Random regression models to estimate genetic parameters for weights in Murrah buffaloes
Abstract
This article reports genetic analysis of the weight at different ages of Murrah water buffaloes, using random regression models (RRM). Models ranging from third to sixth order polynomial were used to describe direct genetic and animal permanent environmental effects. Contemporary group was included as a fixed effect, and a cubic polynomial was used to model the mean curve of the population. The residual was modeled considering a log-linear function. Two models were selected for study of genetic parameters. The first model included third and sixth order polynomials for direct genetic and animal permanent environmental effects (M36). The second model included sixth order polynomials for all random effects (M66). The estimates of heritability varied from 0.16 + 0.04 (44 days) to 0.38 + 0.04 (568 days) for model M36 and from 0.16 + 0.05 (33 days) to 0.42 + 0.05 (600 days) for model M66. Regarding estimates of the correlation for all effects, the magnitude tended to decline with the increase of the time span between measurements. These results indicate that the species has potential for genetic selection based on weight at different ages, since we found favorable genetic variability within the herd, with selection likely to be more efficient at ages near 600 days.
Introduction
The water buffalo (Bubalus bubalis) has an important economic role in many countries, being used for meat, milk and work purposes. Therefore the genetic improvement of this species for milk production and quality has an important economic and social impact worldwide, especially in developing countries (Misra & Tyagi 2007). The interest in the species has increased because of its ability to convert tropical grass into meat or milk, good environmental adaptability and high fertility (Borghese 2005). Based on information from FAOSTAT (2015), the global production of buffalo milk and meat has shown annual growth of 3.60% and 2.56%, respectively, India being the largest producer.
Body growth is an important selection criterion in dairy animals, due to the genetic correlation with reproductive and productive traits (Vercesi Filho et al. 2007). Many studies have reported estimates of genetic parameters for weight at different ages, using single or multi-trait animal models (Malhado et al. 2012; Agudelo-Gómez et al. 2015). Random regression models (RRM) have shown the greatest efficiency for analysis of growth curves, because the weights are measured along a continuous scale (Kirkpatrick et al. 1990). Few studies of buffaloes have been conducted until now (Bolivar et al. 2013). For this reason, we performed a genetic analysis of the weights at different ages of Murrah water buffaloes, up to the age of 600 days, applying RRM.
Materials and Methods
Animal Care and Use Committee approval was not necessary for this study because the data were obtained from an existing database. We used weight data on 2322 male and female Murrah buffaloes, with measurements taken between the ages of 30 and 600 days. The animals were from a herd kept at Tapuio Farm, a participant in the dairy quality program promoted by São Paulo State University (UNESP), Jaboticabal campus. The main activity of Tapuio Farm is the production of mozzarella cheese. It covers an area of 496 hectares and is located in the municipality of Taipú, Rio Grande do Norte state (latitude S 5°33′57.13″ and longitude W 35°37′37.98″). The animals are managed in a Voisin rotational pasture system, with daily grazing in paddocks measuring 0.8 ha with Marandu grass (Brachiaria brizantha cv. Marandu) and Massai grass (Panicum maximum cv. Massai).
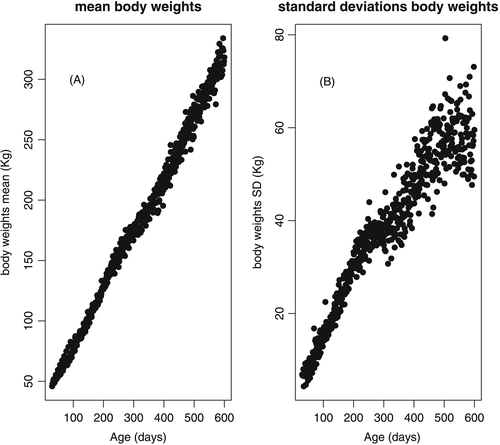
Contemporary groups (CGs) were formed by concatenation of the variables birth year, sex, birth season and weighing year, and after procedures to assure consistency: (i) elimination of CGs with fewer than 10 records; (ii) elimination of records more than 3.5 standard deviations from the mean of the CG; and (iii) elimination of animals with fewer than five weight records. The basic data and statistics are presented in Table 1, and the mean and standard deviation of weights at different ages are presented in Figure 1.
| General description | |
|---|---|
| Number of females with records | 1388 |
| Number of males with records | 934 |
| Number of records (females) | 33 763 |
| Number of records (males) | 13 825 |
| Number of contemporary groups | 221 |
| Number of animals in the relationship matrix | 3093 |
| Number of records per contemporary group (average) | 215.3 |
| Number of records per animal (average) | 20.49 |
| Number of records by age (average) | 83.34 |
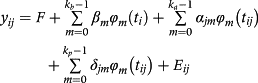
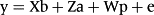
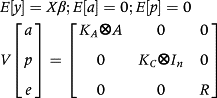
The residuals were modeled considering the heterogeneity of the residual variances, through a quadratic variance function assuming a log-linear model, as described by Misztal et al. (2014). The nomenclature used in the models is Mkakp, where ka and kp are placeholders for the order of the Legendre polynomial used to model the genetic effect and permanent environmental effect, respectively.
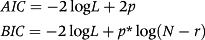
Results and Discussion
The models with a greater number of parameters presented higher values of logL. Two different models (M66 and M36) were selected using the two information criteria employed, AIC and BIC, respectively (Table 2). Regardless of the selection criterion used, for the animal permanent environmental effect the models selected were those with the largest number of parameters. This need for a higher-order polynomial to model this effect was found previously in other studies of dairy and beef cattle (Brotherstone et al. 2000; Meyer 2001). Other studies using random regression to analyze body weight in cattle (Boligon et al. 2010; Souza Júnior et al. 2010) and buffalo (Bolivar et al. 2013) have found the need for higher orders for direct genetic effects than we found.
| Model | ka | kp | Statistical criterion | |||
|---|---|---|---|---|---|---|
| Np | 2logL | AIC | BIC | |||
| M33 | 3 | 3 | 15 | −357669.16 | 357699.16 | 357863.50 |
| M34 | 3 | 4 | 21 | −352741.22 | 352779.22 | 353000.30 |
| M35 | 3 | 5 | 24 | −347543.78 | 347591.78 | 347883.80 |
| M36† | 3 | 6 | 30 | −344188.59 | 344248.59 | 344625.70 |
| M46 | 4 | 6 | 34 | −344130.77 | 344198.77 | 344632.70 |
| M56 | 5 | 6 | 40 | −344111.43 | 344189.43 | 344694.30 |
| M66‡ | 6 | 6 | 45 | −343983.32 | 344073.32 | 344663.30 |
- † Model selected according to the BIC.
- ‡ Model selected according to the AIC.
Analysis of the eigenvalues of the two models chosen (Tables 3 and 4) for direct genetic effect showed that the change in this statistic in M66, above the third order, is small, which can indicate that an M36 model is sufficient to estimate the parameters. However, because of the discrepancy in the number of parameters in the models selected by the different criteria, we considered both in the elucidations about the variance and genetic parameters.
| Effect | 0† | 1 | 2 | 3 | 4 | 5 | λ | |
|---|---|---|---|---|---|---|---|---|
| Direct genetic | 0 | 793.92 | 394.32 | −61.75 | 61.57 | 38.75 | 24.41 | 1004.60 |
| 1 | 0.91 | 234.81 | −14.06 | −94.84 | 24.83 | 18.35 | 46.24 | |
| 2 | −0.44 | −0.18 | 24.84 | −0.74 | −51.07 | −0.33 | 17.01 | |
| 3 | 0.07 | −0.20 | −0.05 | 10.05 | −0.11 | −23.60 | 10.28 | |
| 4 | 0.40 | 0.48 | −0.30 | −0.01 | 11.61 | 65.24 | 2.87 | |
| 5 | 0.35 | 0.49 | −0.03 | −0.30 | 0.78 | 60.39 | 0.22 | |
| Animal permanent environmental | 0 | 1381.50 | 700.76 | −177.13 | −57.38 | 65.54 | 20.40 | 1787.50 |
| 1 | 0.87 | 470.95 | −34.67 | −85.15 | 11.04 | 17.44 | 171.69 | |
| 2 | −0.46 | −0.15 | 107.95 | 13.73 | −39.24 | −74.82 | 90.79 | |
| 3 | −0.17 | −0.44 | 0.15 | 78.51 | 15.30 | −15.99 | 53.06 | |
| 4 | 0.24 | 0.07 | −0.51 | 0.23 | 55.76 | 22.56 | 17.02 | |
| 5 | 0.11 | 0.16 | −0.14 | −0.35 | 0.59 | 26.43 | 0.99 | |
| CR‡ | a = 5.972 | b = 1.017 | c = −0.966 | |||||
- † The values 0–5 are indicators of the order of fit.
- ‡ coefficients of residual function (Misztal et al. 2014).
| Effect | 0† | 1 | 2 | 3 | 4 | 5 | λ | |
|---|---|---|---|---|---|---|---|---|
| Direct genetic | 0 | 808.29 | 398.65 | −53.90 | 1014.10 | |||
| 1 | 0.92 | 230.76 | −0.01 | 50.14 | ||||
| 2 | −0.37 | −0.00 | 26.30 | 11.09 | ||||
| Animal permanent environmental | 0 | 1381.50 | 700.76 | −177.13 | −57.38 | 65.54 | 20.40 | 1787.50 |
| 1 | 0.87 | 470.95 | −34.67 | −85.15 | 11.04 | 17.44 | 171.69 | |
| 2 | −0.46 | −0.15 | 107.95 | 13.73 | −39.24 | −74.82 | 90.79 | |
| 3 | −0.17 | −0.44 | 0.15 | 78.51 | 15.30 | −15.99 | 53.06 | |
| 4 | 0.24 | 0.07 | −0.51 | 0.23 | 55.76 | 22.56 | 17.02 | |
| 5 | 0.11 | 0.16 | −0.14 | −0.35 | 0.59 | 26.43 | 0.99 | |
| CR‡ | a = 5.971 | b = 1.009 | c = −0.972 | |||||
- † The values 0–5 are indicators of the order of fit.
- ‡ coefficients of residual function (Misztal et al. 2014).
The direct genetic variances behaved similarly in the two models, with a rising trend during the animal's lifetime (Fig. 2A). This rising trend of the direct genetic variance has also been reported in studies with buffaloes in Colombia (Bolivar et al. 2013) and Nelore and Tabapuã cattle in Brazil (Albuquerque & Meyer 2001; Nobre et al. 2003; Dias et al. 2006).
Regarding the variances of the animal permanent environmental effect (Fig. 2B), there was also a tendency for increased variances with animal age, with similar curves between the models. In model M36, the variances of the animal permanent environmental effect were slightly higher from 300 to 450 days and lower from 470 to 570 days, after which age they started to increase again, more pronounced in comparison to model M66 (Fig. 2B). Increases in the animal permanent environmental variances, from birth to 600 days, have also been reported by Dias et al. (2006), Boligon et al. (2010) and Souza Júnior et al. (2010) in cattle, and by Bolivar et al. (2013) in buffaloes.
The coefficients of the quadratic variance function assuming a log-linear model used for estimation of residual variance are reported in Tables 3 and 4. The residual variance behaved similarly in the two models (Fig. 2C), where the residual variance increased until near 365 days of age, after which this statistic declined.
The heritability estimates for the body weights at different ages (Fig. 3A) showed similar behavior between the two models, where we can highlight a greater magnitude of the estimates in the initial phase, with subsequent stability and then a renewed increase at ages greater than 400 days. The estimates of heritability varied from 0.16 ± 0.04 (44 days) to 0.38 ± 0.04 (568 days) in model M36 and from 0.16 ± 0.05 (33 days) to 0.42 ± 0.05 (600 days) in model M66. In both models the heritability increased until near weaning (100 days of age), with values of 0.31 ± 0.03 (136 days) and 0.36 ± 0.04 (113 days) for M36 and M66, respectively.
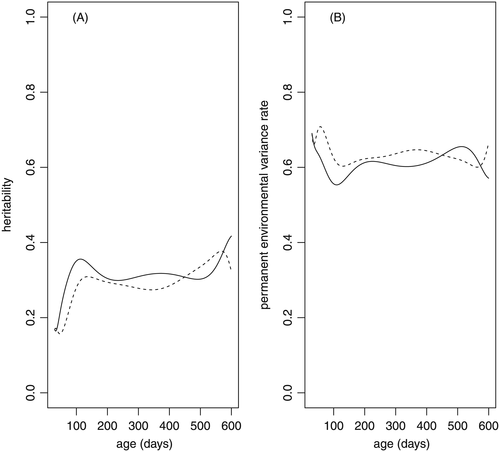
The heritability values found were similar to those reported by Souza Júnior et al. (2010), studying Tabapuã cattle, who found values that fluctuated from 0.15 for weight at birth to 0.45 for weight at 660 days of age. However, the values observed were lower than those estimated for Nelore cattle by Boligon et al. (2010) and for Canchim cattle by Baldi et al. (2010). This lower heritability and the lower orders for direct genetic effects in relation to bovine breeds can be explained by the production system, since we studied dairy animals where the management and feeding regimen are not designed to maximize the growth.
From the estimated heritability values obtained in this study, it was possible to infer that significant genetic gains can be obtained by selecting animals for weight at different ages, which assumed values with medium to high magnitude. From these values, greater genetic differences could be observed between animals near weaning and at older ages (near 600 days). These are the phases where genetic selection will be most efficient. Since the heritability estimates in these periods are similar, the selection based on younger age becomes interesting, due to: (i) the shorter generation interval; and (ii) the small indirect genetic gain in adult weight, arising from the smaller estimated genetic correlation reported in the literature between weight at pre-weaning ages and adult weight (Baldi et al. 2010; Boligon et al. 2010).
With respect to the animal permanent environmental effect, the proportion of this effect in relation to the total variance presented estimates that ranged between 0.60 ± 0.04 (536 days) and 0.71 ± 0.03 (57 days) for model M36 and between 0.55 ± 0.04 (110 days) and 0.69 ± 0.05 (30 days) for model M66 (Fig. 3B). Lower values of these statistics were reported by Souza Júnior et al. (2010), who observed a tendency for growth from birth (0.01) to 160 days of age (0.29) followed by gentle decline to 440 days (0.23) and virtually constant values thereafter (0.24).
The estimates of direct genetic (Table 5), animal permanent environmental (Table 6) and phenotypic correlations (Table 7) showed the same pattern as reported in other studies employing the random regression method (Baldi et al. 2010; Boligon et al. 2010; Bolivar et al. 2013), in which the magnitude tends to diminish with increases in the interval between measurements, assuming the classic ‘saddle’ shape. In general, model M36 presented greater correlation values than model M66. The magnitude of the associations tended to change less with increase of the period between measurements. In buffaloes, Bolivar et al. (2013), analyzing weight values between ages of 240 and 900 days, found estimates varying from 0.71 (300 and 900 days of age) to 0.99 (240 and 270 days). A greater amplitude of values was described by Baldi et al. (2010), who estimated correlations between 0.45 and 0.97, considering weight at birth, weight at weaning, adult weight and weights at ages of 12, 18, 24 and 30 months.
| W100† | W200 | W300 | W400 | W500 | W600 | |
|---|---|---|---|---|---|---|
| W100 | − | 0.88 ± 0.03 | 0.71 ± 0.06 | 0.63 ± 0.07 | 0.64 ± 0.07 | 0.55 ± 0.08 |
| W200 | 0.98 ± 0.01 | − | 0.91 ± 0.02 | 0.80 ± 0.04 | 0.79 ± 0.05 | 0.66 ± 0.07 |
| W300 | 0.95 ± 0.01 | 0.99 ± 0.00 | − | 0.97 ± 0.01 | 0.92 ± 0.03 | 0.86 ± 0.04 |
| W400 | 0.90 ± 0.03 | 0.95 ± 0.01 | 0.99 ± 0.00 | − | 0.97 ± 0.01 | 0.92 ± 0.03 |
| W500 | 0.81 ± 0.04 | 0.87 ± 0.03 | 0.93 ± 0.02 | 0.98 ± 0.01 | − | 0.91 ± 0.04 |
| W600 | 0.67 ± 0.07 | 0.75 ± 0.05 | 0.83 ± 0.04 | 0.91 ± 0.02 | 0.98 ± 0.01 | − |
- † Body weight at 100 (W100), 200 (W200), 300 (W300), 400 (W400), 500 (W500), and 600 (W600) days of age.
- ‡ M36 – third and sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect, respectively.
- § M66 - sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect.
| W100† | W200 | W300 | W400 | W500 | W600 | |
|---|---|---|---|---|---|---|
| W100 | − | 0.70 ± 0.02 | 0.52 ± 0.03 | 0.43 ± 0.03 | 0.41 ± 0.04 | 0.45 ± 0.04 |
| W200 | 0.67 ± 0.02 | − | 0.87 ± 0.01 | 0.66 ± 0.02 | 0.59 ± 0.03 | 0.58 ± 0.04 |
| W300 | 0.45 ± 0.03 | 0.84 ± 0.01 | − | 0.91 ± 0.01 | 0.74 ± 0.02 | 0.74 ± 0.02 |
| W400 | 0.35 ± 0.03 | 0.62 ± 0.02 | 0.91 ± 0.01 | − | 0.90 ± 0.01 | 0.77 ± 0.02 |
| W500 | 0.36 ± 0.03 | 0.56 ± 0.02 | 0.74 ± 0.02 | 0.90 ± 0.01 | − | 0.75 ± 0.02 |
| W600 | 0.41 ± 0.04 | 0.55 ± 0.03 | 0.75 ± 0.02 | 0.77 ± 0.02 | 0.71 ± 0.02 | − |
- † Body weight at 100 (W100), 200 (W200), 300 (W300), 400 (W400), 500 (W500), and 600 (W600) days of age.
- ‡ M36 – third and sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect, respectively.
- § M66 ‑ sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect.
| W100† | W200 | W300 | W400 | W500 | W600 | |
|---|---|---|---|---|---|---|
| W100 | − | 0.70 ± 0.01 | 0.54 ± 0.02 | 0.46 ± 0.02 | 0.46 ± 0.02 | 0.46 ± 0.02 |
| W200 | 0.70 ± 0.01 | − | 0.81 ± 0.01 | 0.65 ± 0.01 | 0.61 ± 0.01 | 0.58 ± 0.02 |
| W300 | 0.55 ± 0.02 | 0.81 ± 0.01 | − | 0.86 ± 0.01 | 0.75 ± 0.01 | 0.74 ± 0.01 |
| W400 | 0.48 ± 0.02 | 0.67 ± 0.01 | 0.86 ± 0.01 | − | 0.87 ± 0.01 | 0.79 ± 0.01 |
| W500 | 0.47 ± 0.02 | 0.62 ± 0.01 | 0.75 ± 0.01 | 0.87 ± 0.01 | − | 0.78 ± 0.01 |
| W600 | 0.47 ± 0.02 | 0.58 ± 0.02 | 0.74 ± 0.01 | 0.78 ± 0.01 | 0.78 ± 0.01 | − |
- † Body weight at 100 (W100), 200 (W200), 300 (W300), 400 (W400), 500 (W500), and 600 (W600) days of age.
- ‡ M36 – third and sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect, respectively.
- § M66 ‑ sixth order of the Legendre polynomial used to model the direct genetic effect and the animal permanent environmental effect.
Based on the results presented, considering that the direct genetic correlations between weight measurements at the various ages have large magnitude, selection for greater direct and indirect genetic gain should be done at ages that presented the highest heritability estimates (100 and 568 days). However, unlike for other bovine species, buffaloes still have not been found to present defined specialization for milk or meat production. Therefore, the way to use the body weight criterion for selection will depend on the production system.
In cattle, the genetic correlation of weight at older ages with reproductive traits has shown favorable association in males (Araujo Neto et al. 2011) and females (Mercadante et al. 2000; Berry et al. 2003; Gaviolli et al. 2012), with magnitudes having low to moderate values. This favorable association with reproductive traits means weight is an additional selection criterion that can be used in dairy production systems. However, studies of dairy cattle have verified an unfavorable association between body weight and milk production. Vercesi Filho et al. (2007), studying mestizo dairy cows, reported genetic correlation estimates of −0.59, −0.73, −0.62 and −0.67 for growth rate with milk yield, fat and protein content and duration of lactation, respectively. Additionally, Brotherstone et al. (2007) described a positive association between growth until weaning of animals and the incidence of mastitis. Therefore, the growth rates of animals should be controlled to achieve reproductive efficiency while maintaining good productive levels, justifying the use of other selection mechanisms such as economic indices or rejection levels.
Conclusions
From the results, we can conclude that for genetic evaluation of body weight in buffaloes, the use of third- and sixth-order Legendre polynomials in RRM is recommended for direct genetic effects and animal permanent environmental effects, respectively. The results of the genetic correlation estimates indicate only small changes in the shape of the growth curve can be achieved by selection at some of the points, and that selection carried out at age of 568 days is more efficient due to the greater heritability, but the selection method depends on the production system adopted (meat or milk).
Acknowledgments
Thanks to the Tapuio Farm for providing the data for the study.




