Role of peroxisome proliferator-activated receptor alpha in the expression of hepatic fatty acid oxidation-related genes in chickens
Abstract
Liver is the most important target organ for investigation of lipid metabolism in domestic fowls. However, little is known about the regulatory mechanism of fatty acid oxidation in chicken liver. In mammals, proliferator-activated receptor alpha (PPARα), a transcription factor, plays an essential role in the regulation of hepatic fatty acid oxidation. The aim of the present study was to investigate the regulatory mechanisms of PPARα-induced gene expression involved in hepatic fatty acid oxidation in chickens in vivo and in vitro. WY14643, a PPARα agonist, significantly increased the messenger RNA (mRNA) levels of carnitine palmitoyltransferase 1a (CPT1a) and acyl-coenzyme A oxidase (ACO), but not long-, middle- and short-chain acyl-coenzyme A dehydrogenase (LCAD, MCAD and SCAD, respectively), hydroxyacyl-coenzyme A dehydrogenase (HAD), and PPARα itself in chicken hepatoma cells. In contrast, WY14643 significantly increased the mRNA levels of CPT1a, ACO, MCAD, SCAD, HAD and PPARα in human hepatoma cells. The mRNA levels of CPT1a and ACO in the liver were significantly increased by 6 h of fasting in chickens, whereas the mRNA levels of LCAD, MCAD, SCAD and HAD were unchanged. These results suggest that, unlike in mammals, CPT1a and ACO might play an important role in PPARα-induced fatty acid oxidation in the liver of chickens.
Introduction
Broiler chickens have been intensively selected over many generations with specific emphasis on increasing body weight. This increased body weight has been accompanied by unintended changes such as increased body fat (Havenstein et al. 2003). In view of the obesity epidemic, chickens that provide several times the fat energy compared with protein seem illogical (Wang et al. 2010). In addition, excessive fat accumulation not only increases inedible products such as visceral adipose tissue but also results in metabolic diseases, which are a serious problem for the poultry industry (Julian 2005). Therefore, the regulatory mechanisms of lipid metabolism in chickens have been a focus of research by poultry nutritionists.
Liver plays a primary role in lipid metabolism in birds. In addition, fatty liver is a serious problem in many types of domestic fowl (Julian 2005). Therefore, liver is the most important target for investigation of lipid metabolism in domestic fowls. Peroxisome proliferator-activated receptors (PPARs) are in the nuclear receptor family of ligand-activated transcription factors. In particular, PPARα plays an essential role in the metabolic adaptation of the liver to fasting situations by inducing gene expression of the rate-limiting enzymes for mitochondrial and peroxisomal fatty acid oxidation, such as carnitine palmitoyltransferase 1a (CPT1a), hydroxyacyl-coenzyme A dehydrogenase (HAD), and acyl-coenzyme A oxidase (ACO) in mammals (Schoonjans et al. 1996; Mandard et al. 2004). Fatty acid oxidation-related genes such as long-, middle- and short-chain acyl-coenzyme A dehydrogenase (LCAD, MCAD and SCAD, respectively), HAD and PPARα itself are also target genes of PPARα (Schoonjans et al. 1996; Kersten et al. 1999; Mandard et al. 2004). Therefore, PPARα plays important roles in fatty acid oxidation in mammals.
The PPARα agonist fenofibrate is used as a medicine for non-alcoholic fatty liver disease in humans (Kostapanos et al. 2013). Chicken liver expresses PPARα, which has high homology with human PPARα (Diot & Douaire 1999). There is evidence that PPARα agonists have complex effects on hepatic lipid metabolism in chickens. For example, the addition of PPARα agonist clofibrate did not influence the messenger RNA (mRNA) levels of the PPARα in the liver in laying hens, although the mRNA levels of CPT1a and ACO were increased (König et al. 2007a). PPARα agonists GW7647 and perfluorooctane sulfonate significantly increased mRNA levels of ACO, but not PPARα or CPT1a, in the liver from chicken embryos (Strömqvist et al. 2012). It is therefore likely that the target genes of PPARα in chickens might differ from those in mammals.
Fasting triggers a complex array of adaptive metabolic responses. Therefore, the effects of fasting on the expression of genes involved in the metabolic adaptations to feeding in the liver have been extensively studied in chickens. We previously showed that 4 h of fasting significantly increased the mRNA levels of PPARα, CPT1a and ACO in the liver in broiler chickens (Saneyasu et al. 2013). However, the mRNA level of PPARα was significantly increased after 2 h of fasting, whereas the mRNA levels of CPT1a and ACO were unchanged (Saneyasu et al. 2013). In addition, we found that the mRNA levels of LCAD, MCAD, SCAD and HAD were unchanged by both 2 and 4 h of fasting in chickens (unpublished data).
In the present study, we focused on the different responses of fatty acid oxidation-related genes between humans and chickens. The results show the first direct evidence that target genes of PPARα might substantially differ between humans and chickens.
Materials and Methods
Cell culture
Vanden Heuvel et al. (2003) demonstrated the direct effect of WY14643 on gene expression in a human hepatoma cell line (HepG2). They chose the short treatment time (6 h) and relatively high concentration of WY14643 (50 μmol/L) in order to focus on early and predominantly transcriptional events. In fact, 24 h incubation of rat hepatoma cells with WY14643 significantly increased not only PPARα target genes but also cholesterol metabolism-related genes (König et al. 2007b). According to these findings, we confirmed the effect of 6 h incubation with WY14643 on hepatoma cell lines. Also, since there is no report showing the effect of WY14643 on the expression of PPARα target genes in chicken hepatocytes, a wider range of concentrations (0–100 μmol/L) of WY14643 was used in a chicken hepatoma cell line (LMH) experiment.
LMH was purchased from American Type Culture Collection (ATCC, Manassas VA, USA; ATCC # CRL-2117). Cells were grown on six-well culture dishes in Waymouth's MB 752/1 containing 10% Fetal Clone III (HyClone, Logan, UT, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin under a controlled atmosphere (95% air and 5% CO2). LMH cells were cultured prior to reaching confluence and then incubated with the medium supplemented with either WY14643 (25, 50 or 100 μmol/L) or vehicle (dimethyl sulfoxide, 0.1% v/v) for 6 h. After removing the cell culture medium, cells were washed twice with cold phosphate buffered saline (PBS), and total RNA was extracted as described in the ‘Real-time PCR analysis’ section.
HepG2 was purchased from ATCC (ATCC # HB-8065). Cells were grown on six-well culture dishes in Dulbecco's modified Eagle's medium containing 10% Fetal Clone III (HyClone), 100 IU/mL penicillin, and 100 μg/mL streptomycin under a controlled atmosphere (95% air and 5% CO2). HepG2 cells were cultured prior to reaching confluence and then incubated with the medium supplemented with either 0 or 50 μmol/L WY14643 for 6 h. After removing the cell culture medium, cells were washed twice with cold PBS, and total RNA was extracted as described in the ‘Real-time PCR analysis’ section.
Animals and diet
Day-old male chicks of a Ross 308 broiler (Gallus gallus domesticus) were purchased from a local hatchery (Ishii Co., Ltd, Tokushima, Japan). They were maintained in an electrically heated brooder at 32°C ± 2°C with an automatically controlled 12-h light : dark cycle (18.00–06.00 hours). They were given free access to water and a commercial chick starter diet (Nippon Formula Feed Mfg. Co., Ltd, Kanagawa, Japan). This study was approved by the Institutional Animal Care and Use Committee and carried out according to the Kobe University Animal Experimentation Regulation.
Sampling and preparation
A total of 24 13-day-old male broiler chicks were weighed and allocated based on body weight to six cages (1725 mm × 425 mm × 320 mm, six birds in each group). Twelve chicks were fasted for 0 or 6 h prior to euthanasia by decapitation. The remaining 12 chicks were fasted for 12 h and refed for 0 or 6 h prior to euthanasia by decapitation. The liver was excised, weighed and frozen immediately using liquid nitrogen for real-time PCR analysis.
Real-time PCR analysis
Total RNA was extracted from the liver and hepatoma cells using Sepazol-RNA I (Nacalai Tesque, Inc., Kyoto, Japan). First-strand complementary DNA (cDNA) was synthesized from 2 μg of DNase I (Ambion Inc., Austin, Texas, USA)-treated total RNA using a ReverTra Ace® qPCR RT Kit (TOYOBO CO. LTD., Osaka, Japan) with random primers. Complementary DNAs of chicken PPARα, CPT1a, ACO and LCAD were amplified with the primers as previously described (Motoki et al. 2012; Saneyasu et al. 2013). Complementary DNAs of chicken MCAD (GenBank accession no. BM426980), SCAD (GenBank accession no. NM_001031246) and HAD (GenBank accession no. XM_418403) were amplified with the primers as follows: MCAD sense, 5′-GCG GAA GGG CGA TGA GT-3′; MCAD antisense, 5′-TCC GTT GGT GAT CCA CAT CTT-3′; SCAD sense, 5′-ATA TCC TCG GGC AGA TTG GA-3′; SCAD antisense, 5′-TGC CAC CAT TCA GCA TTC C-3′; HAD sense, 5′-CAA GCA ACA CTT CAT CCT TGC A-3′; HAD antisense, 5′-GTC CTG CCT GGT GGT TGA GT-3′. Complementary DNAs of chicken lipogenic genes such as sterol regulatory element-binding protein 1 (SREBP1), acetyl-coenzyme A carboxylase α, (ACCα), and fatty acid synthase (FAS) were also amplified with the primers as previously described (Saneyasu et al. 2013). As an internal standard, complementary DNA of chicken ribosomal protein S17 (RPS17) was also amplified with the primers as previously described (Ibuki et al. 2013). Complementary DNAs of human genes, such as CPT1a (GenBank accession no. NM_001031847), ACO (GenBank accession no. NM_001185039), LCAD (GenBank accession no. NM_001608), MCAD (GenBank accession no. NM_000016), SCAD (GenBank accession no. NM_000017), HAD (GenBank accession no. NM_001184705), PPARα (GenBank accession no. NM_001001928) and RPS17 (GenBank accession no. NM_001021), were amplified with the primers as follows: CPT1a sense, 5′-TGG TGG GCG TGA TGA CAA C-3′; CPT1a antisense, 5′-GAG TCC GAT TGA TTT TTG CAA TT-3′; ACO sense, 5′-TCT TCA CTT GGG CAT GTT CCT-3′; ACO antisense, 5′-TTC CAG GCG GGC ATG A-3′; LCAD sense, 5′-AGC TTA TGT GGA TGC CAG AGT TC-3′; LCAD antisense, 5′-TCT TGC AAT CAG CTC CTT CAT TAT-3′; MCAD sense, 5′-GCT GGT GCT GTT GGA TTA GCA-3′; MCAD antisense, 5′-CCT TTC CAG GGC ATA CTT GGT-3′; SCAD sense, 5′-TTT GCC AGC ACG GAC AGA-3′; SCAD antisense, 5′-GGA CCA GGA AGG CAC TGA TG-3′; HAD sense, 5′-GAT TCG CTG GCC TCC ATT T-3′; HAD antisense, 5′-TTT AAT GAC CTC CAC AAG TTT CAT G-3′; PPARα sense, 5′-AAC ATC CAA GAG ATT TCG CAA TC-3′; PPARα antisense, 5′-CCG TAA AGC CAA AGC TTC CA-3′; RPS17 sense, 5′-CGC CAT TAT CCC CTG CAA-3′; RPS17 antisense, 5′-CAG ATG CGT GAC ATA ACC TGC TA-3′. THUNDERBIRDTM SYBR® qPCR Mix was purchased from TOYOBO CO. LTD. (Osaka, Japan), and mRNA expression was quantified in duplicate using the Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) according to the supplier's recommendations.
Data analysis
Data from an in vitro experiment using LMH were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test. Other data were analyzed by Student's t-test. All statistics were performed using a commercial software package (StatView version 5; SAS Institute, Cary, NC, USA, 1998).
Results
We first examined the effects of WY14643 on fatty acid oxidation-related gene expression in chicken and human hepatoma cells. The mRNA levels of CPT1a, ACO, MCAD, SCAD, HAD and PPARα were significantly increased by WY14643 in HepG2 cells (Fig. 1). On the other hand, WY14643 significantly increased the mRNA levels of CPT1a and ACO, but not LCAD, MCAD, SCAD, HAD or PPARα in LMH cells (Table 1). We also analyzed the effects of WY14643 on lipogenic gene expression in chicken hepatoma cells because PPARα influenced the expression of lipogenic genes in mammals (Yoshikawa et al. 2003; König et al. 2009; Fernández-Alvarez et al. 2011). However, WY14643 did not influence the mRNA levels of SREBP1, FAS or ACCα in LMH cells (Table 1).
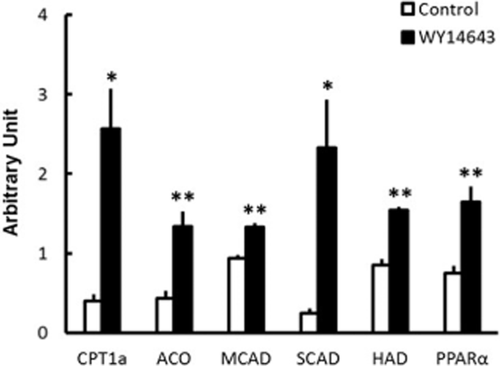
Effects of WY14643 on the messenger RNA levels of fatty acid oxidation-related genes in human hepatoma cells. Data are the means ± SEM. of four wells in each group. Data were analyzed by Student's t-test. *P < 0.05; **P < 0.01.
| Gene | WY14643 (μmol/L) | |||
|---|---|---|---|---|
| 0 | 25 | 50 | 100 | |
| PPARα | 0.97 ± 0.01 | 1.06 ± 0.05 | 0.99 ± 0.12 | 0.76 ± 0.07 |
| CPT1a | 0.66 ± 0.05b | 1.02 ± 0.16ab | 1.28 ± 0.20ab | 1.44 ± 0.20a |
| ACO | 1.38 ± 0.16b | 1.94 ± 0.37ab | 2.56 ± 0.22ab | 2.99 ± 0.42a |
| LCAD | 3.45 ± 0.20 | 4.58 ± 1.00 | 4.88 ± 0.11 | 4.83 ± 0.58 |
| MCAD | 0.38 ± 0.20 | 0.43 ± 0.10 | 0.49 ± 0.12 | 0.61 ± 0.15 |
| SCAD | 2.10 ± 0.10 | 2.10 ± 0.14 | 2.41 ± 0.37 | 1.98 ± 0.12 |
| HAD | 1.11 ± 0.11 | 1.13 ± 0.04 | 1.04 ± 0.03 | 0.87 ± 0.05 |
| SREBP1 | 0.67 ± 0.05 | 0.76 ± 0.10 | 0.97 ± 0.20 | 0.90 ± 0.11 |
| ACCα | 0.73 ± 0.08 | 0.67 ± 0.11 | 0.72 ± 0.20 | 0.82 ± 0.11 |
| FAS | 2.11 ± 0.22 | 2.26 ± 0.44 | 2.48 ± 0.20 | 2.41 ± 0.33 |
- Data are the means ± SEM. of four wells in each group. Data were analyzed by analysis of varaince followed by Tukey-Kramer test. Groups with different letters are significantly different (P < 0.05). PPARα, proliferator-activated receptor alpha; CPT1a, carnitine palmitoyltransferase 1a; ACO, acyl-coenzyme A oxidase; LCAD, long-chain acyl-coenzyme A dehydrogenase; MCAD, middle-chain acyl-coenzyme A dehydrogenase; SCAD, short-chain acyl-coenzyme A dehydrogenase; HAD, hydroxyacyl-coenzyme A dehydrogenase; ACCα, acetyl-coenzyme A carboxylase α; FAS, fatty acid synthase
We next examined the effects of fasting and refeeding on the mRNA levels of fatty acid oxidation-related genes in the liver in chickens. The mRNA levels of CPT1a, ACO and PPARα were significantly increased by fasting, whereas the mRNA levels of LCAD, MCAD, SCAD and HAD were not influenced by fasting (Fig. 2a). The mRNA levels of these enzymes were not changed by refeeding, whereas the mRNA levels of CPT1a, ACO and PPARα were significantly decreased (Fig. 2b).
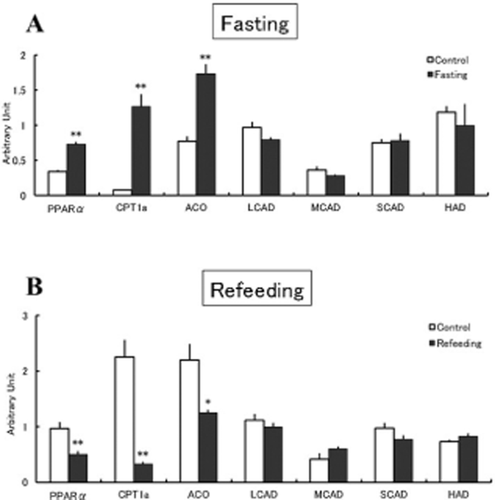
Effects of fasting (a) or refeeding (b) on the messenger RNA levels of fatty acid oxidation-related genes in the liver in chickens. Data are the means ± SEM. of six chicks in each group. Data were analyzed by Student's t-test. *P < 0.05; **P < 0.01.
Discussion
LCAD is a mitochondrial fatty acid oxidation enzyme the expression of which in humans is low or absent in organs known to utilize fatty acids for energy, such as heart, muscle and liver. (He et al. 2007; Chegary et al. 2009; Maher et al. 2010). In the present study, we analyzed the LCAD mRNA level in HepG2 cells, but cDNA of LCAD cannot be amplified by real-time PCR. Therefore, we showed the effects of fasting and WY14643 on LCAD mRNA levels in chicken hepatoma cells (Table 1) and liver (Fig. 2), but not in human hepatoma cells (Fig. 1).
The PPARα agonist WY14643 significantly increased the mRNA levels of CPT1a and ACO in chicken hepatoma cells (Table 1). The addition of the PPARα agonist clofibrate in the diet significantly increased the mRNA levels of CPT1a and ACO in the liver of laying hens (König et al. 2007a). In ovo injection of PPARα agonists GW7647 and perfluorooctane sulfonate significantly increased ACO mRNA levels in the liver of chicken embryos (Strömqvist et al. 2012). These findings and our results suggest that CPT1a and ACO are target genes of PPARα in chickens.
Mitochondrial fatty acid oxidation is the main mechanism providing energy from fatty acids in cells. Long-chain fatty acids are imported into the mitochondrial matrix after the transfer of acyl groups from coenzyme A to carnitine by CPT1a (Bartlett & Eaton 2004). After the transport of fatty acids from the cytosol to mitochondria, they are oxidized by the β-oxidation pathway, in which HAD is considered as the rate-limiting enzyme in mammals (McGarry et al. 1989; Eaton 2002). Very long-chain fatty acids were oxidized in peroxisomes, and ACO, the rate-limiting enzyme of peroxisomal fatty acid oxidation, is transcriptionally regulated by PPARα in mammals (Mandard et al. 2004). CPT1a, ACO, HAD, PPARα and gene for other β-oxidation-related enzymes including LCAD, MCAD, and SCAD, are known to be the target genes of PPARα in mammals (Schoonjans et al. 1996; Kersten et al. 1999; Mandard et al. 2004). Therefore, we examined the effects of WY14643 on the mRNA levels of these genes in chicken hepatoma cells. However, WY14643 did not influence the mRNA levels of LCAD, MCAD, SCAD, HAD or PPARα in chicken hepatoma cells (Table 1), although the mRNA levels of CPT1a and ACO were significantly increased. On the other hand, WY14643 significantly increased the mRNA levels of CPT1a, ACO, MCAD, SCAD, HAD and PPARα in human hepatoma cells (Fig. 1). PPARα agonists did not increase PPARα mRNA levels in laying hens and chicken embryos (König et al. 2007a; Strömqvist et al. 2012). These findings and our results suggest that the target genes of PPARα differ between chickens and humans.
In the present study, the mRNA levels of LCAD, MCAD, SCAD and HAD were unchanged by fasting (Fig. 2a) and refeeding (Fig. 2b) in chickens, although the mRNA levels of CPT1a and ACO were significantly changed. CPT1a and ACO function as rate-limiting enzymes for mitochondrial and peroxisomal fatty acid oxidation, respectively, in mammals (Mandard et al. 2004). Our findings suggest that the transcriptional change of CPT1a and ACO plays an important role in hepatic fatty acid oxidation in chickens.
We previously showed that the mRNA levels of CPT1a and ACO were not increased by 2 h of fasting in chickens (Saneyasu et al. 2013), although the PPARα mRNA level was increased. In the present study, the PPARα agonist WY14643 significantly increased the mRNA levels of CPT1a and ACO, but not PPARα, in chicken hepatoma cells (Table 1), suggesting that PPARα itself is not the target gene of PPARα. All our findings clearly demonstrate that the regulatory mechanisms of fasting-induced gene expression differ between PPARα and its target genes CPT1a and ACO.
Fatty acid is known to be one of the natural ligands of PPARα (Bocos et al. 1995). During fasting, fatty acids are released from white adipose tissue and travel to the liver, where they bind and activate PPARα, which results in stimulation of the fatty acid oxidative pathway in the liver (Mandard et al. 2004). In fact, a significant increase in the mRNA level of PPARα at 8 h of fasting occurred after the significant increase of plasma non-esterified fatty acid (NEFA) concentration at 4 h of fasting in rats (Palou et al. 2008). However, we recently found that PPARα mRNA levels were significantly increased after 2 h of fasting in chickens, although the plasma NEFA concentration was not significantly changed (Saneyasu et al. 2013). In the present study, WY14643, an artificial ligand of PPARα, did not influence the mRNA level of PPARα in chicken hepatoma cells (Table 1), unlike in human hepatoma cells (Fig. 1). These findings suggest that fasting-induced PPARα gene expression in the liver does not depend on elevation of plasma NEFA concentration in chickens.
The transcription factor SREBP1 plays important roles in regulation of the expression of lipogenic genes including ACCα and FAS in the liver in both mammals and chickens (Shimano 2009; Wang et al. 2009). However, several differences between animal species have been demonstrated previously in terms of the regulation of lipogenic gene expression by PPARα. For example, WY14643 increased the mRNA levels of SREBP1 and FAS in rat hepatoma cells (König et al. 2009). The overexpression of PPARα in HEK293 cells has been shown to inhibit mouse SREBP1 promoter activity (Yoshikawa et al. 2003). In humans, PPARα seems to be an activator of the SREBP1 promoter (Fernández-Alvarez et al. 2011). However, in the present study we showed that WY14643 did not influence the mRNA levels of SREBP1, ACCα or FAS in chicken hepatoma cells (Table 1). Our findings provide new insight into the species-specific mechanism underlying the regulation of hepatic lipogenesis.
In summary, the PPARα agonist WY14643 significantly increased the mRNA levels of PPARα, MCAD, SCAD and HAD in human hepatoma cells, but not in chicken hepatoma cells. Fasting significantly increased the mRNA levels of PPARα, CPT1a and ACO, but not MCAD, SCAD or HAD, in chicken liver. These results suggest that the mechanisms underlying the transcription of fatty acid oxidation-related genes in the liver might differ between humans and chickens.




