Differential expression of genes encoding neurotrophic factors and their receptors along the septal-temporal axis of the rat hippocampus
Abstract
The hippocampus plays a key role in learning and emotional regulation. The hippocampus’ function varies along its septotemporal axis, with the septal pole being more frequently involved in spatial learning and memory, and the temporal pole playing a greater role in emotional behaviors. In this study, we present findings aimed at checking the expression level of the genes encoding neurotrophins and their receptors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and their receptors (TrkA, TrkB and TrkC) in the hippocampus along the septotemporal axis. Using real-time PCR, several different expression patterns were observed. Remarkably, the expression of both NT-3 and TrkA genes in the septal hippocampus was higher than in the middle and temporal hippocampus. Higher expression of NT-3 and TrkA may implicate active neurogenesis in the dentate gyrus (DG) of the septal hippocampus because more neurogenesis occurs in the septal than the temporal DG of rats. Finally, the results obtained in this study emphasize the importance of choosing the hippocampal portion along its septotemporal axis for any hippocampal molecular and biochemical experimental studies.
Introduction
The hippocampus plays various roles in brain functions, including memory and learning (Ramirez et al. 2013), and it is also related to emotional disorders, such as depression and schizophrenia (Miyakawa et al. 2003; Tsankova et al. 2006). Also, the hippocampus of farm animals has been analyzed, because the hippocampus is an important region for stress regulation (De Vry et al. 2012). Therefore, the hippocampus has been well-studied by many researchers using various experimental techniques, including molecular and biochemical procedures. Recently, comprehensive techniques, such as proteomics, transcriptomics and metabolomics, have been frequently used to study the mechanisms underlying hippocampal functions (Sugiura et al. 2011; Cajigas et al. 2012). The hippocampus has specific morphological organizations, such as the dentate gyrus (DG), CA3, CA2 and CA1, and many genes are expressed differentially in the regions of the hippocampus (Yoshioka et al. 2012). Thus, precise evaluation of the expression of hippocampal genes should be undertaken following accurate isolation techniques of specific hippocampal regions. For example, molecular studies in the hippocampal DG have been performed using simple but sophisticated dissection techniques to isolate the DG from other hippocampal regions (Hagihara et al. 2009). Commonly, for molecular studies, RNA is isolated from the whole hippocampus, and gene expression was determined using molecular techniques such as real-time PCR techniques (Tsankova et al. 2006). Several studies have revealed that the hippocampus is not a homogenous structure but instead has different properties and functions associated with its septotemporal subregions (Bannerman et al. 2004; Fanselow & Dong 2010). Generally, lesions directed at the septal pole damage spatial learning and memory, whereas lesions of the temporal pole have been determined to be anxiolytic (Moser et al. 1995; Kjelstrup et al. 2002). A genome-wide atlas of gene expression in the adult mouse brain revealed that heterogeneous gene expression in the hippocampus is common (Lein et al. 2007). Molecular expression pattern in the hippocampus of lager animals may be more complicated. Although the information about the hippocampal molecular expression of larger animals is limited, recently the different expression pattern of neurotrophin was observed in the pig hippocampus along its dorsal-ventral axis under stressful conditions (De Vry et al. 2012). Thus, the determination of gene expression in the hippocampal subregions is necessary for the precise evaluation of hippocampal molecular functions.
In this study, we focused on the major neurotrophins and their receptors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and their receptors (TrkA, TrkB and TrkC) because these genes and proteins in the hippocampus have been well studied and characterized. NGF binds to neurotrophic tyrosine kinase receptor type 1 (TrkA) and plays an important role in neuronal cell survival (Levi-Montalcini 1987; Smeyne et al. 1994). BDNF binds to neurotrophic tyrosine kinase receptor type 2 (TrkB) and plays roles in the growth of dendrites and spines (Nibuya et al. 1995). NT-3 binds to TrkA, TrkB and neurotrophic tyrosine kinase receptor type 3 (TrkC) and plays roles in neuronal differentiation and survival (Lamballe et al. 1991). Because the previous study showed that transport stress decreased hippocampal BDNF expression in pigs (Wei et al. 2010), we should pay more attention to the expression of hippocampal neurotrophins and their receptors in farm animals to evaluate their conditions under several stresses.
Currently, only a small volume of RNA samples is required for commercial RNA purification kits. Thus, only a small amount of tissue (approximately 10-20 mg wet weight) is needed for purification. Thus, it is not necessary for the usual molecular research to extract RNA from the whole hippocampus (approximately 200 to 300 mg wet weight in rats) from the point of view of cost saving. Therefore, it is important to choose the subregions of the hippocampus to isolate RNA for evaluation of gene expression, especially in large animals. In this study, we divided the rat hippocampus into six portions along its septal-temporal axis (Fig. 1) and extracted the total RNA from each portion following the evaluation of expression, including genes encoding NGF, BDNF, NT-3, TrkA, TrkB and TrkC using real-time PCR techniques.
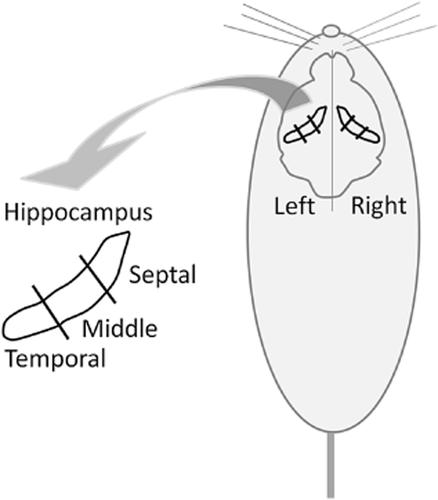
Line drawing of the rat brain shows the septotemporal axis of the hippocampal formation. Whole rat hippocampus was divided into six portions ((right or left side) × (septal, middle or temporal parts)). Each portion was subjected to total RNA extraction.
Materials and Methods
Animals
Five-week-old male Wistar rats were purchased from Charles River (Yokohama, Japan) and housed individually at room temperature (22 ± 1°C), with lights on from 06.00 to 18.00 hours with ad libitum access to food and water. After arrival, they were handled daily for 1 week to habituate them to the environment. The rats were fed a normal powder diet (MF; Oriental Yeast, Tokyo, Japan) for 5 weeks. All experimental procedures followed the guidelines of the Animal Care and Use Committee of Ibaraki University.
Brain sampling
The rats were anesthetized and decapitated. The hippocampus was excised from the brain, and six portions ((right or left side) × (septal, middle or temporal parts)) were separated by sterilized scissors as soon as possible (Fig. 1). Each portion was collected into a sterilized microtube, immediately soaked into liquid nitrogen, and then stored in a deep freezer at −80°C until analysis.
cDNA preparation and real-time PCR
The methods of total RNA extraction and the synthesis of complementary DNA (cDNA) are described elsewhere (Inoue et al. 2008; Tsukahara et al. 2010). Briefly, total RNA was extracted from the respective portions of the hippocampal samples using the QuickGene RNA tissue kit SII (KURABO Industries, Tokyo, Japan) and RNA extraction kit for use with a semi-automated nuclear acid extraction machine (QuickGene810; FujiFilm, Tokyo, Japan). The concentration of the extracted total RNA was measured with a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and 500 ng of the total RNA was used for reverse transcription using a ReverTraAce kit (TOYOBO, Osaka, Japan) with oligo (dT)20 and random primers. All procedures were performed according to the manufacturer's instructions.
Real-time PCR was performed using a Rotor-Gene 6200 (Qiagen, Tokyo, Japan). The primers and Taqman probes used in this study are listed in Table 1. The optimal primers and probes were designed and selected at https://lifescience.roche.com/shop/home. The methods of PCR analyses were similar as previously described (Ushida et al. 2010). Briefly, the expression level of a housekeeping gene, such as glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh), for all samples was evaluated, and the cDNA samples were diluted with ultrapure distilled water (Life Technologies, Tokyo, Japan) to obtain the same Gapdh expression level. PCR was performed with a thermal cycle program with an initial denaturation at 95°C for 30 s followed by 50 cycles of 95°C for 4 s and 60°C for 25 s. The relative expression levels of the mRNAs were calculated by the comparative Ct method (Nishimura et al. 2003). The delta Ct value, the amount of target relative to housekeeping mRNA for each sample, was determined for comparison among the hippocampal segment.
| Gene name | Primers 5′-3′ | Probe number | GenBank accession number |
|---|---|---|---|
| beta-nerve growth factor (NGF) | F tgcagtctcccacctctga | 64 | M36589.1 |
| R ccagggagttcccaatatcc | |||
| Brain-derived neurotrophic factor (BDNF) | F agcgcgaatgtgttagtggt | 92 | NM_012513.3 |
| R gcaattgtttgcctctttttct | |||
| Neurotrophin-3 (NT-3) | F cgacgtccctggaaatagtc | 29 | NM_031073.2 |
| R tggacatcaccttgttcacct | |||
| Neurotrophic tyrosine kinase, receptor, type 1 (TrkA) | F tgtccaagtcagcgtctcc | 20 | NM_021589.1 |
| R caccagtgatgctgttccac | |||
| Neurotrophic tyrosine kinase, receptor, type 2, transcript variant 2 (TrkB) | F cgaggttggaacctaacagc | 115 | NM_001163168.1 |
| R ccttttctggtttgcaatgag | |||
| Neurotrophic tyrosine kinase, receptor, type 3 (TrkC) | F catgtccagggacgtctaca | 42 | NM_019248.1 |
| R cggtacatgatgctttcagg |
- Listed probe numbers indicate the product number of the Universal ProbeLibrary Set, Human and Extension Set sold by Roche Applied Science.
Statistical analysis
Data were analyzed using StatView for Windows (SAS Institute Inc., Cary, NC, USA). All data were analyzed by two-way analysis of variance (ANOVA) following a Tukey-Kramer Honestly Significant Difference (HSD) test. The differences were considered statistically significant when P < 0.05.
Results and Discussion
Expression of neurotrophin genes
To elucidate the expression difference of neurotrophin genes encoding NGF, BDNF and NT-3 in the hippocampus, the rat hippocampus was divided to six portions: right-septal, left-septal, right-middle, left-middle, right-temporal and left-temporal (Fig. 1). The total RNA was extracted from each portion and subjected to real-time PCR for evaluating the expression of the genes as described above. The relative expression of the Ngf, Bdnf and Nt-3 genes is shown in Figure 2A–C. As shown in Figure 2A, there was no significant difference of Ngf gene expression in each portion of the hippocampus. A two-way ANOVA revealed that the hippocampal region (F(2, 42) = 3.086, P = 0.0562) and laterality (F(1, 42) = 1.523, P = 0.2240) and the region × laterality (F(2, 42) = 0.372, P = 0.6914) had no significant effect on the expression of the Ngf gene. On the other hand, there were significant differences in the Bdnf and Nt-3 gene expressions of the portions of the hippocampus (Fig. 2B,C). The hippocampal region (F(2, 42) = 7.592, P = 0.0015) had a significant effect on the expression of Bdnf genes but not laterality (F(1, 42) = 2.117, P = 0.1531) and the region × laterality (F(2, 42) = 1.876, P = 0.1659). Subsequently, Tukey-Kramer HSD post-hoc tests revealed a significant difference in Bdnf gene expression between the septal and middle hippocampus (P < 0.05) (Fig. 2B). Moreover, the hippocampal region (F(2, 42) = 13.188, P < 0.0001) had a significant effect on the expression of Nt-3 genes but not laterality (F(1, 42) = 0.217, P = 0.6435) and the region × laterality (F(2, 42) = 1.976, P = 0.1513). Subsequently, Tukey-Kramer HSD post-hoc tests revealed a significant difference in Nt-3 gene expression between the septal and middle hippocampus and between the septal and temporal hippocampus (P < 0.05) (Fig. 2C). Finally, the Ngf, Bdnf and Nt-3 gene expressions were not different between the left and right hippocampus (Fig. 2A–C).
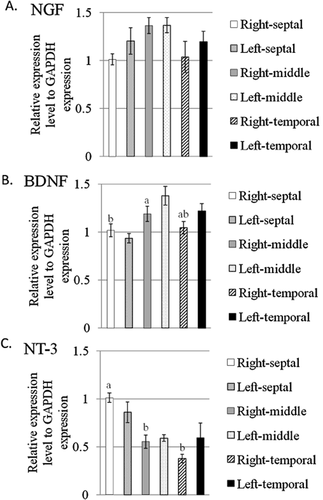
Expression of the genes encoding nerve growth factor (NGF) (A), brain-derived neurotrophic factor (BDNF) (B), and neurotrophin-3 (NT-3) (C) in the septotemporal portions of the hippocampus. All data are expressed as the means ± SEM (n = 8). Columns not sharing the same superscript are significantly different among the portions (P < 0.05; two-way analysis of variance, post-hoc comparison by Tukey-Kramer's Honestly Significant Difference test). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Expression of neurotrophin receptor genes
Next, we elucidated the expression differences of neurotrophin receptor genes encoding TrkA, TrkB and TrkC in the hippocampus. The relative expressions of TrkA, TrkB and TrkC genes are shown in Figure 3A–C. The hippocampal region (F(2, 42) = 22.091, P < 0.0001) and the region × laterality (F(2, 42) = 3.662, P = 0.0342) had significant effects on the expression of TrkA genes but not laterality (F(1, 42) = 0.005, P = 0.9459). Subsequently, Tukey-Kramer HSD post-hoc tests revealed a significant difference in TrkA gene expression between the septal and middle hippocampus and between the septal and temporal hippocampus (P < 0.05) (Fig. 3A). The hippocampal region (F(2, 42) = 5.529, P = 0.0074) had a significant effect on the expression of TrkB genes but not laterality (F(1, 42) = 0.184, P = 0.6703) and the region × laterality (F(2, 42) = 1.426, P = 0.2516). Subsequently, Tukey-Kramer HSD post-hoc tests revealed a significant difference in TrkB gene expression between the septal and temporal hippocampus (P < 0.05) (Fig. 3B). As shown in Figure 3C, there was no significant difference of TrkC gene expression in each portion of the hippocampus. A two-way ANOVA revealed that the hippocampal region (F(2, 42) = 0.475, P = 0.6253), laterality (F(1, 42) = 1.768, P = 0.1908) and the region × laterality (F(2, 42) = 0.531, P = 0.5919) had no significant effect on the expression of the TrkC gene (Fig. 3C). Finally, TrkA, TrkB and TrkC gene expression was not different between the left and right hippocampus (Fig. 3A–C).
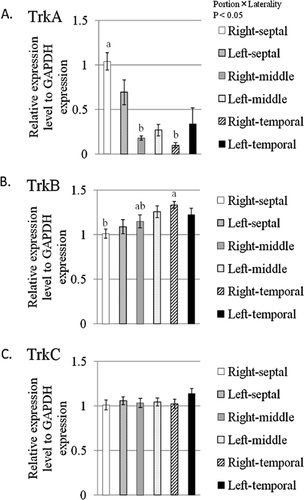
Expression of the genes encoding neurotrophic tyrosine kinase, receptor, type 1 (TrkA) (A), TrkB (B) and TrkC (C) in the septotemporal portions of the hippocampus. All data are expressed as the means ± SEM (n = 8). Columns not sharing the same superscript are significantly different among the portion (P < 0.05; two-way analysis of variance, post-hoc comparison by Tukey-Kramer's Honestly Significant Difference test).
Hippocampal neurotrophins and their receptors have various important roles in neuronal development, neurogenesis and differentiation. These molecules in the hippocampus have been related to learning and memory, emotion, and stress management. Therefore, the precise determination of the expression of these genes is essential for research into the aforementioned areas. The hippocampus has a functional heterogeneity along the septal-temporal axis (Bannerman et al. 2004). Lesion studies in rats suggest that functions related to memory and anxiety regulation may be distributed across the septal-temporal axis (Bannerman et al. 2004). Lesions of the septal hippocampus induce spatial learning deficit without affecting anxiety, whereas temporal lesions affect anxiety without affecting spatial navigation (Bannerman et al. 1999, 2002; Kjelstrup et al. 2002; McHugh et al. 2004). The morphology is another aspect of hippocampal heterogeneity. Septal and temporal regions receive distinct, separable inputs and send their projections to distinct targets. Visual, auditory and sensory association cortices project primarily to the septal hippocampus via the lateral entorhinal cortex, whereas olfactory input is distributed evenly across the septal-temporal axis (Kosel et al. 1981; Witter et al. 1989; Burwell & Amaral 1998). Regarding efferent connections, the temporal hippocampus projects directly to the prefrontal cortex, the amygdala and the shell of the nucleus accumbens, whereas the septal hippocampus does not (Pitkanen et al. 2000). Moreover, the maturation of adult-born neurons in the DG varies in its septal-temporal axis (Snyder et al. 2012). As described above, the different expression levels of Bdnf, Nt-3, TrkA and TrkB have been observed in the hippocampal portions, but left-right asymmetry of hippocampal gene expression of Ngf, Bdnf, Nt-3, TrkA, TrkB and TrkC was not observed in this study (Figs 2, 3). The left-right asymmetry of the hippocampal synapses was observed following differential expression of N-methyl-D-aspartate (NMDA) receptor type B and spine morphology (Shinohara et al. 2008). Thus, precise histological comparisons of the genes encoding neurotrophins and their receptors in the left-right hippocampus should be assessed in future studies. Gene expressions of Nt-3 and TrkA in the septal hippocampus were higher than the middle and temporal hippocampus as shown in Figures 2C and 3A. Because the expression of Nt-3 is largely confined to the hippocampal DG, Nt-3 signals may be up-regulated in the DG of the septal hippocampus (Shimazu et al. 2006). As described above, the septal hippocampus plays an essential role in spatial learning and memory, and up-regulation of Nt-3 and TrkA genes may be profoundly related to spatial navigation ability. Actually, Shimazu et al. (2006) described that NT-3 in the hippocampal DG facilitates spatial memory by regulating neurogenesis. Moreover, previous studies have demonstrated that more neurogenesis occurs in the septal than temporal DG of both rats and mice (Snyder et al. 2009; Jinno 2011). Because NT-3 weakly binds to TrkA, the higher expression of both Nt-3 and TrkA genes may be reflected by the frequent neurogenesis in the DG of the septal hippocampus.
The comparative studies for the hippocampus in larger animals such as domestic animals are important to proceed our knowledge about the human and animal brains, because the brain structure of domestic animals is more similar to the human brain compared to rodents. In particular, the brains of domestic animals are more suitable for studies to elucidate the biological significance of local gene and protein expression in the brain, because of their brain size and due to welfare issues. In the future, we should collect more information about brain function and gene expression in domestic animals.
Conclusion
Here, we observed the different gene expressions of neurotrophins and their receptors along the septal-temporal axis of the rat hippocampus. Therefore, we should take care of tissue-sampling for molecular and biochemical analyzes, especially from the hippocampus of larger animals.
Acknowlegment
We would like to thank Dr. Hiroko Toyoda (Ibaraki University) for helpful comments regarding the manuscript. This research was supported in part by Grants-in-Aid for no. 22580303 to A.T and the research program ‘Ibaraki University Cooperation between Agriculture and Medical Science (IUCAM)’ to A.T and T.G. from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the School of Agriculture, Ibaraki University to A.T.




