Fbxw7β, E3 ubiquitin ligase, negative regulation of primary myoblast differentiation, proliferation and migration
Abstract
Satellite cells attached to skeletal muscle fibers play a crucial role in skeletal muscle regeneration. During regeneration, the satellite cells proliferate, migrate to the damaged region, and fuse to each other. Although it is important to determine the cellular mechanisms controlling myoblast behavior, their regulators are not well understood. In this study, we evaluated the roles of Fbxw7 in primary myoblasts and determined its potential as a therapeutic target for muscle disease. We originally found that Fbxw7β, one of the E3 ubiquitin ligase Fbxw7 subtypes, negatively regulates differentiation, proliferation and migration of myoblasts and satellite cells on muscle fiber. However, these phenomena were not observed in myoblasts expressing a dominant-negative, F-box deleted Fbxw7β, mutant. Our results suggest that myoblast differentiation potential and muscle regeneration can be regulated by Fbxw7β.
Introduction
Satellite cells are adult skeletal muscle stem cells that exist in a quiescent state in undamaged muscle and are activated when the muscle is injured or diseased. The activated satellite cells proliferate to self-renew, and maintain the dormant population, and differentiate into myoblasts. Although myoblasts do not demonstrate capacity for self-renewal, they can proliferate and migrate toward injured regions as they continue to differentiate into new myofibers. Both proliferation and differentiation are major functions of satellite cells with respect to skeletal muscle repair (Relaix & Zammit 2012).
Fbxw7 is a Skp, Cullin, F-box-containing complex (SCF) ubiquitin E3 ligase, highly conserved between humans and mice. It is also known as CDC4, FBW7, FBX3, FBXO30 and SEL10 (Mao et al. 2004), Fbxw7 consists of a common Skp1-Cul1-Rbx1 subunit, a target substrate-recognition subunit of F-box, and seven WD40 repeat domains. In addition, three isoforms of Fbxw7 (α, β and γ) can be produced by alternative splicing of a specific domain at the N-terminal region (Koepp et al. 2001; Moberg et al. 2001; Strohmaier et al. 2001; Spruck et al. 2002; Mao et al. 2004). These isoforms are expressed in different tissues and differentially located within the cell (Welcker et al. 2004; Ye et al. 2004; Matsumoto et al. 2006). In particular, the Fbxw7β and γ isoforms are expressed in the brain, testis, heart and skeletal muscle tissues. However, their interactions and various functional mechanisms have yet to be elucidated.
Fbxw7 is well known as a tumor suppressor gene that targets oncoproteins for ubiquitin-mediated degradation (Nakayama & Nakayama 2006; Akhoondi et al. 2007; Welcker & Clurman 2008). Several Fbxw7 oncoprotein targets, such as cyclin E and c-Myc, are positive cell cycle regulators that control the proliferation of malignant tumor cells. Fbxw7 deficiencies at the chromosomal locus or its low expression levels are frequently seen in malignant tumors (Knuutila et al. 1999; Ranganathan et al. 2011). These abnormalities promote malignant cell cycle progression and contribute to invasion and metastatic phenomena in several types of human cancers (Onoyama et al. 2007; Onoyama & Nakayama 2008; Tsunematsu 2009; Naganawa et al. 2010).
Recently, it was discovered that Fbxw7 participates not only in proteasomal degradation in abnormal cells but also in healthy cell growth, differentiation and embryonic development (Tsunematsu et al. 2004; Wang et al. 2011; Takeishi & Nakayama 2014). This suggests that Fbxw7 has several important functional roles in healthy tissues. The present study investigated the roles of Fbxw7 in skeletal myogenic differentiation to determine whether it may serve as a potential therapeutic target for muscle disease.
Materials and Methods
Animals
Six-week-old C57BL/6NCrljOri male mice were obtained from Orient Bio Inc. (Seungnam, South Korea). The mice were housed and cared for using protocols approved by the Institutional Animal Care and Use Committee under specific pathogen-free conditions at the Korean Institute of Radiological and Medical Science.
Adenoviral clone of Fbxw7β and ΔFbox
Full length of mFbxw7β complementary DNA (cDNA) was constructed commercially using the National Institutes of Health Mammalian Gene Collection library (Accession No. BC131648). ΔFbox clone, a dominant negative mutant of mFbxw7β, was constructed by deletion of F-box domain from exon 2 to 6 of the full length mFbxw7β cDNA (Supplement Fig.1A). These sub-clones were cloned into adenoviral expression vector using pAd/CMV/V5-DESTTM Gateway® Vector (Invitrogen, Carlsbad, CA, USA).
Ectopic adenoviral expression of Fbxw7ß and ΔFbox in myofiber
For each Fbxw7β and ΔFbox, 2 × 108 plaque-forming units (PFU) of adenovirus were injected into 5-6 sites of Anterior tibialis (TA) and Gastrocnemius (Gastroc) muscles by 31G insulin syringes after the mouse was anesthetized with isoflurane. The ectopic adenoviral expressing TA and Gastroc muscles were harvest at 5 days.
Muscle injury and satellite cell myogenic potential
For each mixed solution of 2 × 108 PFU adenovirus mock, Fbxw7β and ΔFbox was injected into 5-6 sites of the TA and Gastroc muscles with 10 μL of cardiotoxin I (CTX, 100 µg/mL in phosphate-buffered saline; Sigma-Aldrich, St. Louis, MO, USA) after the mouse was anesthetized with isoflurane. Satellite cells activated by CTX-induced focal injury were harvested 3 days later post injury and then differentiated into myotubes directly to assay de novo generation of myotubes. Regenerated TA and Gastroc muscles were also harvested 5 days later post-injury, and then de novo embryonic myosin heavy chain-positive myofiber nuclei were analyzed.
Cell culture
Primary myoblasts were cultured from C57BL/6NCrljOri male mice as previously described (Jeong et al. 2013). Briefly, myoblasts were cultured in growth medium containing Ham's F-10 (Gibco, Grand Island, NY, USA), 20% horse serum (Gibco), 6 ng/mL basic fibroblast growth factor (R&D Systems, Minneapolis, MN, USA) and 1% Pen/Strep (Gibco) on extra-cellular matrix (ECM)-coated plates (1:500 dilution; Matrigel Matrix, Corning, CA, USA). For differentiation, culture media were replaced with Minimum Essential Media (MEM) (Gibco) containing 3% horse serum and 1% Pen/Strep for 72 h at 37°C and 5% atmospheric CO2.
RT-PCR
Total RNA from the myoblasts was extracted with TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA), and 500 ng of total RNA was synthesized into cDNA using TakaRa-PrimeScriptTM RT Master Mix (TaKaRa Bio, Shiga, Japan). RT-PCR was performed with a PCR pre-mixture (Bioneer, Daejeon, South Korea) and primers (Supplement Table 1). Quantitation of messenger RNA (mRNA) was performed with FastStart Essential DNA Green Master (Roche, Mannheim, Germany) using a LightCycler®96 System (Roche). The amplification consisted of denaturation at 95°C for 10 sec, annealing at 58°C for 10 sec, and extension at 72°C for 10 sec performed for 40 cycles. Analysis was performed in triplicate, and each result was normalized relative to the HPRT mRNA level.
Proliferation assay
Myoblasts were seeded into 24-well plates at 1 × 104 cells/well. The next day the cells were then infected with adenovirus carrying either Fbxw7β or ΔFbox at a multiplicity of infection (MOI) of 200 for 90 min in growth media. During adenovirus infection, the 24-well plate was gently shaken to help the viruses attach to the cells. The cells were counted with a hemocytometer at 48 h, 72 h and 96 h post-adenoviral infection.
Wound healing assay
Myoblasts were seeded into 24-well plates at 1 × 105 cells/well. After infection at an MOI of 200 with adenovirus carrying either Fbxw7β or ΔFbox, a line was drawn through each well with a 200 μL pipet tip (Fronza et al. 2009). The migration activity of the myoblasts was analyzed by measuring the width of the line over time.
Western blotting
Proteins were prepared with 1% sodium dodecyl sulfate (SDS) lysis buffer. Denatured proteins were separated with 8% or 10% SDS polyacrylamide gel electrophoresis (PAGE) and transferred into Nitrocellulose Blotting membrane (GE Healthcare, Little Chalfont, England). Each primary antibody, Cdc4 (Santa Cruz, sc-33196), glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling, 2118), Zeb1 (Cell Signaling, 3396), Vimentin (Cell Signaling, 5741), N-cadherin (Abcam, ab12221), E-cadherin (Cell Signaling, 3195), Slug (Cell Signaling, 9585), cyclin E (Cell Signaling, 4129) and c-Myc (Santa Cruz, sc-40), was treated into 5% DifcoTM Skim Milk (BD, Sparks, MD, USA) blocked membrane at 4°C overnight. Tris-buffered saline-Tween 20-washed membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz, sc-2004 and sc-2005) at room temperature for 1 h. The antigen-antibody conjugated was detected with ImageQuant™ LAS 4000 mini (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) after treatment of ImmobilonTM Western (Millipore Corporation, Billerica, MA, USA).
Histological analysis
All mice myofiber tissues were frozen in Optimal Cutting Temperature compound (Tissue-Tek, Sakura Finetek, CA, USA) and cryosectioned at 10-15 µm using a Cryocut microtome (Leica, Nussloch, Germany). Hematoxylin (Sigma-Aldrich) and eosin (Sigma-Aldrich) staining of muscle sections were performed according to the manufacturer's instructions.
Statistical analysis
All statistical analyses were performed in triplicate using Student's t-test or analysis of variance. *P < 0.05, **P < 0.01 and ***P < 0.005 were considered statistically significant.
Results
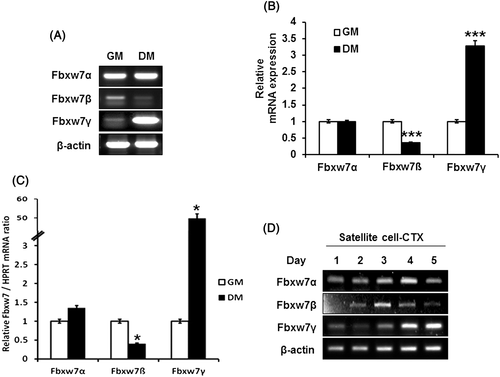
Endogenous Fbxw7β and γ expression are inversely regulated during myoblast differentiation
Fbxw7 is known to be expressed in several tissues. According to a previous report, Fbxw7α and γ are strongly expressed in skeletal muscle, while Fbxw7β is expressed in the brain and weakly expressed in the testis (Matsumoto et al. 2006). In this study, we addressed Fbxw7β expression in different myoblast differentiation states. To evaluate the endogenous mRNA expression levels of Fbxw7α, β and γ in the myoblast and myotube states, we switched the mouse primary cultured myoblast from growth medium to differentiation medium for 72 h and conducted RT-PCR or quantitative (q)RT-PCR. As expected, Fbxw7α was highly expressed and its expression did not show any considerable difference between myoblasts and myotubes (Fig. 1A and quantified in 1B). However, Fbxw7β was weakly expressed before differentiation, and its expression was mostly diminished after differentiation. In contrast, the expression of Fbxw7γ was greatly increased after differentiation. These results were supported by qRT-PCR experiments (Fig. 1C). Moreover, we evaluated endogenous Fbxw7 expression in satellite cells after CTX injury. Fbxw7 subtypes, β and γ, were regulated reciprocally (Fig. 1D). Therefore, these data suggest that Fbxw7 expression changes according to the differentiation state of myoblasts, and that this fine-tuning could play a role in myoblast differentiation.
Differentiation capacity of myoblasts and satellite cells is diminished by Fbxw7β overexpression
Based on the results described earlier, we hypothesized that the expression of Fbxw7β and γ regulates myoblast differentiation. However, it is already known that Fbxw7β is rarely expressed in skeletal muscle, while Fbxw7γ is only expressed in heart and skeletal muscle (Matsumoto et al. 2006). Therefore, we focused on the function of Fbxw7β in myoblasts and satellite cells. To assess the function of Fbxw7β in myoblast differentiation, myoblasts were transduced with adenovirus of Fbxw7β and ΔFbox. We confirmed mRNA expression of both Fbxw7β and ΔFbox in myoblasts by RT-PCR (Fig. 2A, quantified in 2B) and ectopic expression of Fbxw7β in protein levels (Fig. 2C). However, the ectopic expression of ΔFbox could not detect in SDS-PAGE. We assumed that the primary antibody was somehow not suitable to detect the modified three-dimensional structure of ΔFbox protein. To evaluate the function of Fbxw7β during myoblast differentiation, Fbxw7β- and ΔFbox-overexpressing myoblasts were cultured with differentiation medium for 72 h. As shown in Fig. 2D, myoblasts transduced with the control adenovirus highly formed multinucleated myotubes as we expected, whereas the fusion index for Fbxw7β-transduced myoblasts was decreased by 70% as compared with control. To evaluate the side effect of adenovirus, green fluorescent protein (GFP) expressing adenovirus was transduced MOI-dependently into myoblast to analyze the adenovirus effect on myogenic differentiation. GFP expressing myoblasts highly formed multinucleated myotubes as a control (Supplement Fig. 2A and quantified in S2B). Myoblasts expressing ΔFbox were less affected (Fig. 2D and quantified in 2E). These data were repeated with shFbxw7β adenovirus infection. shFbxw7β expressing myoblast showed the same fusion indices as compared to control in our pre-experiments (data not shown).
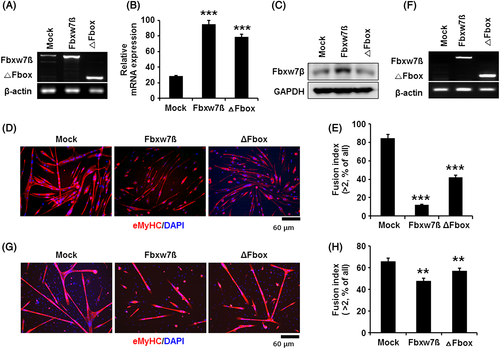
To determine whether Fbxw7β overexpression affects satellite cell activation and differentiation with respect to muscle regeneration in vivo, we injected CTX and Fbxw7β-carrying adenovirus together into TA and Gastroc muscles of mice (Jeong et al. 2013) and their expressions were detected by RT-PCR (Fig. 2 F). Three days after muscle injury and adenoviral injection, the regenerative capacity of the activated satellite cells was analyzed by assessing their ability to form myotubes ex vivo. We found that Fbxw7β-carrying adenovirus injected satellite cells formed lower rates of de novo myotubes than the control (Fig. 2G and quantified in 2H). These data demonstrate that Fbxw7β inhibits differentiation capacity of myoblasts and response of satellite cells to regeneration to de novo myotubes.
Fbxw7β inhibits proliferation and migration of myoblasts
To determine whether Fbxw7β also regulates the proliferation rate of myoblasts, we counted the myoblasts transduced with the Fbxw7β- and ΔFbox-carrying adenovirus. Myoblasts overexpressing Fbxw7β displayed a decreased growth rate over time compared with controls. However, the growth rate of ΔFbox-expressing myoblasts, in which Fbxw7 enzyme activity was abolished, was similar to that of controls (Fig. 3A and 3B). To determine whether Fbxw7β also regulates satellite cell proliferation, we seeded muscle myofibers transduced with CTX and Fbxw7β- and ΔFbox-carrying adenoviruses onto ECM-coated slides at 3 days post-injury. In agreement with the myoblast data, the satellite cells overexpressing Fbxw7β grew at a lower rate than the controls (Fig. 3C).
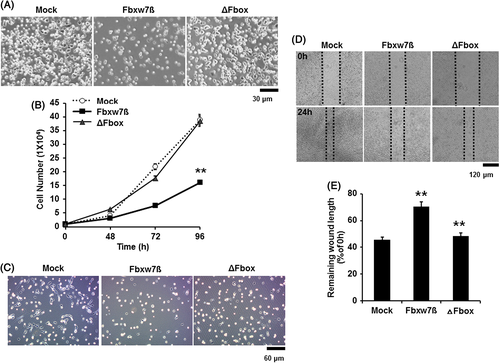
During muscle regeneration, activated satellite cells move to the damaged site and fuse with damaged muscle fibers. Therefore, we evaluated myoblast migration capacity using a wound healing assay to determine whether Fbxw7β could modulate myoblast migration. We seeded myoblasts onto culture plates and then generated a wound using a 200 μL pipet tip. Migration of myoblasts was observed for 24 h in growth medium. As shown in Figure 3D, movement of myoblasts was reduced by almost 1.5-fold when Fbxw7β was overexpressed (Fig. 3D and quantified in 3E). However, the migration of ΔFbox-expressing myoblasts was similar to that of the control. These results suggest that Fbxw7β negatively regulates the proliferation and migration of myoblasts and satellite cells. To further elucidate the mechanism underlying the decreased migration ability, Epithelial-mesenchymal transition (EMT) molecules were analyzed in Fbxw7β- and ΔFbox-expressing myoblasts. EMT molecules such as vimentin, E-cadherin and Snai2 (also known as Slug) are involved in migration and cancer invasion (Hills & Squires 2011; Masszi & Kapus 2011; Barriere et al. 2014; Joosse et al. 2014). According to previous reports, N-cadherin is an important factor in myoblast migration for homing and differentiation (Cifuentes-Diaz et al. 1993; Cifuentes-Diaz et al. 1994). To determine whether the expression of any of these EMT molecules alter in myoblasts by overexpression of Fbxw7β, we conducted RT-PCR and Western blot after transduction with each viral vector. We found that overexpression of Fbxw7β did not significantly change the expression of these EMT molecules (Supplement Fig. 3A and 3B). These data suggest that regulating migration of myoblasts and satellite cells of Fbxw7β is not related to EMT molecules but related to another mechanism.
Discussion
Fbxw7 is well known as a tumor suppressor that causes degradation of several target oncoproteins such as c-Myc, c-Jun and cyclin E (Koepp et al. 2001; Moberg et al. 2001; Strohmaier et al. 2001; Spruck et al. 2002; Mao et al. 2004). However, the function of Fbxw7 in muscle satellite cells has not been reported. In this study, we evaluated primary myoblast myogenic activity while manipulating Fbxw7β expression. Fbxw7β expression is inactivated by promoter hypermethylation in primary breast cancer and glioma cell lines (Gu et al. 2007; Akhoondi et al. 2010). In addition, in myotubes and myofibers, endogenous Fbxw7β expression does not occur, in contrast to its weak but continuous expression in myoblasts. Therefore, we overexpressed Fbxw7β and its dominant negative mutant of ΔFbox in primary myoblasts to verify its function.
Understanding and regulating myogenesis is expected to be important for treating and curing muscle degeneration, especially because satellite cells in muscle fibers diminish in number and activity with aging and in some diseases (Krauss 2010; Jeong et al. 2013). Few proteins are known as regulators of myoblast differentiation, including p38 MAPK Stac3, and p53 (Molchadsky et al. 2008; Carlson et al. 2009; Pijet et al. 2013; Li et al. 2014; Tierney et al. 2014). In this study, we found that overexpression of Fbxw7β inhibited not only muscle regeneration of myofibers (Fig. S4A, quantified in S4B) but also differentiation, proliferation and migration in myoblasts and satellite cells. Thus, we suggest that Fbxw7β is another negative regulator of myogenesis. Notably, F-box-deleted dominant negative mutant of Fbxw7β, ΔFbox, did not inhibit myogenesis. Unfortunately, even ΔFbox proteins somehow could not be detected in SDS-PAGE; its functional expression was detected with RNA levels in a mouse muscle disease model (data is not shown). These together strongly suggest that the F-box, a variable substrate recognition subunit, plays a key role in the effects of Fbxw7β.
EMT is associated with embryo implantation, embryogenesis and organ development (Newgreen & Minichiello 1995; Newgreen & Minichiello 1996; Thompson & Levin 2010; Hughes et al. 2014). Recently, various studies have reported EMT program activation during tumor invasion and metastasis; however, the EMT is still poorly understood (Kalluri & Weinberg 2009). In myoblasts, N-cadherin is involved in migration and homing (Brand-Saberi et al. 1996) and Snai2 is known to act downstream of MyoD (Zhao et al. 2002). However, we did not find that Fbxw7β significantly regulates these EMT molecules.
Fbxw7 degrades positive regulators of the cell cycle such as cyclin E, c-Myc, Notch and c-Jun by mediating their ubiquitination. Our data showed that Fbxw7β expressing myoblasts did not degrade cyclin E and c-Myc despite its decreased growth rate: however, the identity of the molecules binding to Fbxw7β remains to be resolved. In conclusion, our data suggest that cellular function of mammalian skeletal myoblast is negatively regulated by Fbxw7β expression. Future experiments using knock-out animals and muscle disease in case of, especially, abnormal over-expressed Fbxw7β in myofibers could further clarify the relationship between Fbxw7β and disease-induced muscle degeneration.
Acknowledgments
This research was supported by Basic Science Research Program (No. 2013R1A1A2009473 to HK) through the National Research Foundation of Korea funded by MISP and No. 2012M2A2A7010422 from the Nuclear Research & Development Program of the National Research Foundation grant (to SH) and No. 2014R1A1A1002599 from Basic Science Research Program (to JJ) through the National Research Foundation of Korea funded by MISP.




