MicroRNA-21 and microRNA-214 play important role in reproduction regulation during porcine estrous
Abstract
Normal estrous cycle is crucial for porcine reproduction, and microRNA is closely related to regulation of estrous cycle in porcine ovaries. In this study, we found that the expression of miR-214 in porcine ovaries was higher than in many other tissues, and miR-21 expression in ovaries was significantly higher than in the uterus and pituitary. Meanwhile, miR-21 was upregulated and miR-214 was downregulated in the ovaries of high litter size (YH) pigs compared with low litter size (YL) pigs. Moreover, the lowest expression of miR-21 and miR-214 occurred on Days 14 and 7 of the estrous cycle and was expressed at greater levels in the granulosa cells of subordinate follicles than in dominant follicles on Day 3 of the estrous cycle. Bioinformatics analysis showed that miR-21 and miR-214 might target several genes that involved in the mTOR signaling, apoptosis, and steroid biosynthesis pathways, and they play important roles in maintaining the porcine estrous cycle. The qPCR and western blot analysis indicated that miR-214 inhibited the expression of SCARB1 gene in the transcriptional level, but not affected the SCARB1 gene's protein level. Our research findings indicated that miR-21 and miR-214 played important roles in reproduction regulation during porcine estrous.
1 INTRODUCTION
The normal estrous cycle is of great significance to reproductive performance in mammals. Recent studies have shown the important roles played by microRNA (miRNA) in the regulation of the estrous cycle. Knockdown of microRNA-21 (miR-21) expression in mice was found to inhibit the ovulation rate but increase the rate of apoptosis (Carletti, Fiedler, & Christenson, 2010). Yang et al. (2012) found that miR-21 was significantly upregulated during the transition from germinal vesicle to metaphase II-arrested porcine oocytes in vitro. A study in bovine oocytes reported that microRNA-214 (miR-214) was expressed at greater levels in immature oocytes than in matured oocytes (Tesfaye et al., 2009). It has been suggested that miR-214 may contribute to the regulation of steroidogenesis by targeting the low-density lipoprotein receptor gene in rats (Hu et al., 2013). In addition, our previous high-throughput sequencing data showed that miR-21 and miR-214 were differentially expressed between the ovaries of high-prolific and low-prolific pigs (Zhang et al., 2015). These studies show that miR-21 and miR-214 might play important roles in the regulation of reproduction in ovaries, but currently, there is no research on the expression patterns of miR-21 and miR-214 and their interaction with target genes in porcine ovaries during the estrous cycle. In this study, we investigated the expression patterns of miR-21 and miR-214 and verified the regulation relationship between miR-214 and its target gene (SCARB1) in porcine ovaries during estrous cycle.
2 MATERIALS AND METHODS
All experimental procedures and sample collection were approved by the Institutional Animal Care and Use Committee of Anhui Agricultural University, Hefei, China, under permit no. ZXD-P20150613.
2.1 Animal and tissue collection
First, four 5-month-old prepubertal Yorkshire gilts were selected at a local abattoir. Immediately after slaughter, 5 g samples of the heart, liver, spleen, lung, kidney, fat, muscle, ovaries, uterus, oviduct, pituitary, and hypothalamus were collected from the pig carcasses (Table S1a). Second, intact ovaries were collected from six multiparous Yorkshire sows in diestrus (no estrus symptoms) and divided into two groups: high litter size (YH: TNB & NBA > 13.0; n = 3; TNB represents the total number born, and NBA represents the number born alive) and low litter size (YL: TNB & NBA < 5.5; n = 3) groups (Table S1b), representing pigs with high and low fecundity, respectively. Third, the estrus cycle was divided into days and the day of onset of estrus symptoms (standing reflex) was recorded as Day 0. Then, the intact bilateral ovaries were collected from three natural estrous Yorkshire gilts on Days 3, 7, 14, and 20 of the estrous cycle, respectively (Table S1c). One of the ovaries from each gilt was frozen in liquid nitrogen for total RNA extraction, and the contralateral ovaries were transported to the laboratory in a thermos flask containing warm saline solution (0.9% NaCl) for follicle classification and granulosa cell (GC) collection. All the samples were rapidly harvested from carcasses and immediately frozen in liquid nitrogen and stored at −80°C until extraction of total RNA. All the pigs had ad libitum access to water and feed and were humanely killed.
2.2 Follicle classification and GC collection
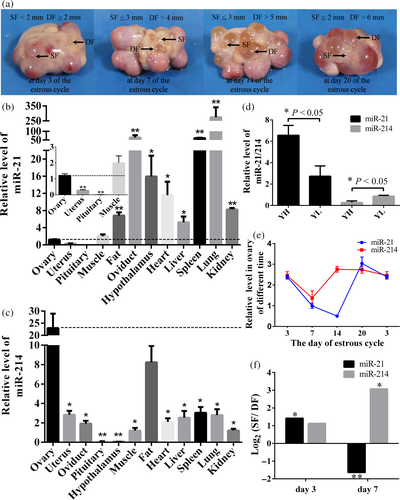
One ovary from each pig obtained on Days 3, 7, 14, and 20 of the estrous cycle was washed once in 70% ethanol and thrice in 0.9% NaCl solution within 1 hr of collection. The ovarian follicles were measured using a caliper and classified as dominant follicles (DFs) or subordinate follicles (SFs) depending on their diameter (Figure 1a), as recommended earlier (Garrett & Guthrie, 1996). The GCs were collected from porcine ovaries using a syringe, and they were washed with PBS and cultured in DMEM/F12 (1:1) with 15% fetal bovine serum in an incubator with 5% CO2 in air at 37°C. Penicillin (100 U/ml) and streptomycin (100 μg/ml) were used in the cell culture system.
2.3 Total RNA extraction and quantitative real-time PCR
Total RNA was extracted using TRIZOL, and the RT reactions were performed with Prime Script™ RT kit (TaKaRa, Tokyo, Japan) according to manufacturer protocol. Real-time PCR was performed with SYBR Premix Ex Taq in a reaction volume of 20 μl. The β-actin gene, GAPDH gene, and U6 small nuclear RNA were used as reference genes in qPCR analysis, and the relative expression levels were calculated using the 2−ΔΔCt method.
All primers used for quantitative real-time PCR (qPCR) are listed in Table S2. And all data were presented as the mean ± standard error; significance tests were performed using t test in SPSS 22.0 for Windows (IBM, Chicago, IL). A p value of <0.05 was considered statistically significant, and p < 0.01 was considered highly statistically significant. Co-expression analysis between miRNAs and their target genes was performed by Pearson correlation coefficient (r), and a p value of <0.05 represents significant difference.
2.4 miR-214 mimics and inhibitor transfection
miR-214 mimics, miR-214 negative control (miR-NC), and miR-214 inhibitor were synthesized and purified by GenePharma Co. (Shanghai, China). Granular cell transfection was performed with Lipofectamine 2000 Reagent (Invitrogen, Shanghai, China) following manufacturer protocol. Briefly, cells were seeded in six-well plates 1 day prior to transfection. When the cells reached 80% confluence, miRNAs were transfected into the cells at different final concentrations. All experiments were performed in triplicate.
2.5 Western blotting
Cell lysate was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. Protein expression was analyzed by western blotting with primary antibodies against SCARB1 (EP1556Y; abcam, CA) and then incubated with a secondary antibody. Blots were finally developed with enhanced chemiluminescent substrate. After draining the solutions, chemiluminescence was detected using a luminescent image analyzer LAS-4000 (Fujifilm, Tokyo, Japan).
2.6 Bioinformatics analysis
The miRNA target genes were predicted from the miRanda (http://www.microrna.org), TargetScan (http://www.targetscan.org), and RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) databases. Then, the common genes within the three databases were combined with our previous RNA sequencing results (Huang et al., 2016; Zhang et al., 2015) to determine the intersection gene. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed using the DAVID database (http://david.abcc.ncifcrf.gov). Finally, interaction analysis between miRNAs and target genes was performed using the STRING database (http://string-db.org), and a protein–protein interaction (PPI) network was constructed using Cytoscape software (http://www.cytoscape.org).
3 RESULTS
3.1 Tissue expression profiles of miR-21 and miR-214 in prepubertal gilts
The expression level of miR-21 in the ovary was significantly higher than that in the uterus and pituitary (p < 0.01), but lower than that in other tissue samples (p < 0.05 or p < 0.01; Figure 1b). The highest and lowest expression levels of miR-21 appeared in the lung and pituitary, respectively. miR-214 was expressed at significantly greater levels in the ovary than in the other tissues (p < 0.05 or p < 0.01; Figure 1c).
3.2 Differential expression of miR-21 and miR-214 in ovaries of YH and YL
We found that the expression level of miR-21 in both YH and YL groups was higher than that of miR-214. The expression of miR-21 in YH group was significantly higher (p < 0.05) than that in YL group, while expression pattern of miR-214 was opposite with miR-21 (Figure 1d).
3.3 Expression pattern of miR-21 and miR-214 in porcine ovaries during the estrous cycle
To examine the temporal expression patterns of miR-21 and miR-214 in the ovary, the expression levels of these two miRNAs were measured on Days 3, 7, 14, and 20 of the estrous cycle. Our results revealed that the lowest expression levels of miR-21 and miR-214 occurred on Days 14 and 7 of the estrous cycle, respectively (Figure 1e). In addition, miR-21 and miR-214 were expressed in ovarian GCs obtained from the DFs and SFs at Days 3 and 7 ovaries of the estrous cycle. The expression levels of miR-21 and miR-214 were all greater in the GCs obtained from SFs than from DFs on Day 3 of the estrous cycle. However, on Day 7, miR-21 expression was lower (p < 0.01) and miR-214 expression was greater (p < 0.05) in SFs than in DFs, respectively (Figure 1f).
3.4 Functional enrichment and negative correlation between miR-21/miR-214 and target genes engaged in regulating estrous cycle
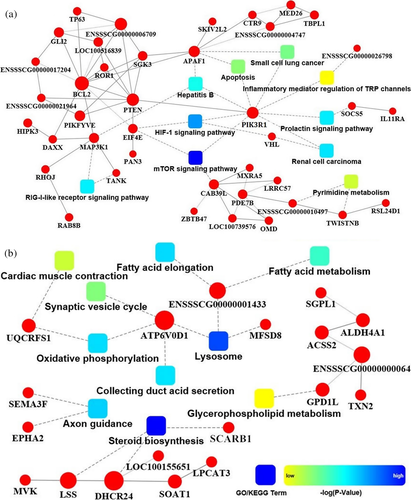
A total of 162 and 66 potential target genes were predicted for miR-21 and miR-214, respectively (Table S3). These findings were predicted by GO, KEGG, and PPI analyses. The interaction networks indicated that the mTOR signaling pathway, which involves three key genes, namely calcium-binding protein 39-like (CAB39L), phosphatidylinositol 3-kinase regulatory subunit alpha (PIK3R1), phosphatase and tensin homolog (PTEN), was the most significantly enriched signaling pathway of miR-21. The apoptosis pathway was significantly enriched by B-cell lymphoma 2 (BCL2), apoptotic protease activating factor 1 (APAF1), and PIK3R1, which were potentially targeted by miR-21 (Figure 2a). The steroid biosynthesis pathway was the most significantly enriched pathway in miR-214, and this pathway contained three genes: scavenger receptor class B member 1 (SCARB1), lanosterol synthase (LSS), and 24-dehydrocholesterol reductase (DHCR24; Figure 2b).
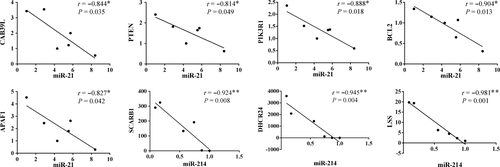
Then, we selected all the eight genes that were enriched in the above-mentioned three pathways for co-expression analysis in the ovaries of high and low litter size pigs. The results show that when the expression of miR-21 or miR-214 is increased, the corresponding expression of the target gene will decrease in the ovaries, especially for the SCARB1, LSS, and DHCR24 genes which have a high negative correlation coefficient with their corresponding miRNAs (r < −0.9, p < 0.01; Figure 3).
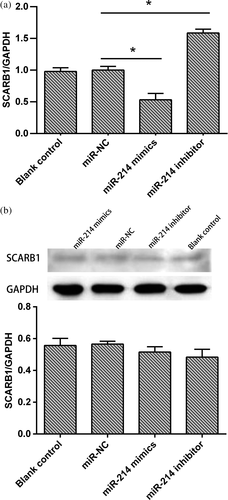
3.5 miR-214 inhibits SCARB1 gene's expression at the transcriptional level in GCs
To determine whether the SCARB1 gene is a true target of miR-214, GCs were transfected with miR-214 mimics, miR-214 inhibitor, or miR-NC. Real-time PCR results showed that miR-214 overexpression in porcine GCs resulted in a reduction of SCARB1 mRNA level, whereas the inhibition of miR-214 significantly increased SCARB1 mRNA level (p < 0.05; Figure 4a). Western blotting analysis showed that there were no significant changes in SCARB1 protein level with transfection of miR-214 mimics and miR-214 inhibitors in GCs (Figure 4b).
4 DISCUSSION
Mammalian organs exhibit uniquely spatiotemporal miRNA expression patterns; therefore, miRNA expression profiles can be used as a novel class of biomarkers for the diagnosis and prognosis of cancer and many other diseases. Understanding the spatiotemporal specificity of miRNA expression is an important step to elucidate their function. Here, we determined the expression profiles of miR-21 and miR-214 in 12 organs and ovaries at different days of the porcine estrous cycle. Interestingly, our results revealed that miR-21 expression in ovaries was lower than that in all the other organs except the uterus and pituitary, while miR-214 expression in the ovaries was greater than that in any of the other organs. These results revealed the spatially specific expression of miR-21 and miR-214 in the ovaries, suggesting that they have an important role in ovarian function.
It is known that luteinizing hormone (LH) surge is activated for up to 20 days before estrus, and the LH peak is observed at 6–7 hr after the onset of estrus (Henricks, Guthrie, & Handlin, 1972). The plasma progesterone level remained at the baseline level until 3 days after the LH level peaked, and then remained elevated until 14 days in gilts with a normal estrous cycle duration. At luteolysis, the plasma progesterone concentrations declined from high to baseline concentrations over a 2-day period (Eiler & Nalbandov, 1977; Garrett & Guthrie, 1996; Knox, Vatzias, Naber, & Zimmerman, 2003; Noguchi et al., 2010). miR-21 has been identified as an antiapoptotic LH-induced factor in preovulatory GCs, and it ensures both the survival and differentiation of these cells (Carletti et al., 2010). The role of miR-214 in the regulation of cell apoptosis and gonad steroidogenesis (such as progesterone) has been reported previously (Tian, Zeng, Li, Wu, & Wang, 2015; Wang et al., 2014). Here, we showed that the miR-21 expression level increased at the same time as the LH concentration increases before, during, and 3–4 days after ovulation. There was a clear inverse relationship between the miR-214 expression level and plasma progesterone concentration in the luteal phase: the miR-214 expression was the lowest, while the plasma progesterone concentration was high in this phase. Combined with previous studies and our results, miR-21 and miR-214 may regulate the secretion of LH and progesterone, respectively, whereas LH and progesterone have a direct effect on ovarian physiological functions such as follicle growth, development, and ovulation in mammals. Therefore, miR-21 and miR-214 play an important regulatory role in mammalian reproductive activities.
Follicular recruitment was identified during two periods of the estrous cycle: from the late luteal to the follicular phase and during the early luteal phase (Noguchi et al., 2010). The percentage of atretic follicles has been found to notably increase from 5% on Days 3 and 5 to 41% on Day 7 (Garrett & Guthrie, 1996). Thus, we can affirm that Day 3 of the estrous cycle is the follicular recruitment phase and Day 7 of the estrous cycle is the follicular atretic phase. Our results showed that the expression of miR-21 in GCs in SFs exhibited a 2.7-fold increase on Day 3 of the estrous cycle and a 3.1-fold decrease on Day 7 of the estrous cycle. These results illustrated that the miR-21 probably plays an important regulatory role in follicle recruitment and atresia. It has been noted that the concentration of three sex steroids (estrogen, progestin, and androgen) in the follicular fluid increases with increase in follicle size, and the steroid concentrations between the SFs and DFs significantly differed (Eiler & Nalbandov, 1977). In the present study, we found that miR-214 expression in the GCs was 2.1-fold greater in SFs at Day 3 of the estrous cycle and 8.4-fold greater in SFs at Day 7 of the estrous cycle. It is interesting to speculate that miR-214 plays a key role in atretic follicles and GC steroidogenesis. Although further validation of these findings is required, miR-214 may result in dysregulation of cholesterol metabolism or steroid synthesis to induce programmed cell death in GCs of SFs (Lasuncion, Martin-Sanchez, Canfran-Duque, & Busto, 2012).
Recent findings have shown that miRNA is provided with cell- and tissue-specific target genes (Li et al., 2016; Zhang et al., 2013). Therefore, we selected previously published RNA-seq data for porcine ovaries (Zhang et al., 2015) for predicting the target genes of miR-21 and miR-214. The results revealed that some of the target genes were involved in the mTOR signaling, apoptosis, and steroid biosynthesis pathways. The mTOR pathway is a key regulator of cell growth and proliferation, and increasing evidence suggests that mTOR controls ovarian follicle growth by regulating GC proliferation (Sarbassov, Ali, & Sabatini, 2005; Yu, Yaba, Kasiman, Thomson, & Johnson, 2011). The CAB39L, PIK3R1, PTEN, BCL2, and APAF1 have been indicated to play important roles in cell growth, proliferation, and apoptosis (Adams & Cory, 1998; Boudeau et al., 2003; Li et al., 1997; Tamura et al., 1998; Vivanco & Sawyers, 2002). Si et al. (2007) used a mouse model of breast cancer and found that miR-21 can inhibit apoptosis by regulating BCL2. Similarly, Meng et al. (2007) reported that inhibition of miR-21 increased PTEN expression and decreased the proliferation, migration, and invasion of human hepatocellular cancer cells. Furthermore, studies have shown that miR-21 promoted glioma invasion partly by targeting APAF1 (Gabriely et al., 2008; Papagiannakopoulos, Shapiro, & Kosik, 2008). Although CAB39L and PIK3R1 were previously shown to be targets of other miRNAs (Huang et al., 2015; Tatarano et al., 2011), there is no evidence indicating that these genes are miR-21 targets. Therefore, we have a bold conjecture that miR-21 may regulate the enrichment of the three pathways (mTOR signaling, apoptosis, and steroid biosynthesis) among the five genes (CAB39L, PIK3R1, PTEN, BCL2, and APAF1) to influence the recruitment and development of porcine follicles. Several miR-214 targets were found to be directly associated with steroid hormone synthesis, especially SCARB1, a physiologically relevant lipoprotein receptor that has been shown to mediate cholesterol uptake in steroidogenic tissue for hormone production (Jimenez, Binelli, Bertolin, Pelletier, & Murphy, 2010). Complete deficiency of SCARB1 has been associated with reduced fertility in female mice (Trigatti et al., 1999). The LSS gene, which encodes the key steroidogenic enzymes of LSS (2,3-oxidosqualene-lanosterol cyclase), catalyzes the first step in the biosynthesis of cholesterol, steroid hormones, and vitamin D (Natter et al., 2005). The DHCR24 catalyzes the C-24 reduction of sterol intermediates during cholesterol biosynthesis and has also been involved in cell growth, senescence, and cellular response to oncogenic and oxidative stress (Daimiel et al., 2013).
Research has shown that miR-214 is directly involved in lactoferrin expression and lactoferrin mediated cancer susceptibility (proapoptotic activities) in mouse mammary epithelial cells (Liao, Du, & Lönnerdal, 2010). Downregulation of miR-214 may result in an increase in the expression of its target genes SLC11A1 and LILR during Salmonella typhi infection of the swine (Bao et al., 2015). In our study, qPCR and western blotting were used to study the expression of SCARB1 regulated by miR-214 at transcriptional and protein level. The results confirm that the SCARB1 gene is a miR-214 target and that miR-214 regulates SCARB1 gene expression at the transcriptional level but not at the protein level. The miRNA regulates genes expression through two typical patterns including degrade mRNA and posttranscriptional repression. There are one-to-many regulating relationship between miRNAs and target genes. Thus, one target gene could be blinded with more than one miRNAs. In this study, perhaps, other miRNAs or regulatory elements are engaged in the regulation of the SCARB1 gene, which results in no significant difference at protein level. Of course, further studies should be performed deeply to demonstrate the accurate regulation between miR-214 and SCARB1 gene.
This is the first study to determine the expression profiles of miR-21 and miR-214 in 12 organs in pigs and dynamic expression in ovaries during the porcine estrous cycle. Our studies revealed the importance of these two miRNAs in the regulation of ovarian function and in the induction of ovarian GCs to induce follicle development, ovulation, luteinization, and steroid biosynthesis, all of which are critical for female fertility. The results may also provide evidence of the posttranscriptional regulatory mechanism of genes and pathways by miR-21 and miR-214 in porcine ovaries and provide new potentially therapeutic targets in ovarian dysfunction. In the future, experiments such as gain-of-function and loss-of-function experiments should be designed to investigate the regulatory mechanisms of miR-21 and miR-214 on ovarian function.
ACKNOWLEDGMENTS
This research was supported by grants from the National Natural Science Foundation of China (grant numbers 31402037 and 31572377), the Anhui Provincial Science and Technology Major Project (no. 17030701061), the Anhui Provincial Natural Science Foundation (grant number 1508085QC52), the Open Fund of Anhui Province Key Laboratory of Local Livestock and Poultry, Genetical Resource Conservation and Breeding (grant number AKLGRCB2017003), and the Open Science Foundation of Key Laboratory of Farm Animal Genetic Resources and Germplasm Innovation of Ministry of Agriculture (grant number nzdsys2016-x).




