Determination of bacteria constituting ruminal fibrolytic consortia developed on orchard grass hay stem
Abstract
To determine the relationship between Fibrobacter succinogenes and other rumen bacteria, the bacterial community structure on fiber was analyzed by using two different materials. These were ruminally incubated orchard grass hay stems without and with preincubation with F. succinogenes (natural and artificial consortia, respectively). The natural consortium mainly consisted of Firmicutes (56.6%) and Bacteroidetes (33.1%), while the artificial consortium showed a significantly higher proportion of Firmicutes (85.5%) and a lower proportion of Bacteroidetes (4.6%). At species or genus level, Butyrivibrio fibrisolvens, the U2 group, Ruminococcus albus and Lachnospiraceae incertae sedis made up a higher proportion in the artificial consortium. The most dominant bacterial group was the Butyrivibrio-Pseudobutyrivibrio-Lachnospiraceae incertae sedis group, which accounted for 19.7% in the natural and 29.5% in the artificial consortium. Within the genus Butyrivibrio, the phylogenetic groups SA and VA2 and phylogeny-undefined Butyribivrio, but not VA1, were detected at high frequency in the artificial consortium. These results suggest that ecological and possibly functional relationships exist in the rumen among F. succinogenes, a subset of B. fibrisolvens, the U2 group, R. albus and Lachnospiraceae incertae sedis.
Introduction
The phylum Fibrobacteres is recognized in various environments including the rumen, soil, seawater, landfills, lake water and termite guts, and there is interest in its contributions to material cycles (McDonald et al. 2009; Ransom-Jones et al. 2012). In the phylum Fibrobacteres, the rumen-habiting species Fibrobacter succinogenes, especially phylogenetic group 1 (Amann et al. 1992) is considered to have a prominent role in the degradation of plant polysaccharides (Michalet-Doreau et al. 2002; Shinkai et al. 2007). On the plant material in the rumen, a number of bacterial cells associate and develop functional partnerships for nutrient processing, which is called a consortium (Koike et al. 2003; Brulc et al. 2009). F. succinogenes is frequently observed with other, morphologically different bacterial cells on plant materials digested in the rumen (Cheng et al. 1984; Shinkai & Kobayashi 2007).
To reveal aspects of the cooperative interaction, several bacteria, archaea and fungi have been co-cultured with F. succinogenes, and some of the co-cultures synergistically accelerated fiber digestion (Miron et al. 1994; Rychlik & May 2000; Chen & Weimer 2001; Fukuma et al. 2012). However, the ecological and physiological importance of these microbes and F. succinogenes are unproven. It is difficult to determine the consortium member actually working with F. succinogenes in the rumen, because most rumen bacteria have not yet been cultured (Edwards et al. 2004). In the present study, two different fibrolytic consortia were experimentally prepared and analyzed to identify the bacteria potentially involved with F. succinogenes in fiber digestion.
Materials and Methods
Bacteria and animal
F. succinogenes S85 (ATCC 19169), a type strain of F. succinogenes, belonging to its phylogenetic group 1, was purchased from the American Type Culture Collection. Succiniclasticum ruminis SE10 (DSMZ9236) was purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. The F. succinogenes strain was anaerobically maintained in Avicel medium (Shinkai et al. 2010) in a Hungate tube at 37°C. S. ruminis was cultured the same way but in Succiniclasticum-specific medium (Medium 666 offered by DSMZ).
A ruminally fistulated wether (73.0 kg in body weight), which had been fed orchard grass hay ad libitum and 200 g of concentrate (Monster 16; Mercian, Tokyo, Japan) once daily (08.30 hours) for 2 months, was employed for a nylon bag experiment and rumen fluid collection (see below). The wether received appropriate care as described in the Hokkaido University Guidelines for Animal Experiments.
Natural and artificial consortia
The details for preparation of natural and artificial consortia (defined below) were previously described (Shinkai et al. 2010). In brief, 2 g of orchard grass hay stem in 15 mm lengths, cut longitudinally into two to three pieces, were enclosed in a nylon mesh bag (5 × 10 cm with 50 μm pore size). The nylon bag was immersed in the rumen of the sheep before morning feeding and incubated for 24 h. The incubated stems were washed twice with 10 mL of anaerobic dilution solution (Bryant & Burkey 1953) and used as the material on which the fibrolytic consortium was naturally formed (natural consortium).
Another type of consortium was prepared as follows: F. succinogenes cells grown on Avicel and filter paper were transferred into 5 mL of basal medium containing 0.1 g of orchard grass hay stem (4–6 pieces per tube). The culture was incubated for about 20 h until the culture supernatant became turbid. Three pieces of the incubated hay stems were withdrawn from the culture, and the excess culture medium was removed. At this stage, coverage of the inner surface of the hay stems by F. succinogenes cells was confirmed by microscopy. Then, the stems covered by F. succinogenes were incubated in the tube for 24 h with 5 mL of rumen fluid taken before morning feeding and filtered through two layers of cheesecloth to form the fibrolytic consortium. After incubation, the stems were withdrawn from the culture, washed twice with 10 mL of anaerobic dilution solution and used as the material on which the fibrolytic consortium was artificially formed (artificial consortium). The hay stems on which either the natural or artificial consortium developed were stored at −80°C until DNA extraction.
Preparation of the natural and artificial consortia was conducted at the same time and repeated on three different days for a total of three replicates of each consortium for analysis.
DNA extraction
Total bacterial DNA from the hay stems with consortia was extracted by bead-beating with surfactant as previously described (Shinkai et al. 2010). The extracted DNA was purified by chromatography on hydroxyapatite Bio-Gel HTP Gel (Bio-Rad, Hercules, CA, USA) followed by gel filtration through Microspin S-200R HR Columns (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The purified DNA was quantified with a DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech, San Francisco, CA, USA). DNA from each sample (10 ng) was pooled equally (30 ng in total for three replicates of each consortium) and used for the construction of the clone library, while DNA from each sample was used for real time PCR analysis.
Clone library and sequence analysis
The bacterial 16S rRNA gene (16S rDNA) in natural and artificial consortia was amplified by PCR with bacterial universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1525R (5′-AAGGAGGTGWTCCARCC-3′) using a TaKaRa Ex-taq polymerase system (Takara Bio Inc., Yokkaichi, Japan). The PCR conditions were as follows: 15, 25 and 35 cycles of 94°C for 30 s for denaturation, 58°C for 30 s for annealing and 72°C for 90 s for extension, except for 5 min denaturation in the first cycle and 7 min extension in the last cycle. Since the target PCR product was visually confirmed by electrophoresis only for 35 PCR cycles, the cycle for the clone library construction was set at 35. The PCR product was electrophoretically separated in an agarose gel (Nusieve GTG agarose, BioWhittaker Molecular Application, Rockford, ME, USA), purified using a QIAEX II Gel Extraction Kit (QIAGEN, Hilden, Germany), and ligated to vector pGEM-T Easy (Promega, Madison, WI, USA). The ligation product was introduced into Escherichia coli JM109 and screened on a LB-agar plate with 100 μg/mL ampicillin, 80 μg/mL X-Gal (5-bromo-4-chloro-3-indolyl- β -D-galactopyranoside) and 0.5 mmol/L IPTG (isopropyl-β-D-thiogalactopyranoside). White colonies were randomly selected from each clone library to isolate plasmids with the Wizard Plus SV miniprep system (Promega). Plasmid inserts were sequenced using an Applied Biosystems 3730xl DNA Analyzer at The Dragon Genomics Center (Takara Bio). The sequences were compared with those available in the DNA database using the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/) and RDP Classifier tool available at the Ribosomal Database Project (RDP, http://rdp.cme.msu.edu/). A phylogenetic tree was drawn with the multiple sequence alignment tool CLUSTAL W and the neighbor-joining method via the DDBJ (http://www.ddbj.nig.ac.jp/index-e.html). The 16S rDNA sequences and accession numbers of reference strains were obtained from the GenBank database.
Primer design and real-time PCR
Primers for Succiniclasticum ruminis were newly designed. The 16S rDNA sequences of related bacteria were imported to the CLC Main Workbench (CLC bio, Aarhus, Denmark) for complete alignment and selection of species-specific sequences targeting S. ruminis. The designed primer sequences Succinic180Fw (5′-AACCTGCCCCCCGGATT-3′) and Succinic530Rv (5′-TACCGTCATTGCTTCCCGTTG-3′) were checked for self-complementary sequence according to Kibbe (2007) and compared with sequences available in the NCBI database via the BLAST program and Probe Match tool available at the RDP. To check experimental specificity, the primer set was tested in PCR using 28 rumen bacterial strains (Mitsumori et al. 2003).
The real-time PCR assay was conducted with the standard curve method using a serially diluted standard template (Applied Biosystems, User Bulletin #2 2001). Preparation of template plasmid was as described by Koike et al. (2007). In brief, the 16S rDNA of strain S. ruminis SE10 was PCR-amplified with the specific primer set described above and ligated into vector pCR2.1 (Invitrogen, Carlsbad, CA, USA). The ligated plasmid was cloned into E. coli INVαF’ (Invitrogen). Isolated purified plasmid with the correct insert was quantified using the DyNA Quant 200 fluorometer.
Real-time PCR was performed with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) and the LightCycler system (Roche, Mannheim, Germany). The primers and their targets are presented in Table 1. The amplification conditions of Koike et al. (2007), Boeckaert et al. (2008) and Ohene-Adjei et al. (2008) were followed. The melting curve of PCR products was monitored by heating from 70°C to 95°C at 0.1°C intervals at the end of the real-time PCR to check the specific amplification.
| Target species/group | Sequence (5′ → 3′) | Annealing temp. (°C) | Reference | |
|---|---|---|---|---|
| Total bacteria | Fw | CCTACGGGAGGCAGCAG | 60 | Muyzer et al. 1993. |
| Rv | ATTACCGCGGCTGCTGG | |||
| Fibrobacter succinogenes | Fw | GGTATGGGATGAGCTTGC | 60 | Tajima et al. 2001. |
| Rv | GCCTGCCCCTGAACTATC | |||
| Butyrivibrio-Pseudobutyrivibrio-Lachnospiraceae incertae sedis group | Fw | GYGAAGAAGTATTTCGGTAT | 55 | Boeckaert et al. 2008. |
| Rv | CCAACACCTAGTATTCATC | |||
| Ruminococcus albus | Fw | CCCTAAAAGCAGTCTTAGTTCG | 55 | Koike et al. 2007. |
| Rv | CCTCCTTGCGGTTAGAACA | |||
| Ruminococcus flavefaciens | Fw | TCTGGAAACGGATGGTA | 55 | Koike et al. 2007. |
| Rv | CCTTTAAGACAGGAGTTTACAA | |||
| Succiniclasticum ruminis | Fw | AACCTGCCCCCCGGATT | 60 | This study |
| Rv | TACCGTCATTGCTTCCCGTTG | |||
| Selenomonas ruminantium-Mitsuokella multiacida group | Fw | TGCTAATACCGAATGTTG | 57 | Tajima et al. 2001. |
| Rv | TCCTGCACTCAAGAAAGA | |||
| Group U2 | Fw | CTAGGTGTAGGGGGTATC | 60 | Koike et al. 2010. |
| Rv | GCTGCCCTCTGTCGTTG | |||
| Treponema bryantii | Fw | AGTCGAGCGGTAAGATTG | 57 | Tajima et al. 2001. |
| Rv | CAAAGCGTTTCTCTCACT | |||
| Methanogenic archaea | Fw | GAGGAAGGAGTGGACGACGGTA | 63 | Ohene-Adjei et al. 2008. |
| Rv | ACGGGCGGTGTGTGCAAG |
Statistical analyses
The library comparison program in RDP was run to compare the compositional differences between the natural and artificial consortia by setting the confidence threshold values at 80%. Differences were considered to be significant at P < 0.05. The relative proportion of bacterial species or phylogenetic groups out of the total bacteria was calculated and compared based on 16S rDNA copy number using Student's t-test. Statistical significance was defined as P < 0.05.
Nucleotide sequence accession numbers
The 16S rDNA sequences obtained from natural and artificial consortia were deposited in DDBJ nucleotide sequence databases under accession numbers AB494738 through AB494959 and AB781606 through AB781655.
Results
The taxonomic profile of 16S rDNA clone libraries constructed from natural and artificial consortia are shown in Table 2. A total of 267 sequences were obtained (136 sequences from the natural consortium and 131 from the artificial consortium). Due to this limited number of sequences employed, the library analysis was semi-comprehensive but supposed to provide compositional information of bacterial consortia.
| Taxonomic rank | Proportions in each clone library (%) | Library comparisona | |
|---|---|---|---|
| Natural consortium | Artificial consortium | ||
| Phylum Firmicutes | 56.6 | 85.5 | * |
| Order Clostridiales | 55.1 | 85.5 | * |
| Family Lachnospiraceae | 22.1 | 64.9 | * |
| Genus Butyrivibrio | 5.1 | 22.1 | * |
| Genus Pseudobutyrivibrio | 3.7 | 4.6 | NS |
| Genus Lachnospiracea incertae sedis | 0.0 | 5.3 | * |
| unclassified Lachnospiraceae | 11.8 | 32.1 | – |
| Family Ruminococcaceae | 15.4 | 13.0 | NS |
| Genus Ruminococcus | 2.2 | 3.1 | NS |
| Genus Saccharofermentans | 5.1 | 7.6 | NS |
| unclassified Ruminoccaceae | 8.1 | 1.5 | – |
| Family Veillonellaceae | 6.6 | 4.6 | NS |
| Genus Succiniclasticum | 2.2 | 1.5 | NS |
| Genus Selenomonas | 1.5 | 0.0 | NS |
| unclassified Clostridiales | 11.0 | 3.1 | – |
| Phylum Bacteroidetes | 33.1 | 4.6 | * |
| Order Bacteroidales | 31.6 | 3.8 | * |
| Family Prevotellaceae | 0.7 | 0.0 | NS |
| Family Porphyromonadaceae | 0.7 | 0.0 | NS |
| unclassified Bacteroidales | 30.1 | 3.1 | – |
| Phylum Spirochaetes | 5.1 | 5.3 | NS |
| Phylum Proteobacteria | 2.2 | 1.5 | NS |
| Phylum Planctomycetaceae | 0.7 | 0.0 | NS |
| Phylum Synergistetes | 0.0 | 0.8 | NS |
| Phylum Fibrobacteres | 0.0 | 0.0 | NS |
| Unclassified bacteria | 2.2 | 2.3 | – |
- a Library comparison program in the Ribosomal Database Project: Release 10 (http://rdp.cme.msu.edu/index.jsp) was used, setting the confidence threshold values at 80%. Values less than 0.05 were considered significant and expressed as ‘*’. ‘NS’, not significant; ‘–’, not available.
The natural consortium was comprised of the phylum Firmicutes (56.6%) and Bacteroidetes (33.1%), followed by Spirochaetes (5.1%) and Proteobacteria (2.2%). More than half of the clones in the natural consortium consisted of unclassified bacterial clones, including unclassified Bacteroidales (30.1%), unclassified Lachnospiraceae (11.8%), unclassified Clostridiales (11.0%) and unclassified Ruminococcaceae (8.1%). Clones affiliated with Fibrobacteres were not detected in either library. The phylum Firmicutes was detected at a higher frequency in the artificial consortium than the natural consortium (85.5% vs 56.6%, P < 0.05). At the genus level, Butyrivibrio (22.1% vs 5.1%) and Lachnospiraceae incertae sedis (5.3% vs 0%), both belonging to Firmicutes, also showed a higher detection in the artificial consortium, contributing to the higher detection of Firmicutes. On the other hand, the phylum Bacteroidetes was detected at a lower frequency in the artificial consortium than the natural consortium (4.6% vs 33.1%, P < 0.05). Of unclassified bacterial groups, only unclassified Lachnospiraceae were detected at higher levels in the artificial than the natural consortium (32.1% vs 11.8%). No characteristic cluster was formed within the unclassified Lachnospiraceae group in the phylogenetic analysis (data not shown).
Quantitative PCR data on the bacterial species and groups detected dominantly in the consortia are shown in Figure 1. The total bacterial 16S rDNA copy number in the natural and artificial consortia were, respectively, 3.98 × 105 and 4.87 × 105 per nanogram DNA. On the basis of the 16S rDNA copy number, F. succinogenes accounted for 5.2% of total bacteria in the natural consortium and 13.6% in the artificial consortium. Of the quantified bacterial species and groups, the most abundant was the Butyrivibrio-Pseudobutyrivibrio-Lachnospiraceae incertae sedis group in both consortia (19.7% and 29.5% in the natural and artificial consortia, respectively). The artificial consortium was higher in proportion of F. succinogenes, the Butyrivibrio-Pseudobutyrivibrio-Lachnospiraceae incertae sedis group, the U2 group and Ruminococcus albus, but lower in the proportion of S. ruminis and Selenomonas ruminantium (P < 0.05). The relative abundance of Ruminococcus flavefaciens, Treponema bryantii and methanogenic archaea did not differ between the two consortia.
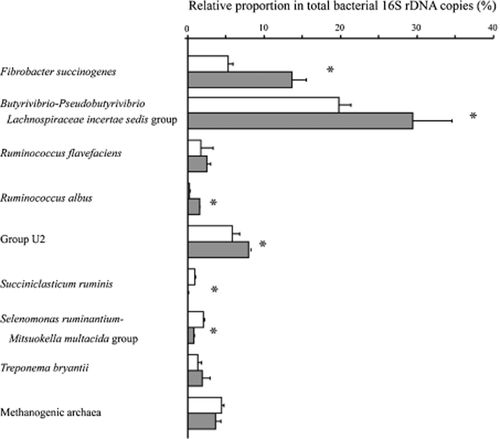
Quantitative difference of microbial species and groups between natural and artificial consortia determined by real-time PCR targeting 16S rDNA. The abundance was expressed by the proportion of the 16S rDNA copy number of the target to that of total bacteria. Asterisk indicates a statistical difference between natural (white) and artificial (gray) consortia (P < 0.05).
The phylogenetic distribution of 48 clones classified into genera Butyrivibrio and Peudobutyrivibrio detected in natural and artificial consortia is shown in Figure 2. Clones were affiliated with Butyrivibrio fibrisolvens and Pseudobutyrivibrio but not Butyrivibiro crossotus. Clones of butyrivibrios were distributed in the groups of SA (14 clones), VA2 (12 clones), VA1 (11 clones) and phylogeny-undefined Butyrivibrio (PUB, nine clones). The detected number of clones was higher in the artificial consortium than the natural consortium in groups of SA (13 vs 1), VA2 (8 vs 4) and PUB (7 vs 2), but was not much different in group VA1 (6 vs 5).

Phylogenetic placement of 16S rDNA sequences within the genus Butyrivibrio and Pseudobutyrivibrio detected in the natural and artificial consortia. The strains given in bold letters are type strains. Clone numbers are preceded with ‘A’ and ‘N’, indicating a clone of the artificial consortium and natural consortium, respectively. The accession numbers are shown in parenthesis. The sequence of Aquifex pyrophilus (accession no. NR_029172) was used as an outgroup. The bootstrap value representing the confidence percentage (≥ 80%) is shown at the nodes. Group PUB is an abbreviation of phylogeny-undefined Butyrivibrio group. The other Butyrivibrio/Psudobutyrivibrio groups were defined by Paillard et al. (2007).
Discussion
The fiber-associated bacteria in the rumen consist of not only fibrolytic bacteria, but also abundant non-fibrolytic and uncultured bacteria (Koike et al. 2003; Jami & Mizrahi 2012). In the present study, expecting to identify the consortium members having close relationships with F. succinogenes group 1, an F. succinogenes-enriched artificial consortium was prepared and its microbial composition was determined. A natural consortium was used as a control. Although direct comparison between natural and artificial consortia is not possible due to the difference in preparation conditions, information from two consortia may provide insights for relationships within rumen bacteria. Thus, through the analysis of these materials, it was assumed that bacteria detected in both consortia may be important for fiber degradation and that bacteria detected at high frequency in the artificial consortium might have a particularly strong linkage to F. succinogenes.
No F. succinogenes-affiliated sequence was detected in clone library analysis, even in the F. succinogenes-enriched artificial consortium (Table 2). The PCR bias of the primer set used in the present study was evaluated precisely in the rumen bacterial sequences, and found to be specific to the F. succinogenes sequence (Tajima et al. 2001; Khafipour et al. 2009) Therefore, detection frequency of F. succinogenes in the clone library constructed by these universal primers is not a useful index. However, in the present study the dominance of F. succinogenes in the artificial consortium (Fig. 1) was confirmed at levels 2.6 times higher than in the natural consortium by real-time PCR quantification. This shows successful enrichment of F. succinogenes in developing an artificial consortium dominated by F. succinogenes.
F. succinogenes has a prominent role in plant polysaccharide digestion but uses only cellulose and its hydrolysis products (Suen et al. 2011; Ransom-Jones et al. 2012). The consortium-forming non-fibrolytic bacteria are probably able to utilize solubilized xylo-oligosaccharides and cellobionic acid produced by F. succinogenes (Matulova et al. 2005; Nouaille et al. 2009). Butyrivibrio and Pseudobutyrivibrio, active utilizers of xylan and its hydrolysates (Miron et al. 1994), were abundantly detected in analyses of the clone library and quantitative real-time PCR in both natural and artificial consortia. In addition to the phylogenetic and physiologic groups of Butyrivibrio defined as VA1, VA2 and SA, the PUB group has been proposed for descriptive purposes (Paillard et al. 2007). A symbiotic relationship in fiber digestion is found in co-cultures of F. succinogenes group 1 strains and the PUB group (Miron & Ben-Ghedalia 1993a,b). In the present study, not only PUB but also SA and VA2 were detected at higher levels in the artificial consortium than the natural consortium, suggesting their positive interaction with F. succinogenes. Although a co-culture study is obviously necessary, SA and VA2 may also interrelate (possibly cooperate) with F. succinogenes group 1 in the degradation of plant polysaccharides.
In the family Ruminococcaceae, the U2 group was abundantly detected in both consortia, although its proportion was higher in the artificial consortium. The strain R-25 belonging to the U2 group enhances fiber digestibility in co-culture with the F. succinogenes group 1, although R-25 itself does not show the ability to digest fibers (Fukuma et al. 2012). Since the U2 group is located in the solid fraction of rumen digesta by associating with plant materials (Koike et al. 2010), the present results confirm the importance of this group as a member of a fibrolytic community dominated by F. succinogenes.
The major fiber digesters R. albus and R. flavefaciens were detected in both consortia. R. albus but not R. flavefaciens, was present at a higher proportion in the artificial consortium. This differing response may be partly due to the fact that R. flavefaciens is a strong competitor of F. succinogenes (Shi et al. 1997; Chen & Weimer 2001). S. ruminis and S. ruminantium in the family Veillonellaceae were present at a higher proportion in the natural consortium. This might be because they can utilize succinate and lactate that are released from F. succinogenes, two ruminococcal species and the U2 group (Sawanon & Kobayashi 2006; Sawanon et al. 2011; Fukuma et al. 2012). Methanogenic archaea and T. bryantii were also detected in both consortia. These are reported to show synergism with F. succinogenes (Rychlik & May 2000).
One of the important characteristics of both consortia was a high proportion of unclassified bacteria, for example, unclassified Bacteroidales, unclassified Lachnospiraceae, unclassified Clostridiales and unclassified Ruminococcaceae. The genus Lachnospiraceae incertae sedis is also poorly characterized phylogenetically and physiologically because of the lack of isolate (Huws et al. 2011). Despite of the abundance of these unclassified bacteria in the consortia, physiological information on them is limited due to the difficulties in the isolation, cultivation and maintenance of such rumen bacteria (Kobayashi 2006). However, the bacteria detected in the artificial consortium may suggest participation in supporting F. succinogenes. This possibility awaits further clarification.
There is a possibility that not only a direct relationship with F. succinogenes, but also other factors affect the composition of the consortium. Many Butyrivibrio isolates produce bacteriocins that show strong and broad-spectrum inhibitory activity against inter- and intra-species competitors, except for F. succinogenes group 1 (Kalmokoff et al. 1996; Rychlik & Russell 2002). Butyrivibrios and other bacteria producing bacteriocin might partially select and define the consortium members in the rumen (Krause et al. 2003).
In the present study, the natural consortium consisted of the phylum Firmicutes, Bacteroidetes, Spirochaetes, Proteobacteria and Fibrobacteres with many unclassified bacteria. The artificial consortium showed higher incidence of the phylum Firmicutes, especially B. fibrisolvens groups, the U2 group, R. albus and genus Lachnospiraceae incertae sedis, which suggests functional interrelationships between these bacteria and F. succinogenes in the rumen. These findings will contribute to further understanding of the microbial network for degrading plant materials in the rumen.
The present study had a methodological limitation that affects the bacterial composition of the artificial consortium, that is, pre-association of F. succinogenes with the fibers may limit the bacterial-attachable surface area on the plant material. This may reduce accessibility for tightly attaching bacteria but possibly not loosely attaching bacteria. However, the bacteria identified in the artificial consortium might include close associates of F. succinogenes to a larger extent, reflecting its community structure.
Acknowledgments
This study was supported in part by Grant-in-Aid for Scientific Research (numbers 15580231 and 17380157 to Y.K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of this report.




