospermic or polyspermic according to the number of swollen sperm heads and pronuclei in the cytoplasm.
2.4 IVC and assessment of blastocyst quality
The remaining denuded zygotes were subsequently transferred to Porcine zygote medium (PZM-5, Research Institute for the Functional Peptides Co.). Approximately, 50 zygotes were cultured continuously in 500 μl of PZM-5 covered with mineral oil in 4-well dishes. After culturing the embryos for 3 days, they were subsequently incubated in 500 μl of porcine blastocyst medium (Research Institute for the Functional Peptides Co.) covered with mineral oil for 4 days in 4-well dishes. The incubation of zygotes and embryos was conducted at 39°C in a humidified incubator containing 5% CO2, 5% O2, and 90% N2. To evaluate the developmental stage of the fertilized oocytes, all embryos were fixed on day 7 (day 0 = insemination) and stained with Hoechst 33342 to assess the quality of the embryos by cell counting. The embryos that had a blastocoel and were cleaved to more than sixteen cells were classified as blastocysts.
2.5 Measurement of the plasma AMH level
The AMH concentrations of all stored plasma samples were measured by enzyme-linked immunosorbent assay (ELISA) using a commercially available porcine AMH ELISA kit (Elabscience Biotechnology Inc., Houston, TX, USA) according to the manufacturer's instructions. AMH concentrations were determined in 100 μl of undiluted plasma. The intra and interassay coefficients of variation were < 10%. The absorbance measurement was determined at 450 nm by an iMark microplate reader (Bio-Rad, Hercules, CA, USA).
2.6 Experimental design
2.6.1 Experiment 1: Comparison of oocyte quality and follicle numbers by pig breed type
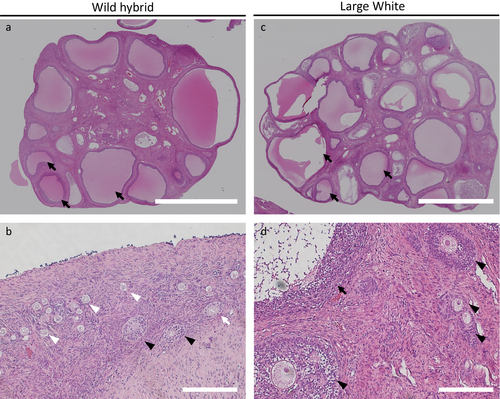
To evaluate the quality of oocytes and the number of follicles, a pair of fresh ovaries from the two breeds of gilts at the follicular phase was transversely cut in half using a scalpel blade. One half of each ovary was used for the collection of COCs to examine the number of classified COCs according to the method described above. The number of COCs that was collected from a pair of hemi-ovaries from one gilt was totaled for the calculation to obtain the number of COCs per ovary. The remaining 2 hemi-ovaries were fixed in 4% paraformaldehyde neutral buffer solution (Wako) for histological examination. Each ovary was manually embedded in paraffin. The paraffin sections were prepared and stained with hematoxylin and eosin using conventional techniques. The cutting plane of each hemi-ovary, in other words, the cross-section of the center of each ovary, was evaluated, and the number of follicles at each stage was counted. The follicles were classified as follows: primordial follicles, follicles with one layer of flattened pregranulosa cells that surrounded the oocytes; primary follicles, follicles with one layer of cuboid granulosa cells; secondary follicles, follicles with two or more layers of granulosa cells and no vesicular spaces; and vesicular follicles, follicles with vesicular spaces (Figure 2). An average number of each class of follicle per cross-section was calculated.
2.6.2 Experiment 2: Comparison of the maturation, fertilization and development of oocytes by pig breed type
To evaluate the meiotic maturation, fertilization, and development abilities of the porcine oocytes from the two breed types, the COCs that were classified in experiment 1 were subsequently cultured separately according to COC grade and pig breed. These COCs were used for IVM, IVF, and IVC to evaluate the rates of maturation, fertilization, monospermy, cleavage and blastocyst formation and the cell number of the blastocysts.
2.6.3 Experiment 3: Comparison of the plasma AMH level by pig breed type
Plasma samples of wild hybrid gilts and Large White gilts, whose ovaries were used in experiments 1 and 2, were used for the measurement of plasma AMH levels. We excluded plasma samples from gilts who were determined to be in the luteal phase of the estrous cycle.
2.7 Statistical analysis
The experiments were repeated more than four times to evaluate the maturation, fertilization and development of the oocytes. All percentage data were subjected to arcsine transformation before performing an analysis of variance (ANOVA). The data were tested by ANOVA, followed by a Fisher's protected least significant difference test, using StatView software (Abacus Concepts, Berkeley, CA). The proportions of AMH-detected gilts were analyzed using chi-squared analysis with Yates’ correction. Differences with a probability value (p) of 0.05 or lower were considered statistically significant.
3 RESULTS
3.1 Comparison of the quality of ovaries by pig breed type
As shown in Table 1, the total number of oocytes collected from wild hybrid gilts was significantly lower (p < 0.05) than that from Large White gilts. The numbers of oocytes collected from wild hybrid gilts were significantly lower (p < 0.05) than those from Large White gilts, irrespective of the COC grade. In particular, approximately 45% of the COCs collected from Large White gilts were grade A oocytes, whereas the percentage of COCs with the same grade in wild hybrid gilts was approximately 22%. The COCs collected from wild hybrid gilts were morphologically similar with that from Large White gilts (Figure 1).
| Breed types | Number of evaluated giltsa | Number of oocytes per ovaryb | |||
|---|---|---|---|---|---|
| COC grade | Total | ||||
| A | B | C | |||
| Wild hybrid | 24 | 15.0 ± 1.5c | 16.8 ± 1.5c | 36.9 ± 3.5c | 68.7 ± 5.7c |
| Large White | 18 | 58.0 ± 8.4c | 23.2 ± 4.1c | 46.3 ± 6.7c | 127.6 ± 18.0c |
- a The gilts at the luteal phase were excluded.
- b Numbers of oocytes per ovary are expressed as mean ± SEM. COC: cumulus oocyte complex.
- c Values with different superscripts in the same COC grade differ significantly (p < 0.05).
As shown in Table 2, the average number of primordial follicles in ovary cross-sections was significantly higher (p < 0.05) in wild hybrid gilts than in Large White gilts. However, the numbers of secondary follicles and vesicular follicles from wild hybrid gilts were significantly lower (p < 0.05) than those from Large White gilts.
| Breed types | Number of evaluated giltsa | Number of follicles per cross sectionb | ||||
|---|---|---|---|---|---|---|
| Follicular stage | Total | |||||
| Primordial follicle | Primary follicle | Secondary follicle | Vesicular follicle | |||
| Wild hybrid | 24 | 132.9 ± 18.9c | 16.1 ± 3.1 | 14.9 ± 3.6c | 15.1 ± 1.1c | 179.0 ± 23.3 |
| Large White | 18 | 54.8 ± 10.7c | 14.7 ± 3.7 | 36.3 ± 7.3c | 39.7 ± 4.2c | 145.6 ± 21.5 |
- a The gilts at the luteal phase were excluded.
- b Number of follicles per ovary cross section are expressed as mean ± SEM.
- c Values with different superscripts in the same follicular stage differ significantly (p < 0.05).
3.2 Comparison of the maturation, fertilization and development of oocytes by pig breed type
As shown in Table 3, significantly more grade C COCs that were collected from Large White gilts reached metaphase II (MII) compared to those from wild hybrid gilts (31.7% vs. 12.3%, p < 0.05). For grade A and grade B COCs, however, there were no significant differences in the MII rates of oocytes between wild hybrid gilts and Large White gilts. The rates of total fertilization and monospermic fertilization of the oocytes from wild hybrid gilts and Large White gilts were also comparable between each COC grade. In grade A and grade C COCs, the blastocyst formation rates of the oocytes collected from Large White gilts (12.2% and 4.3% for grade A and grade C, respectively) were significantly higher (p < 0.05) than those from wild hybrid gilts (4.8% and 0.3% for grade A and grade C, respectively). In grade B COCs, the cleavage rate of the oocytes collected from wild hybrid gilts (76.1%) was significantly higher (p < 0.05) than that from Large White gilts (49.2%), but no significant difference was observed in blastocyst formation rate.
| Breed types | COC grade | In vitro maturation | In vitro fertilization | In vitro culture | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of examined oocytes | Number (%)b of matured oocytes | Number of examined oocytes | Number of oocytes | Number of examined oocytes | Number of embryos | Number of cells in blastocyst | ||||
| Fertilized (%) | With monospermy (%)c | Cleaved (%) | Developed to blastocysts (%) | |||||||
| Wild hybrid | A | 38 | 29 (72.9 ± 10.5) | 28 | 16 (60.6 ± 13.5) | 7 (41.8 ± 14.1) | 177 | 141 (79.6 ± 7.2) | 7 (4.8 ± 2.3)d | 39.1 ± 4.1 |
| B | 47 | 22 (48.3 ± 16.2) | 28 | 12 (44.9 ± 12.5) | 9 (67.9 ± 15.6) | 216 | 165 (76.1 ± 2.9)d | 1 (0.6 ± 0.6) | 33.6 | |
| C | 80 | 10 (12.3 ± 5.3)d | 46 | 3 (7.8 ± 3.1) | 1 (40.0 ± 30.6) | 466 | 230 (47.8 ± 7.8) | 1 (0.3 ± 0.3)d | 53.3 | |
| Large White | A | 207 | 126 (66.0 ± 7.3) | 115 | 59 (52.6 ± 6.4) | 32 (52.8 ± 5.5) | 631 | 529 (84.0 ± 1.6) | 78 (12.2 ± 1.7)d | 38.8 ± 1.7 |
| B | 91 | 16 (23.0 ± 10.5) | 94 | 11 (12.6 ± 5.7) | 6 (46.7 ± 29.1) | 222 | 108 (49.2 ± 7.1)d | 1 (0.5 ± 0.7) | 17 | |
| C | 185 | 58 (31.7 ± 5.5)d | 140 | 2 (1.7 ± 1.1) | 2 (100) | 519 | 211 (44.0 ± 6.7) | 20 (4.3 ± 1.2)d | 27.9 ± 1.6 | |
- a Four replicated trials were carried out for oocytes collected from Wild hybrid gilts and five times for oocytes collected from Large White gilts.
- b Percentages are expressed as mean ± SEM.
- c The monospermic fertilization rate was defined as a ratio of the number of monospermic oocytes and the total number of fertilized oocytes.
- d Values with different superscripts in the same COC grade differ significantly (p < 0.05).
3.3 Comparison of the plasma AMH level by pig breed type
The proportion (8.3%, 2/24) of wild hybrid gilts with >0.16 ng/ml AMH level in plasma tended to be lower (p < 0.1) than that (33%, 6/18) of Large White gilts. The average AMH levels in the plasma collected from wild hybrid gilts and Large White gilts were 0.18 and 0.42 ± 0.09 ng/ml, respectively. When the mean numbers of follicles in the ovary cross-sections from gilts with detectable AMH levels were evaluated in each breed type, the ovaries collected from wild hybrid gilts and Large White gilts contained 19.0 and 46.2 vesicular follicles, respectively.
4 DISCUSSION
The national average litter size of European wild breeds has been reported to be 3.5–6.2 per sow (Gayet et al., 2016; Orlowska, Rembacz, & Florek, 2013), which is lower than that of the Large white (10.6 per sow) and Duroc × Large White breeds (11.6 per sow) (Canario et al., 2006). It has been suggested that cross-breeding with the European wild boar influences the litter size of sows (Klimienė & Klimas, 2010). Therefore, the low litter size of wild hybrid sows that were produced by a cross with the Japanese wild boar was presumed to be due to the genetic background that originated from the wild boar.
It has been known that oocyte effects, uterine capacity, and placental development are key factors for limiting porcine reproduction (Vallet, McNeel, Johnson, & Bazer, 2013). Uterine capacity, including uterine length, which is highly variable among pigs, has an influence on litter size in pigs and selecting for increased uterine length is an effective strategy to improve reproductive performance (Chen & Dziuk, 1993; Wu, Hentzel, & Dziuk, 1987). Although the previous study has indicated that the selection based on uterine capacity have the advantage to improve reproductive capacity compared with the selection based on ovulation rates (Freking, Leymaster, Vallet, & Christenson, 2007), the oocytes factors, such as number of ovulations, fertilization rate, and embryonic survival, also influence the litter size (Bennett & Leymaster, 1989). The ovulation rate of European wild breeds is 5.14–8.27 per sow (Gethöffer, Sodeikat, & Pohlmeyer, 2007), whereas the ovulation rate of Large White breeds is 13.7–14.4 per sow (Bolet et al., 1986). In this study, we found that wild hybrid gilts had significantly lower numbers of COCs collected from their ovaries and of secondary and vesicular follicles in their ovary sections compared to Large White gilts. However, a higher number of primordial follicles were observed in wild hybrid gilts than in Large White gilts. These results indicate that the low number of COCs that were collected from wild hybrid gilts might result from a higher frequency of follicular regression. Although the uterine length and placental factors of wild hybrid and Large White gilts were not evaluated in the present study, we guess that the low litter size of the wild hybrid breed may result in part from low numbers of vesicular follicles.
Moreover, our results showed that although the rates of maturation and fertilization of grade A oocytes collected from wild hybrid gilts were comparable with those from Large White gilts, the blastocyst formation rate of grade A oocytes from wild hybrid gilts significantly decreased. Cytoplasmic maturation is responsible for the capacity to undergo early embryonic development (Yamauchi & Nagai, 1999). Oocytes retrieved from small- to medium-sized follicles can mature to a state in which they can be fertilized, but full developmental competence of the retrieved oocytes continues to be a challenge (Lonergan & Fair, 2016). Therefore, the low developmental competence of morphologically good quality oocytes from wild hybrid gilts may be due to insufficient cytoplasmic maturation.
Circulating AMH levels have been reported to be positively associated with the ovarian population of gonadotropin-responsive follicles in prepubertal hormone-treated goats and cattle (Monniaux et al., 2011; Rico et al., 2011). AMH is an important regulator of the early growth of follicles through its inhibitory effects on the recruitment of primordial follicles into the pool of growing follicles (Durlinger et al., 1999). In cows and goats, the numbers of 3–7 mm diameter follicles in the cow and 1–5 mm diameter follicles in the goat are strongly related to AMH levels (Monniaux et al., 2011; Rico et al., 2009). However, circulating AMH levels are highly variable between individual animals, and the estrous cycle and age/parity affect circulating AMH levels (Koizumi & Kadokawa, 2017; Rico et al., 2011). Moreover, in pigs, corpora lutea also expresses AMH and thus may contribute to the circulating AMH levels during the luteal phase of the estrous cycle (Almeida et al., 2018). In this study, therefore, the plasma samples were collected only from gilts in the follicular phase, and the samples from gilts in the luteal phase were excluded. Our results demonstrated that the percentage of AMH-detected gilts in the wild hybrid breed tended to be lower than that in the Large White breed. The ovaries from wild hybrid gilts with detectable AMH levels had fewer vesicular follicles than those from Large White gilts with detectable AMH levels. Moreover, the average numbers of collectable COCs in all of the wild hybrid gilts decreased compared with those of the Large White gilts (Table 1). Therefore, these results indicate that plasma AMH levels may be related to the number of vesicular follicles that supply the collectable COCs for IVP.
In conclusion, our results demonstrate that wild hybrid gilts have lower numbers of vesicular follicles and COCs with morphologically good quality, and the developmental competence of oocytes is lower than that of Large White gilts. Moreover, a low percentage of AMH-detected gilts in the wild hybrid breed may be related to a low number of vesicular follicles.
ACKNOWLEDGMENTS
This study was supported in part by the “Funds for the Development of Human Resources in Science and Technology” under MEXT, through the Home for Innovative Researchers and Academic Knowledge Users (HIRAKU) consortium, JSPS KAKENHI Grant Numbers JP17H03938, JP17K19325, and JP18K12062, and as a research project funded by SATREPS (Science and Technology Research Partnership for Sustainable Development).




