Improved porcine model for Shiga toxin-producing Escherichia coli infection by deprivation of colostrum feeding in newborn piglets
Abstract
Porcine edema disease (ED) is a toxemia caused by enteric infection with Shiga toxin 2e (Stx2e)-producing Escherichia coli (STEC). ED occurs most frequently during the weaning period and is manifested as emaciation associated with high mortality. In our experimental infection with a specific STEC strain, we failed to cause the suppression of weight gain in piglets, which is a typical symptom of ED, in two consecutive experiments. Therefore, we examined the effects of deprivation of colostrum on the sensitivity of newborn piglets to STEC infection. Neonatal pigs were categorized into two groups: one fed artificial milk instead of colostrum in the first 24 h after birth and then returned to the care of their mother, the other breastfed by a surrogate mother until weaning. The oral challenge with 1011 colony-forming units of virulent STEC strain on days 25, 26 and 27 caused suppression of weight gain and other ED symptoms in both groups, suggesting that colostrum deprivation from piglets was effective in enhancing susceptibility to STEC. Two successive STEC infection experiments using colostrum-deprived piglets reproduced this result, leading us to conclude that this improved ED piglet model is more sensitive to STEC infection than the previously established models.
Introduction
Porcine edema disease (ED) is an enterotoxemia caused by infection with Shiga toxin-producing Escherichia coli (STEC), which specifically produces Shiga toxin 2 (Stx2) variant Stx2e. ED is one of the major diseases affecting weaning piglets and causes generalized edema, emaciation and sudden death in severe cases. This results in significant economic loss for hog raisers (Watanabe & Yoshiura 2001).
In order to develop a vaccine or other interventional technique against ED, an appropriate and reliable animal model of STEC infection is required. Oral STEC challenge to piglets is the most straightforward model of ED (Bosworth et al. 1996; Bertschinger et al. 2000; Makino et al. 2001). As ED is a toxemia caused by Stx2e, the injection of crude or purified toxin can reproduce the symptoms (MacLeod et al. 1991; Oanh et al. 2012). In addition, naturally infected piglets in ED-endemic herds have been used as a model to evaluate the efficacy of vaccine candidates (Johansen et al. 1997).
We previously presented an oral infection model in piglets using an enteric-coated capsule filled with the STEC strain that produces Stx2e and heat-stable enterotoxin (ST) (Tsukahara et al. 2005). However, to assess the precise efficacy of a vaccine against ED, the STEC strain producing Stx2e alone is more suitable for use as a virulent challenge strain. Therefore, we examined the reproducibility of ED by using an Stx2e-only producing STEC in our piglet model. However, the results were inconsistent, especially in regard to suppression of weight gain, which is one of the most important objective parameters used to assess ED symptoms. Therefore, in an effort to improve the reproducibility of ED symptoms in our conventional STEC infection model (Tsukahara et al. 2005), we deprived newborn piglets of colostrum in order to diminish the protective immunity provided by maternal breastfeeding. In this paper, we present the results of serial experiments conducted both before and after 24 h deprivation of colostrum in our porcine STEC infection model.
Materials and Methods
Pigs
Pregnant sows (Landrace × Large White) impregnated by Duroc boars were purchased from the same commercial pig farm and housed individually in fallowing pens at Kyodoken Institute (Fukuchiyama, Kyoto) for all experiments. All sows were healthy, and stx2 genes were not detected in their feces. They were fed a commercial diet (Bree-Meal Maxim, Feed One, Yokohama, Japan) throughout the study. After delivery, neonatal piglets were kept under the following particular experimental conditions.
All piglets were weaned at 21 days old and transferred to concrete pens with brooders for each individual experimental group. They were fed a commercial diet for weaning piglets (SDS No. 1; Feed One) ad libitum. Animal handling was performed in accordance with the guidelines for animal studies at Kyodoken Institute. All experiments were approved by the Experimental Animal Committee of Kyodoken Institute with approval numbers EB094047, EB094085, EB104047, EB104062 and EB104078 for experiments 1, 2, 3, 4 and 5, respectively.
Experiments 1 and 2: STEC infection to colostrum-fed piglets
Six piglets delivered from three sows and eight piglets delivered from two sows were used in experiments 1 and 2, respectively. Newborn piglets were kept under the care of their own sows after birth, including colostrum intake and subsequent nursing. Four piglets were challenged with STEC in each experiment, while two and four piglets were used as unchallenged controls in experiments 1 and 2, respectively.
Experiment 3: STEC infection to colostrum-deprived or surrogate mother-fostered piglets
Nine piglets delivered from two sows were used in this experiment. After delivery, six piglets were immediately separated from their sows. Three piglets were fed artificial milk (Meiji Hohoemi, Meiji, Tokyo, Japan) instead of colostrum for 24 h at 2–3 h intervals, then returned to their own sow for nursing (group A). In another group, three piglets were fostered by a surrogate mother until weaning (group B). The six piglets who did not receive their mother's colostrum were challenged with STEC at 25 days old. The remaining three untreated piglets nursing with sows until weaning were considered to be the unchallenged controls (group C).
Experiments 4 and 5: STEC infection to colostrum-deprived piglets
Seven piglets delivered from two sows and five piglets delivered from two sows were used in experiments 4 and 5, respectively. The newborn piglets were separated from their sows immediately after birth and fed artificial milk instead of colostrum as described earlier for group A in experiment 3. After 24 h, the piglets were returned to their own sows for nursing. Four and three piglets were challenged with STEC in experiments 4 and 5, respectively; three and two piglets were kept as unchallenged controls in their respective experiments.
STEC challenge and detection in the feces
STEC strain KY010 (serotype O139:H1, stx2e+, fadA+, paa+, elt−, est−) isolated from a pig with symptoms of ED (Cao et al. 1994) was used in all the experiments in this study. The STEC infection procedure was the same as that previously described (Tsukahara et al. 2005). Briefly, weaned piglets (21 days old) were transferred to another experimental laboratory at Kyodoken Institute and placed in isolated pens for each group. After 4 days of acclimatization, the piglets (25 days old) belonging to the designated infection groups were orogastrically inoculated using an enteric-coated capsule (Sunsho, Tokyo, Japan) filled with 1011 colony-forming units (cfu) of STEC for 3 consecutive days. The same capsules filled with saline were administered to the control piglets. The excretion of STEC was measured in the feces on days 0, 3 and 11 after infection by quantitative real-time PCR for the stx2e gene as previously described (Tsukahara et al. 2007).
Clinical and pathological observations
The piglets were weighed at birth, weekly before inoculation with STEC, and every 2 days after inoculation. Clinical symptoms were individually evaluated once daily throughout the study. The criteria used for clinical scoring was as described in our previous report (Tsukahara et al. 2005).
All piglets were euthanized 11 days post-inoculation by exsanguination under general anesthesia induced with an intraperitoneal injection of sodium pentobarbital (Somnopentyl, Kyoritsu, Tokyo, Japan). Following a ventral midline incision, the digestive system and other organs were evaluated for the presence of gross abnormalities. The intestines were fixed in 10% formalin, embedded in paraffin wax, and sectioned into 4 μm-thick slices. Then, the sections were stained with hematoxylin–eosin to visualize any histopathologic abnormalities under light microscopy (Tsukahara et al. 2005).
Statistical analyses
Student's t-test was applied to analyze the differences in daily weight gain between the groups in experiments 2 and 4. The differences in daily weight gain and clinical scores in experiment 3 were analyzed using the Kruskal‑Wallis test. When significant differences were detected, a non-parametric least significant difference post hoc comparison was used. Differences among means were considered significant at P < 0.05. All data were analyzed using STATCEL (OMS, Saitama, Japan) or StatLight 2000 (Yukms, Tokyo, Japan), an add-on application for Microsoft Excel (Microsoft, Seattle, WA, USA).
Results and Discussion
In all experiments, palpebral edema, which is a typical clinical symptom of ED, was observed in STEC-infected groups, while it was absent in the uninfected controls (data not shown). The stx2e gene was not detected in the feces of any of the piglets on day 0. More than 106 cfu of STEC were detected per gram of wet feces in all infected piglets on day 3. This amount gradually decreased toward day 11, indicating that STEC colonization was well-established in their intestines by that time (data not shown). No significant histopathologic abnormalities were observed in the intestines of any of the infected or control piglets (data not shown).
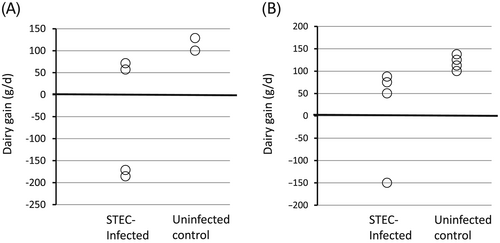
In experiment 1, although two out of four piglets showed a dramatic decrease in body weight from day 0 (day of challenge) to day 7, the other two piglets showed almost the same growth profiles as the piglets in the uninfected control group (Fig. 1A). In experiment 2, only one out of four STEC-infected piglets lost weight (Fig. 1B; P = 0.12). These results suggest that the single Stx2e toxin producing strain (STEC KY010) used in these experiments might be only weakly pathogenic for piglets as compared to the Stx2e/ST double-producing strain (1362–1) used in our previous piglet infection model (Tsukahara et al. 2005).
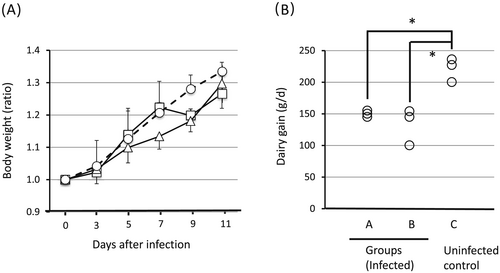
Colostrum is known to be an important source of not only nutrition but also immune components for newborn piglets (Wagstrom et al. 2000). We hypothesized that, although these sows were negative for STEC, the immunoglobulins (IgG or IgA) or lymphoid cells specific to ubiquitous E. coli present in the colostrum might decrease the sensitivity of the piglets to STEC infection. The following observations have been reported: (i) colostral IgG content is 10- to 100-times higher than usual during the first 24 h after delivery (Klobasa et al. 1987); (ii) maternal pathogen-specific IgG is transferred to piglets via colostrum and provides protection against a challenge infection (Ward et al. 1996); and (iii) maternal lymphoid cells are transferred to the mother's own offspring via colostrum within 24 h after birth (Bandrick et al. 2008), but not to other neonates (Tuboly et al. 1988). Therefore, in experiment 3, we examined the effects of complete colostrum deprivation during the first 24 h after birth (group A) and nursing by a surrogate mother just after birth (group B). The body weights of the uninfected control piglets increased uniformly, while those of the piglets infected with STEC did not predictably increase in both groups A and B. It was noted that all three infected piglets in group A lost weight on day 9 (Fig. 2A), which is consistent with other studies that report the appearance of clinical symptoms of ED approximately 1 week after oral infection with STEC (Smith & Halls 1968). Although the weight change was less dramatic than in group A, piglets in group B showed only a slow increase in body weight after infection as compared to the daily weight gain of uninfected piglets (Fig. 2A, B). Furthermore, according to the results of mean daily weight gain during the STEC infection period, this was significantly lower in group A (P < 0.05) and group B (P < 0.01) than that in group C (Fig. 2B). These results suggested that the deprivation of colostrum was effective in making the piglets more sensitive to STEC infection. All infected piglets except one in group A developed palpebral edema, while all piglets except one in group B developed dyspnea (Table 1). Respiratory failure is reported to be one of the typical symptoms associated with ED (Mulei & Ngatia 1999). We previously reported that artificial STEC infection resulted in development of tubercles in the lungs and interstitial pneumonia in a piglet (Tsukahara et al. 2005). With regard to convenience, among the improved ED piglet models, we preferred group A to group B. We next examined the reproducibility of this model through two subsequent experiments.
| Days after infection | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical scores | Groups | Piglet nos. | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total scores |
| Palpebral edema† | A | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||
| 3 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 15 | ||
| B | 4 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 10 | |
| 5 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ||
| 6 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | ||
| C | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Respiratory‡ | A | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| 2 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 8 | ||
| 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||
| B | 4 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 8 | |
| 5 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | ||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| C | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
- † Edemal score in palpebra: 0, normal; 1, mild; 2, moderate; 3, severe.
- ‡ Respiratory score: 0, normal; 1, slightly quick; 2, quick.
- Group A = artificial milk administration and STEC infection; Group B = fostered and STEC infection; Group C = STEC uninfected control.
- Total scores mean sum of the scores from day 1 to day 11.
- The P-value of Kruskal‑Wallis test in ‘total scores in palpebral edema’ is 0.11, whereas that in ‘total scores in respiratory’ is 0.07.
- According to the results of post hoc comparison, total scores in respiratory tended to be higher in Group A than those in Group C.
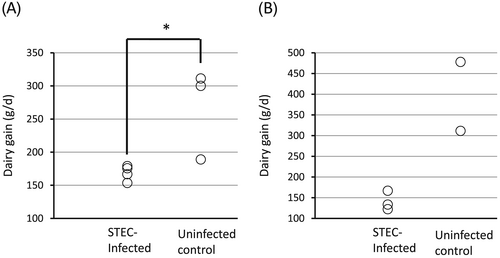
In experiments 4 and 5, all newborn piglets were isolated from their mother just after birth and without receiving colostrum. They were fed artificial milk for 24 h, then placed back in the care of their own sow. The piglets received an oral STEC challenge on days 25, 26 and 27. The daily weight gain of the STEC infected group was clearly lower than that of the uninfected controls in both experiments (Fig. 3; P = 0.03 in experiment 4). Weight loss on day 9 was again observed in two out of three infected piglets in experiment 5 (data not shown). All infected piglets showed palpebral edema while none was seen in the uninfected controls in both experiments (data not shown).
Thus, we concluded that deprivation of colostrum is reproducibly effective in enhancing the symptoms of ED caused by oral STEC challenge in piglets. A recent paper showed that porcine colostrum contained immune-related proteins such as interleukin-18 (IL-18), which were not detected in mature milk (Ogawa et al. 2014). The intestinal cells in neonatal piglets are reported to be too immature to produce IL-18, although they are able to produce interferon-γ by responding to exogeneous IL-18 (Muneta et al. 2002). Further, a very recent study demonstrated that colostrum ingestion during the first 24 h of life significantly increased fecal IgA, plasma IgG and blood B cells in newborn piglets (Ogawa et al. 2016). These reports support the conclusions drawn from our study. The improved piglet infection model described in this study can be applied for the development of vaccines, probiotics and other interventional techniques against ED.
Acknowledgments
The authors thank Messrs Sachio Chomei and Kei-ichi Ohya of Kyodoken Institute for providing care for the animals. This work was supported by a Grant-in-Aid from the ‘Development of fundamental technologies for production of high-value materials using transgenic plants’ project managed by Ministry of Economy, Trade and Industry, Japan.




