Effects of addition of Aspergillus oryzae culture and 2-hydroxyl-4-(methylthio) butanoic acid on milk performance and rumen fermentation of dairy cows
Abstract
To investigate effects of Aspergillus oryzae culture (AOC) and 2-hydroxy-4-(methylthio) butanoic acid (HMB) on milk performance and rumen fermentation of dairy cows. Sixty-four multiparous Chinese Holstein cows were randomly allocated into four experimental diets: (i) Control diet; (ii) AOC diet: 5 g AOC/day per head; (iii) HMB diet: 25 g HMB/day; and (iv) AH diet: 5 g AOC plus 25 g HMB/day. Added HMB tended to increase the yield of milk protein (P = 0.06) and 3.5% fat-corrected milk (P = 0.08) and milk fat content (P = 0.09). Milk fat yield (P = 0.03) and the contents of milk protein (P = 0.05) were increased by adding HMB. The cows fed on AOC diet had a tendency for higher body weight (BW) gain (P = 0.08). Addition of AOC, HMB and AH increased content of microbial protein (MCP) and total volatile fatty acids (VFA) (P < 0.01) in rumen fluid. Populations of rumen fungi, Fibrobacter succinogenes and Ruminococcus flavefaciens relative to total bacterial 16S rDNA (P ≤ 0.03) and activity of carboxymethylcellulase (CMCase) (P < 0.01) were increased with added AOC or HMB. It is inferred that added AOC or HMB can increase the contents of MCP and total VFA potentially by stimulating rumen microbe populations and CMCase activity.
Introduction
Many of feed additives such as direct-fed microbials (DFM) and protected amino acids (AA) are introduced to improve the nutrient availability and consequently the performance of ruminant livestock. The use of Aspergillus oryzae culture (AOC), a nonbacterial DFM, has continued as an interest in milk performance for lactating cows (Higginbotham et al. 1993; Kim et al. 2006). Inclusion of AOC containing α-amylase activity tended to improve milk yield (Harrison & Tricarico 2007). However, Higginbotham et al. (2004) reported that feeding AOC had no effect on lactation performance and rumen parameters in multiparous Holstein cows. Therefore, the effects of AOC on milk production and rumen fermentation were variable. The mode of action of AOC is still unclear.
Methionine (Met) has been considered as the limiting AA for dairy cattle (NRC 2001). Several methods have been developed to protect Met from rumen degradation. 2-hydroxy-4-(methylthio) butanoic acid (HMB) is a Met supplement, aimng to maximize Met absorption in the intestinal tract while minimizing losses in the rumen (Schwab et al. 2001). Response of lactating cows to added HMB includes the increase in milk yield (Piepenbrink et al. 2004) and milk fat percentage (Huber et al. 1984) and no or nonsignificant effect on milk yield and composition (St-Pierre & Sylvester 2005; Karnati et al. 2007). It was observed that responses of HMB (40-50% protection of rumen degradation) on rumen fermentation were variable. The rumen volatile fatty acid (VFA) proportion was not influenced by added HMB in some studies (Vázquez-Añón et al. 2001; Wilson et al. 2008), while the change in rumen VFA profile was observed in other studies (Noftsger et al. 2003; Chung et al. 2006).
Supplementing AOC may enhance utilization of fiber, and adding HMB probably supplies Met and balances AA to improve protein utilization in the rumen. It is well-known that fiber and protein are two important nutrients for ruminants. Our hypothesis is that adding AOC and HMB may achieve a synergistic improvement in lactating performance by simultaneously enhancing utilization of fiber and protein. To our knowledge, there has been no trial in which both AOC and HMB were allocated as supplementations to enhance rumen fermentation and milk performance at the same time. Thus the objective of this study is to investigate the synchronized effects of added AOC and HMB on rumen fermentation and milk performance of lactating dairy cows.
Materials and Methods
Experimental animals and design
The use of animals was approved by the Animal Care Committee, Zhejiang University, China. Sixty-four multiparous Chinese Holstein cows (body weight = 520 ± 10 kg; days in milk (DIM) = 136 ± 5; milk yield = 31 ± 0.3 kg/day; parity = 2.5 ± 0.4) were divided into 16 blocks of four cows based on DIM, parity and milk yield, and were randomly allocated to one of four dietary treatments in a 2 × 2 factorial arrangement. Animals were housed in a tie-stall barn, and fed and milked at 07.00, 14.00 and 20.00 hours. All cows had free access to drinking water. Feed was offered ad libitum to 5% orts. The experimental period consisted of 2 weeks adaptation and 6 weeks test. The ingredients of the experimental control diet are presented in Table 1.
| Ingredient | Treatment† | |||
|---|---|---|---|---|
| Control | AOC | HMB | AH | |
| Corn silage | 12.7 | 12.7 | 12.7 | 12.7 |
| Grass hay | 15.1 | 15.1 | 15.1 | 15.1 |
| Alfalfa hay | 12.1 | 12.1 | 12.1 | 12.1 |
| Dried distillers grains with solubles | 4.07 | 4.07 | 4.07 | 4.07 |
| Beet pulp | 9.33 | 9.33 | 9.32 | 9.32 |
| Ground corn grain | 26.5 | 26.5 | 26.5 | 26.5 |
| Soybean meal, 46.3% crude protein | 8.96 | 8.96 | 8.95 | 8.95 |
| Cottonseed meal | 4.48 | 4.48 | 4.48 | 4.47 |
| Wheat bran | 2.24 | 2.24 | 2.24 | 2.24 |
| Concentrated feed‡ | 4.48 | 4.48 | 4.48 | 4.47 |
| Amaferm§ | 0 | 0.02 | 0 | 0.02 |
| MFP¶ | 0 | 0 | 0.10 | 0.10 |
- † AOC = Aspergillus oryzae culture supplement; HMB = 2-hydroxy-4-(methylthio) butanoic acid (HMB) supplement; AH = AOC and HMB supplement.
- ‡ Formulated to provide (per kg DM) 1% crude protein, 15% ether extract, 6% crude fiber, 7% Ca, 1.3% P, 10% salt, 3% Mg, 1.5% K, 1% Met, 180-345 mg Cu, 190-330 mg Fe, 950-1,800 mg Zn, 350-650 mg Mn, 80 000-145 000 IU of vitamin A, 20 000-39 000 IU vitamin D3 and 700 IU vitamin E.
- § Amaferm contained 5-10% AO fermentation product and 90-95% wheat bran provided by Biozyme, Inc., St. Joseph, MO.
- ¶ MFP contained 84% D, L-HMB and was provided by Novus International Inc., St. Louis, MO.
Calcium salts of HMB (MFP, Novus Int. Inc., St Louis, MO, USA) was added as a source of rumen-protected Met. The treatments were: Control diet, AOC (Amaferm, Biozyme Inc., St, Joseph, MO, USA) diet: 5 g AOC/day per head was added into control diet; HMB diet: 25 g HMB/day per head was added into control diet; AH diet: 5 g AOC plus 25 g HMB/day per head were added into control diet.
Sampling collection and measurements
Offered and refused feed was weighed once a week throughout the experiment to determine dry matter intake (DMI). The feed samples were collected weekly. All samples were dried in a forced-air oven at 65°C for 24 h and stored in sealed plastic containers at -20°C until analyses. Dried samples were ground through a 40 mesh screen in a Cyclotec mill (Tecator 1093; Tecator AB, Hoganas, Sweden) before analysis. The contents of dry matter (DM), crude protein (CP) and ash were analyzed according to the Association of Official Analytic Chemists (1990). The acid detergent fiber (ADF) and neutral detergent fiber (NDF) were determined according to Van Soest et al. (1991). Dietary compositions were calculated according to the chemical analysis and inclusion rate of ingredients (Table 2). At the beginning and end of the test period, every cow was weighed on a weighing scale before morning feeding in order to calculate the change in BW.
| Composition† | Treatment‡ | |||
|---|---|---|---|---|
| Control | AOC | HMB | AH | |
| CP (% of DM) | 15.9 | 15.9 | 16.0 | 16.0 |
| NDF (% of DM) | 33.8 | 33.8 | 33.7 | 33.7 |
| ADF (% of DM) | 19.0 | 19.0 | 18.9 | 18.9 |
| RDP (% of DM) | 10.5 | 10.5 | 10.6 | 10.6 |
| RUP (% of DM) | 5.4 | 5.4 | 5.4 | 5.4 |
| Ca (% of DM) | 0.69 | 0.69 | 0.70 | 0.70 |
| P (% of DM) | 0.40 | 0.40 | 0.40 | 0.40 |
| NEL (Mcal/kg of DM) | 1.57 | 1.57 | 1.57 | 1.57 |
| Met (% of MP) | 1.98 | 1.98 | 2.14 | 2.14 |
| Lys (% of MP) | 6.45 | 6.45 | 6.43 | 6.43 |
- † Calculated based on individual feedstuffs in Ministry of Agriculture, P.R. China (2004).
- ‡ AOC = Aspergillus oryzae culture supplement; HMB = 2-hydroxy-4-(methylthio) butanoic acid (HMB) supplement; AH = AOC and HMB supplement.
- CP, crude protein; DM, dry matter: NDF, neutral detergent fiber; ADF, acid detergent fiber; Rumen degradable protein; RUP, rumen undegradable protein; NEL, net energy for lactation; Met, methionine; MP, metabolizable protein; Lys, lysine
Daily milk yield (three milkings) was recorded every weekend of the test period by using milk-sampling devices (Waikato Milking Systems NZ Ltd., Waikato, Hamilton, New Zealand), and two 50 mL aliquots of the milk sample were collected daily and pooled in a proportion of 4:3:3 for three milkings. One sample with added Bromopol (milk preservative, D&F Control Systems, San Ramon, CA, USA) was stored at 4°C and analyzed for fat, protein and lactose by infrared analysis (Laporte & Paquin 1999) using a spectrophotometer (Foss-4000; Foss, Hillerød, Denmark), and for the somatic cell count using a cell counter (Fossmatic 400; Foss Electric). The other sample without Bromopol was stored at -20°C for the analysis of milk urea nitrogen (Wang et al. 2010).
At the middle and end of the experiment, samples of rumen contents (100 to 150 mL) from six cows of each treatment selected randomly were obtained according to the method of Shen et al. (2012) at 2 h after the morning feeding. Samples were filtered through four layers of cheesecloth and pH was determined immediately using a pH meter (S40 SevenMulti; Mettler Toledo, China). The samples were separated and stored at –20°C for further analysis of ammonia-N, VFA and microbial protein (MCP) using methods described by Hu et al. (2005). Briefly, rumen ammonia-N concentration was determined by steam distillation into boric acid and titration with dilute hydrochloric acid. The VFA concentration was analyzed using a gas chromatograph (GC-2010; Shimadzu Corporation, Kyoto, Japan) equipped with a flame-ionization detector. For measurement of VFAs, 2 μL of supernatant obtained from rumen fluid by centrifuging at 20 000 × g for 10 min at 4°C were injected into a 2 m × 3 mm glass column packed with Porapak Q (80 mesh; Agilent Technologies Inc., Santa Clara, CA, USA). Nitrogen was used as a carrier. The temperature of the injector/detector and the column were 200°C and 180°C, respectively. For determination of MCP, 8 mL sample of the fermentation medium was centrifuged at 20 000 × g for 20 min at 4°C to obtain the supernatant. The MCP was determined by the method of Zinn and Owens (1986) based on purine, and the content of the MCP was estimated from the ratio of purines to nitrogen of isolated bacteria, and the supernatant yeast RNA was used as a standard.
For determination of the relative quantity to total bacterial 16S rDNA of protozoa, fungi, F. succinogenes, R. flavefaciens and R. albus, six aliquots of 1.5 mL rumen fluid were sampled and stored at -80 °C immediately. Total DNA was isolated using a genomic DNA kit (Axygen Biosciences, Union City, USA) following the manufacturer's instructions. The amplifying primer sets of total bacteria, fungi, F. succinogenes, R. flavefaciens, R. albusand, protozoa are listed in Table 3, as described by Denman and McSweeney (2006); Koike and Kobayashi (2001) and Denman et al. (2007). The species-specific qPCR was performed using the ABI 7500 PCR system (Applied Biosystems, USA) with fluorescence detection of SYBR green dye. Samples were run under the amplification conditions as described by Mao et al. (2010). Populations of protozoa, fungi, Fibrobacter Succinogenes, Ruminococcus Flavefaciens and R. albus were expressed as a proportion of total rumen bacterial 16S rDNA according to the equation: Relative quantification of target = 2-(Ct target - Ct total bacteria) × 100, where Ct represents threshold cycle (Pfaffl 2001).
| Target species | Forward/Reverse | Primer sequence |
|---|---|---|
| Total bacteria† | F | CGGCAACGAGCGCAACCC |
| R | CCATTGTAGCACGTGTGTAGCC | |
| Fungi† | F | GAGGAAGTAAAAGTCGTAACAAGGTTTC |
| R | CAAATTCACAAAGGGTAGGATGATT | |
| Ruminococcus flavefaciens† | F | CGAACGGAGATAATTTGAGTTTACTTAGG |
| R | CGGTCTCTGTATGTTATGAGGTATTACC | |
| Fibrobacter succinogenes† | F | GTTCGGAATTACTGGGCGTAAA |
| R | CGCCTGCCCCTGAACTATC | |
| Protozoa‡ | F | GCTTTCGWTGGTAGTGTATT |
| R | CTTGCCCTCYAATCGTWCT | |
| R. albus§ | F | CCCTAAAAGCAGTCTTAGTTCG |
| R | CCTCCTTGCGGTTAGAACA |
Enzyme extraction was performed according to the methods by Giri et al. (2005). Rumen liquor sample was sonicated in an ice bath for 10 min at 20 kHz. The resultant samples were subjected to centrifugation at 24 000 × g for 20 min at 4°C. The clear supernatant was used for rumen enzyme activity determination by measuring reducing sugar formation. Substrates used were sodium carboxymethylcellulase (0.5%) for carboxymethylcellulase (CMCase), α-starch (0.5%) for amylase, and xylan (0.5%) for xylanase, respectively. The assay mixture of 0.4 mL substrate solution and 0.2 mL supernatant was incubated at 39°C for 30 min. The reaction was stopped by the addition of 0.6 mL 3,5-dinitrosalicylic acid reagent. The tubes were placed in a boiling water bath for exactly 5 min and cooled in water. The absorbance was read at 540 nm. The D-glucose was used as the standard for testing CMCase and amylase, and D-xylose for xylanase, respectively. All assays were carried out in triplicate. The units of enzyme activity were expressed as 1 µmol glucose or xylose equivalents per min per mL enzyme supernatant.
Statistical analysis
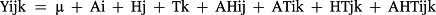
Results
Effects of added AOC and HMB on feed intake, milk performance
Feed intake and milk performance are summarized in Table 4. The DMI was not influenced by added AOC and HMB (P > 0.05) with an average of 21.6 kg/day. Neither AOC nor HMB affected milk yield. Supplementation of AOC increased milk lactose content (P = 0.04) and tended to increase BW gain (P = 0.08). Added HMB increased the contents of protein (P = 0.05) and total solid (P = 0.02) and the yield of milk fat (P = 0.03). There was the tendency to increase milk fat content (P = 0.09) and the yields of milk protein (P = 0.06) and 3.5% fat-corrected milk (FCM) (P = 0.08) due to added HMB. Added AOC plus HMB increased the yield of milk fat in comparison with the Control diet. The interaction was not observed between AOC and HMB in all tested variables except milk lactose content (P = 0.04).
| Item | Treatment† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | AOC | HMB | AH | AOC | HMB | A × H | ||
| DMI (kg/day) | 21.6 | 21.6 | 21.8 | 21.5 | 0.36 | 0.83 | 0.91 | 0.70 |
| Milk production (kg/day) | ||||||||
| Milk yield | 28.8 | 29.9 | 30.2 | 30.1 | 0.96 | 0.59 | 0.42 | 0.49 |
| Milk protein | 0.88 | 0.88 | 0.94 | 0.93 | 0.025 | 0.98 | 0.06 | 0.81 |
| Milk fat | 1.00a | 1.04a | 1.07a | 1.09b | 0.029 | 0.28 | 0.03 | 0.69 |
| 3.5% FCM | 28.6 | 29.8 | 30.5 | 30.8 | 0.82 | 0.36 | 0.08 | 0.60 |
| Milk composition (%) | ||||||||
| Protein | 3.07a | 2.98a | 3.12b | 3.11b | 0.043 | 0.29 | 0.05 | 0.35 |
| Fat | 3.50 | 3.50 | 3.59 | 3.66 | 0.075 | 0.64 | 0.09 | 0.59 |
| Lactose | 4.83a | 4.95b | 4.93b | 4.93b | 0.029 | 0.04 | 0.17 | 0.03 |
| Total solid | 12.5a | 12.4a | 12.8a | 12.9b | 0.16 | 0.81 | 0.02 | 0.62 |
| Milk urea nitrogen | 13.5 | 13.2 | 13.4 | 13.4 | 0.29 | 0.60 | 0.89 | 0.55 |
| Somatic cell score | 3.75 | 3.25 | 3.62 | 3.35 | 0.333 | 0.25 | 0.97 | 0.74 |
| Body weight gain | 0.64 | 0.78 | 0.56 | 0.81 | 0.110 | 0.08 | 0.81 | 0.62 |
- a ,
- b Means within a row with different superscripts differ (P < 0.05).
- † AOC = Aspergillus oryzae culture supplement; HMB = 2-hydroxy-4-(methylthio) butanoic acid (HMB) supplement; AH = AOC and HMB supplement.
- DMI, days in milk; FCM, fat-corrected milk
Effects of added AOC and HMB on rumen fermentation
Effects of added AOC and HMB on the pH, ammonia-N, MCP and VFA in rumen fluid are shown in Table 5. The content of MCP and total VFA in rumen fluid was enhanced when AOC and HMB were added (P < 0.01). Further, the other three diets had higher MCP and total VFA compared with the Control diet. The molar proportion of VFA was not affected by added AOC (P > 0.05). Added HMB increased the proportion of acetate (P < 0.01) and decreased the proportion of propionate (P = 0.01), hence increased ratio of acetate : propionate (P < 0.01).
| Item | Treatment† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | AOC | HMB | AH | AOC | HMB | A × H | ||
| pH | 6.30 | 6.41 | 6.36 | 6.28 | 0.051 | 0.74 | 0.51 | 0.07 |
| Ammonia-N (mg/dL) | 14.7 | 14.6 | 14.6 | 15.8 | 0.99 | 0.57 | 0.54 | 0.54 |
| Microbial protein (mg/mL) | 3.38a | 3.75b | 3.73b | 3.90b | 0.062 | <0.01 | <0.01 | 0.09 |
| Total volatile fatty acid (mmol/L) | 61.9a | 68.0b | 71.4b | 73.7b | 1.20 | <0.01 | <0.01 | 0.13 |
| Molar proportion (mol/100 mol) | ||||||||
| Acetate | 66.7a | 67.8a, b | 69.1b | 68.9b | 0.54 | 0.46 | <0.01 | 0.26 |
| Propionate | 21.5b | 20.8a, b | 19.2a | 19.3a | 0.69 | 0.67 | 0.01 | 0.59 |
| Butyrate | 11.8 | 11.4 | 11.6 | 11.8 | 0.25 | 0.68 | 0.73 | 0.33 |
| A:P | 3.22a | 3.41a, b | 3.65b | 3.64b | 0.114 | 0.42 | <0.01 | 0.39 |
- a ,
- b Means within a row with different superscripts differ (P< 0.05).
- † AOC = Aspergillus oryzae culture supplement; HMB = 2-hydroxy-4-(methylthio) butanoic acid (HMB) supplement; AH = AOC and HMB supplement.
- A:P, acetate-to-propionate ratio.
Effects of added AOC and HMB on rumen microbes and their enzyme activities
No effects on the population of protozoa and R. albus relative to total bacterial 16S rDNA are observed in response to AOC or HMB addition (Table 6). Adding AOC and HMB increased the population of fungi (P < 0.01), F. succinogenes (P ≤ 0.03) and R. flavefaciens (P < 0.01). The AH diet had a bigger effect on fungi, F. succinogenes compared with Control diet (P < 0.05). Cows fed AH diet had higher R. flavefaciens in comparison with cows fed the other three diets (P < 0.05). There were no AOC × HMB interactions for rumen microbes tested in our study (P > 0.05).
| Item | Treatment† | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Control | AOC | HMB | AH | AOC | HMB | A × H | ||
| % total bacterial 16 S rDNA | ||||||||
| Protozoa | 0.13 | 0.11 | 0.11 | 0.08 | 0.028 | 0.35 | 0.42 | 0.69 |
| Fungi (×10-3) | 3.85a | 4.19b | 4.16b | 4.39b | 0.881 | <0.01 | <0.01 | 0.50 |
| Fibrobacter succinogenes | 0.20a | 0.24a, b | 0.23a | 0.28b | 0.016 | <0.01 | 0.03 | 0.96 |
| Ruminococcus flavefaciens (×10-2) | 1.19a | 1.48b | 1.36b | 1.60b | 0.044 | <0.01 | <0.01 | 0.61 |
| R. albus ( ×10-2) | 1.46 | 1.57 | 1.59 | 1.54 | 0.092 | 0.78 | 0.62 | 0.37 |
| Enzyme activities (IU) | ||||||||
| Carboxymethylcellulase | 0.39a | 0.45b | 0.43b | 0.57b | 0.011 | <0.01 | <0.01 | <0.01 |
| Amylase | 1.43a, b | 1.52b | 1.40a | 1.47b | 0.033 | 0.02 | 0.20 | 0.73 |
| Xylanase | 1.37 | 1.36 | 1.31 | 1.27 | 0.069 | 0.76 | 0.30 | 0.83 |
- a ,
- b Means within a row with different superscripts differ (P< 0.05).
- † AOC = Aspergillus oryzae culture supplement; HMB = 2-hydroxy-4-(methylthio) butanoic acid (HMB) supplement; AH = AOC and HMB supplement.
- IU, enzyme activity releasing 1 µmol of reducing sugar per minute per mL supernatant of rumen fluid.
Effects of addition of AOC and HMB on the activity of CMCase, amylase and xylanase are summarized in Table 6. Added AOC and HMB increased CMCase activity (P < 0.01), and there was an interaction between AOC and HMB (P < 0.01). Adding AOC and HMB alone or both improved CMCase activity compared with the control diet. In addition, cows fed AH diet had higher CMCase compared with cows fed AOC diet and HMB diet (P < 0.05). Treatment AH had the highest CMCase activity. Amylase activity significantly increased with the addition of AOC (P = 0.02). No effect of AOC and HMB was observed on xylanase activity (P > 0.05).
Discussion
In the current study, adding HMB affected milk protein content and milk fat yield, tended to increase milk fat content and the yields of milk protein and 3.5% FCM, which were different from AOC. Production responses to HMB reported in the literature vary significantly among studies. Griel et al. (1968) reported the positive effects of HMB on milk performance in dairy cows. Our results agreed with the study by Broderick et al. (2009) who concluded addition of rumen-protected Met increased milk fat yield and tended to increase milk fat content and milk protein yield. Piepenbrink et al. (2004) suggested adding HMB was beneficial for increasing milk production. However, others indicated no effect of HMB on DMI and milk production (Noftsger et al. 2003; Lee et al. 2015). A rumen-protected form of HMB increased milk production and milk protein yield, without affecting DMI of dairy cows, for which Met was estimated to be the first limiting AA (Sklan & Tinsky 1996). Wang et al. (2010) reported supplementation of HMB increased milk yield and milk fat content without corresponding effect on DMI. The improvement of HMB on milk fat was mainly relevant with the metabolic pathways of Met and its methyl donor, S-adenosyl-methionine (Benefield et al. 2009; Wang et al. 2010).
Addition of AOC and HMB to the ration of dairy cows improved the concentration of MCP in the present study. Our study showed for the first time that AOC supplementation had a positive effect on rumen MCP in dairy cows. This may be due to the improvement of microbial activity. However, more studies had suggested the possible effect of HMB on rumen MCP synthesis. Lee et al. (2015) reported that HMB decreased dietary crude protein digestibility and increased microbial N outflow from the rumen. The study with 13C-labeled HMB infused intravenously showed that the Met analog can serve as a source of Met and also has a Met-sparing effect in dairy cows, with 15% of the milk protein Met originating from direct conversion of HMB to Met (Lapierre et al. 2011). It is inferred that HMB as a rumen-protected Met can increase concentration of rumen MCP and milk protein potentially by releasing Met in the intestine. Thus, supplementation of HMB increased milk protein content, presumably though enhanced ruminal MCP synthesis.
Total VFA increased with the supplementation of AOC and HMB. Molar proportions of individual VFA had no differences when adding AOC. However, rumen fermentation pattern was altered as shown by the increase in the ratio of acetate to propionate with supplementing HMB. Altered patterns in VFA profiles suggested differing availability and use of carbohydrates by ruminal microorganisms in response to altered dietary nutrient concentrations (Broderick et al. 2008). The increase in acetate molar proportion was consistent with the increase in milk fat. Seymour et al. (2005) showed content of milk fat had a positive association with rumen acetate-to-propionate ratio. Martin et al. (2013) reported that HMB supplementation increased VFA concentrations in the rumen as well as the ruminal abundance of F. succinogenes and R. flavefaciens. VFAs are the end products of rumen microbial fermentation and represent the main supply of metabolizable energy for ruminants, suggesting increased energy supply to support milk production or BW gain. In the experiment, cows supplemented AOC tended to increase BW gain, implying that more energy was used for BW gain instead of milk production. It has been suggested that when energy is available with limited AA supply, energy utilization efficiency is decreased for milk production, and more energy is partitioned to body tissue (Huhtanen 1998). It is inferred that the diet supplemented with AOC may have enough energy for milk production, but was limited in its AA supply compared with HMB, resulting in a tendency to increase energy utilized for BW. However, adding HMB had the opposite effect on milk production and BW compared with AOC, despite the increase of VFA. The reasons for the different responses to the addition of AOC and HMB are not clear. This may be due to the different effect on the individual proportion of VFA and the acetate : propionate ratio. Further, the increase in BW gain observed with AOC supplementation may be related to the increase of amylase activity, which may have provided more readily fermentable energy.
Relative populations of fungi, F. succinogees and R. flavefaciens and CMCase activity increased, which meant rumen microbial activity may be affected by AOC and HMB. There was no effect on populations of protozoa and R. albus, but the reason is not very clear. To our knowledge, this study is the first to show an in vivo increase in ruminal F. succinogees and R. flavefaciens following AOC supplementation. The effects of AOC on stimulating rumen fermentation were consistent with the results of previous studies (Beharka & Nagaraja 1998; Chang et al. 1999). The improvement of CMCase and amylase may be the result of increase of fungi, F. succinogees and R. flavefaciens. Although AOC does not produce the enzymatic machinery to completely depolymerize structural carbohydrates to simple sugars, it does produce enzymes that cause partial depolymerization (Boing 1983) and it aids rumen cellulytic bacteria in complete depolymerization of cellulosic material to simple sugars (Autrey et al. 1975). Schmidt et al. (2004) found that AOC accelerated the production and maturation of zoospores of Neocallimastix frontalis EB 188, along with an elevated production of protein and CMCase. Current data indicated that HMB had a positive effect on rumen microbial activity. Actually, HMB did not totally escape rumen microbial metabolism and could be a source of Met for rumen microorganisms. Previous studies indicated 40-50% ruminal escape of HMB (Vázquez-Añón et al. 2001; Koenig et al. 2002), which showed a significant amount of HMB was metabolized in the rumen. Because of the ruminal degradability, HMB could affect microbial fermentation by a direct stimulatory effect of Met on certain cellulolytic bacteria or through an indirect effect on the noncellulolytic species in the rumen (Martin et al. 2013). The exact mode of action of HMB in the rumen is not clear, but may be probably related to the stimulatory effect on microbial growth, particularly of some cellulolytic bacteria.
The synergetic effect between AOC and HMB was only found in CMCase activity not in MCP, VFA, rumen microbes composition or performance. The reason was not clear. The increase activity of CMCase was primarily attributed to the growth of cellulolytic bacteria and thus resulted in an increased rumen fiber digestibility. In our study, AOC or HMB significantly increased the relative populations of fungi, F. succinogees and R. Flavefaciens. It is inferred the combined digestibility of fungi, F. succinogenes and R. flavefaciens on the ration fiber component may together improve the activity of CMCase. So the greatest production of CMCase was observed in the AH diet. CMCase (endoglucanase) is one of the cellulolytic enzymes which act synergistically to hydrolyse cellulose. A detailed study with the other cellulolytic enzymes profile (exoglucanase, β-glucosidase) would be useful to explain the synergetic effect between AOC and HMB on CMCase activity.
Conclusion
Added AOC or HMB could improve the synthesis of MCP and formation of VFA in the rumen of dairy cows by stimulating rumen fungi, F. succinogenes, R. flavefaciens and CMCase activity, which may be associated with the enhancement of performance production. Added AOC trends toward increased BW gain of the cows. Added HMB increased milk protein content and milk fat yield in dairy cows. The synergetic effect between AOC and HMB was only found in CMCase activity.
Acknowledgments
This experiment was supported by the grants from the National Basic Research Program of China Ministry of Science and Technology (Grant No. 2011CB100801) and from Novus. Int. Inc. The authors gratefully thank all of the staff of the Hangzhou Hangjiang Dairy Herd (Hangzhou, China) for their assistance in the feeding, milking and care of the animals.




