Increased ectopic fat cells in the longitudinal muscularis layer of the oviduct isthmus in obese Japanese Black cows
Abstract
In obese humans, mesenchymal stem cells differentiate to become ectopic fat cells in muscles. These ectopic fat cells inhibit the contraction of vascular smooth muscles. Stem cells have been recently identified in the human oviduct, a structure important in reproduction. We therefore investigated the number of Oil Red O (ORO)-positive cells in the oviducts of control Japanese Black cows (n = 6; body condition score [BCS], 3.0 on a 5-point scale) compared to those with diet-induced obesity (n = 5; BCS, 4.0). We stained the ampulla and isthmus collected on the second day after ovulation with ORO and then counted the positive cells in each layer in 10 cross-sections of the ampulla or isthmus. The obese group (23.4 ± 3.4 in the 10 sections) had larger numbers of ORO-positive cells in the longitudinal muscularis of the isthmus (P < 0.05) than did the control group (15.0 ± 2.4). ORO-positive cells were also observed in all other layers of the isthmus and ampulla; however, the number of cells in these layers did not differ significantly between obese cows and controls. Whether this observed increase in ORO-positive cells in the oviducts of obese cows affects their reproduction warrants further study.
Introduction
In obese animals, the major site of fat accumulation is subcutaneous adipose tissue, but triglyceride deposition has been found in ectopic sites such as the viscera and cardiac and skeletal muscles in mice, rats and humans (Gastaldelli & Basta 2010; Carobbio et al. 2011). The presence of ectopic fat cells is an important risk factor for various diseases. A recent study on obese humans has revealed that ectopic fat cells can develop from mesenchymal stem cells even in smooth muscles (e.g. aorta) (Xue et al. 2010). Ectopic fat cells in blood vessels have been observed to inhibit the contraction of vascular smooth muscles in mice, rats and humans (Szasz & Webb 2012). The presence of mesenchymal stem cells in the human oviduct has been recently reported (Indumathi et al. 2013). However, whether ectopic fat cells are present in oviducts has not been fully investigated.
The oviduct is an active structure that maintains and modulates sperm capacitation, fertilization and early embryonic development during transport to the uterus (Ellington 1991; Rodriguez-Martinez 2007; Leese et al. 2008; Lloyd et al. 2009; Besenfelder et al. 2012). This structure has five layers, as follows, from innermost to outermost: (1) mucosal epithelium, (2) lamina propria, (3) circular muscularis, (4) longitudinal muscularis and (5) serosa (Pauerstein et al. 1974; Ellington 1991). After fertilization, embryonic development up to the 8- and 16-cell stages takes place within the ampulla; the embryo then passes quickly through the isthmus and enters the uterus (El-Banna & Hafez 1970). For rapid passage of the developing embryo, the isthmus has thick layers of muscle (Pauerstein et al. 1974; Ellington 1991). Thus, any abnormality in the oviduct has the potential to affect embryogenesis.
The conception rate after artificial insemination is declining in Japanese Black cows, a breed famous for genetic improvements that produce marbled beef (unpublished data of Livestock Improvement Association of Japan). In our previous field study, we estimated the relationship between embryo quality and a five-point body condition score (BCS) (Kadokawa et al. 2008) in Holstein heifers; the study revealed that obese donors produced fewer excellent-grade, transferable embryos than did control donors after superovulation. In addition, this study showed that the collected embryos exhibited delays in development, as indicated by an increased number of morulas and a decreased number of blastocysts (Kadokawa et al. 2008). Therefore, obesity may be an important factor in the suppression of reproduction in cattle.
Oil Red O (ORO) staining is the primary staining method for detecting ectopic fat cells. Little is known about the presence of ORO-positive cells in the oviduct, but several studies (Wordinger et al. 1977; Witkowska 1979; Henault & Killian 1993a,b) have reported the presence of ORO-positive cells in the ampullary and isthmic epithelia of dairy and beef cows. However, these studies did not report the presence or absence of ORO-positive cells in the muscle layers of the oviduct. In this study, we compared the number of ectopic fat cells in the ampullary and isthmic sections of oviducts of normal Japanese Black cows to that of cows with diet-induced obesity.
Materials and Methods
Animals and treatment
The experiments were performed in accordance with the Guiding Principles for the Care and Use of Experimental Animals in the Field of Physiological Sciences (Physiological Society of Japan) and approved by the Committee on Animal Experiments of the School of Veterinary Medicine, Yamaguchi University.
Multiparous Japanese Black cows (4 years of age after three parturitions, n = 11) were housed in a free-stall barn. Their calves were separated to wean at 2 months after normal parturition. At the time of weaning, the cows' BCS (shown as mean ± standard error of the mean [SEM]), on a five-point scale (Ferguson et al. 1994), was 2.50; mean body weight was 463 ± 2 kg. The body weight required 11.2 Mcal of metabolizable energy (ME) as a maintenance level, according to the Japanese feeding standard (Agriculture, Forestry and Fisheries Research Council Secretariat 2008). Dietary adjustments were used to cause obesity in some of the cows. The cows were randomly allocated to one of the following 2 groups: (1) control group (n = 6), which was fed 12.3 Mcal of ME (110% of the maintenance level) by using Italian ryegrass hay (84.2% dry matter [DM]), 2.30 Mcal/kg DM of ME, 13.3% crude protein [CP]) plus concentrate (86.6% DM, 3.82 Mcal/kg DM of ME, 21.3% of CP); or (2) obese group (n = 5), which was fed 23.9 Mcal of ME (as commonly used fattening level and as 213% of the maintenance level) by using the same Italian ryegrass hay plus a greater amount of the same concentrate. Water and mineral blocks were provided ad libitum. Absence of disease, including reproductive disease, was confirmed by daily observation or by weekly rectal palpation.
After 6 months of the dietary treatments, the cows were weighed and the BCS was measured. The cows were then slaughtered on the second day after ovulation induced by dinoprost (Pronalgon F; Pfizer, Tokyo, Japan) – the time when oocytes are in the ampulla (El-Banna & Hafez 1970). The oviducts on the ipsilateral side of ovulation were collected within 15 min of slaughter. The tissues surrounding the oviduct were removed carefully, and the oviducts were washed with phosphate-buffered saline (PBS) to collect a 2.5-cm section of the ampulla (at 5 cm from the infundibulum) or isthmus (at 3 cm from the uterotubal junction).
Histochemistry
We obtained cross-sections of the oviduct samples by using an embedding medium (Tissue-Tek O.C.T. compound; Sakura Finetechnical Co. Ltd, Tokyo, Japan) and a cryostat (CM1900; Leica Microsystems Pty Ltd, Wetzlar, Germany), as described in a previous study of ORO staining of beef cow oviducts (Wordinger et al. 1977). Sections of 14-μm thickness were placed on adhesive-coated slides (Poly-L-lysine-coated Superfrost; Matsunami Glass, Osaka, Japan) and attached by air-drying. We obtained 10 sections each from the ampulla and isthmus of each cow. The collected sections were separated by at least 200 μm. We did not collect serial sections because the cross-sections of the oviduct were nearly circular; it was thus difficult to identify angles in the serial sections, precluding identification of ORO-positive cells in different sections.
The sections were treated with 60% isopropanol for 1 min and stained with 0.18% ORO solution (154–02072; Wako Chemicals, Osaka, Japan; dissolved in 60% isopropanol) for 15 min. The sections were then washed and counterstained with Mayer's hematoxylin solution (131–09665; Wako Chemicals) for 5 min. The stained sections were mounted on microscope slides without a coverslip and observed under a microscope (Optiphoto; Nikon, Tokyo, Japan) attached to a charged-coupled device camera (DS-Fi2; Nikon) and its controller (Nis-Element; Nikon). We counted the number of ORO-positive cells in the epithelium, lamina propria, circular muscularis and longitudinal muscularis layers in the 10 cross-sections of the oviduct samples.
Data analysis
Data were analyzed using Statview version 5.0 for Windows (SAS Institute, Inc., Cary, NC, USA). A non-paired t-test was used to evaluate the significance of differences between the obese and control groups in the number of ORO-positive cells in each layer of the 10 cross-sections of the ampulla or isthmus. A P-value < 0.05 was considered statistically significant.
Results
At the time of slaughter, the BCS was 3.0 (body weight, 501 ± 2 kg) in the control group and 4.0 (body weight, 563 ± 3 kg) in the obese group.
ORO-positive cells were observed in all layers of the oviducts, especially in the longitudinal muscularis of the ampulla (Fig. 1A) and isthmus (Fig. 1C, D, F) of obese cows. In contrast, the control cows had fewer ORO-positive cells in both the ampulla (Fig. 1B) and the isthmus (Fig. 1E). The largest ORO-positive cells had diameters of more than 100 μm. The ORO-positive cells were observed both as aggregations (Fig. 1A, D, F) and as separate cells (Fig. 1C).
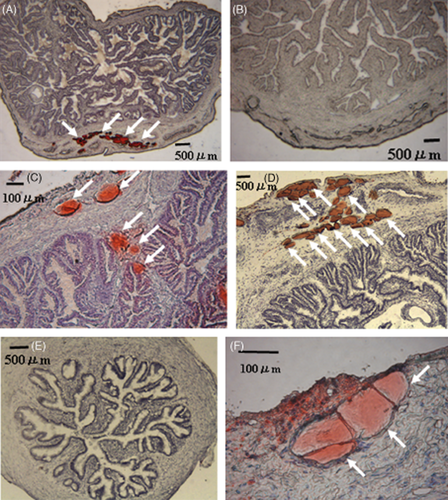
Microscopy of representative Oil Red O (ORO)-positive-stained cells showing red color in the ampulla (A) or isthmus (C, D, F) of obese cows; ORO-negative cells are shown in the ampulla (B) or isthmus (E) of control cows.
Figure 2 shows the number of ORO-positive cells in the 10 ampullary sections of the oviducts of the control or obese cows. ORO-positive cells were observed in the mucosal epithelium (Fig. 2A), lamina propria (Fig. 2B), circular muscularis (Fig. 2C) and longitudinal muscularis layers (Fig. 2D) of the ampulla. There was no significant difference in the number of ORO-positive cells in these ampullary layers. In both control and obese cows, the longitudinal muscularis layers had more ORO-positive cells than did the other three layers.
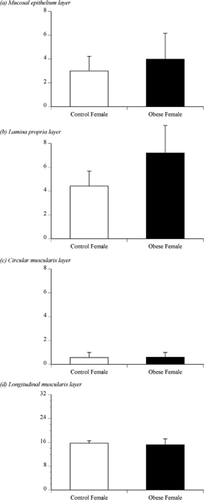
The number of Oil Red O (ORO)-positive-stained cells as counted in 10 sections in the mucosal epithelium, lamina propria, circular muscularis or longitudinal muscularis layers of the ampullae of control or obese cows.
Figure 3 shows the number of ORO-positive cells in the 10 isthmic sections of the oviducts of the control or obese cows. ORO-positive cells were observed in the mucosal epithelium (Fig. 3A), lamina propria (Fig. 3B), circular muscularis (Fig. 3C) and longitudinal muscularis layers (Fig. 3D) of the isthmus. There was no significant difference in the number of ORO-positive cells in the mucosal epithelium, lamina propria or circular muscularis. In both control and obese cows, the longitudinal muscularis had more ORO-positive cells than did the other three layers. In addition, the obese cows had more ORO-positive cells in the longitudinal muscularis of the isthmus than the control cows (P < 0.05; Fig. 3D).
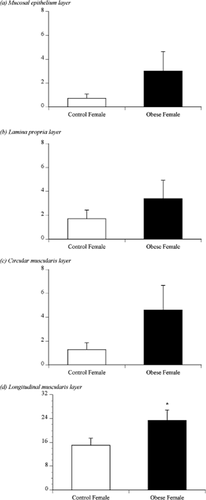
The number of Oil Red O (ORO)-positive-stained cells as counted in 10 sections in the mucosal epithelium, lamina propria, circular muscularis, and longitudinal muscularis layers of the isthmus of control or obese females. The asterisk indicates significant differences (P < 0.05) between control and obese females.
Discussion
Previous studies (Wordinger et al. 1977; Witkowska 1979; Henault & Killian 1993a,b) have reported the presence of ORO-positive cells in the ampullary and isthmic epithelia of dairy and beef cows. However, these studies did not report the presence or absence of ORO-stained cells in other layers of the oviduct. Our findings suggest that obese cows had increased numbers of ectopic fat cells in the longitudinal muscularis layer of the isthmus.
Adipocytes are derived from mesenchymal stem cells (Gimble et al. 2013) via preadipocytes (Kadokawa et al. 2007). Muscles also contain mesenchymal stem cells (Aldahmash et al. 2012; Gimble et al. 2013) and the oviduct isthmus has thick layers of muscle (Pauerstein et al. 1974; Ellington 1991). A recent study has reported the presence of stem cells in the human oviduct (Indumathi et al. 2013). Therefore, the longitudinal muscularis layer of the bovine oviduct isthmus may also have such stem cells, which have the potential to become ectopic fat cells.
Preadipocytes require high-energy substrates to mature into adipocytes (Kadokawa et al. 2007; Gimble et al. 2013). Epithelial cells in the chicken oviduct can be induced to differentiate into adipocytes by incubation in energy-rich medium (Khuong & Jeong 2011). Similarly, the epithelial oviduct cells observed in obese cattle in this study may have developed into ectopic fat cells in response to the high-energy substrates in their environment.
The largest diameter of mature bovine adipocytes is more than 100 μm (Kadokawa et al. 2007). On the basis of the size of the adipocytes observed by us, we conclude that the adipocytes in the oviducts were mature. Ectopic fat cells in blood vessels have been observed to inhibit vascular smooth muscle contraction in other animals (Szasz & Webb 2012). Further studies are thus required to determine whether the observed ORO-positive cells inhibit muscle contraction in the oviduct.
The Japanese Black cows used in this study are a breed famous for genetic improvements that produce marbled beef. The breed thus has a greater ability to produce ectopic fat cells in the skeletal muscle than do other breeds (Oyama 2011). Further study of ectopic fat cells in the reproductive organs of other breeds is required.
Cattle reproduction has been studied primarily in lean cows (Roche et al. 2009). Our previous study on ovarian function by using superovulation (Kadokawa et al. 2008) demonstrated that obese embryo donors produced fewer excellent-grade embryos than did lean and normal donors. Their embryos also exhibited slower development in the oviduct. Recent studies suggest that the condition of the oviduct contributes significantly to reproductive performance in cows (Rizos et al. 2010; Maillo et al. 2012). Embryos collected from the oviducts of diet-induced obese female mice showed delayed embryogenesis, higher lipid content and apoptosis rates and a lower survival rate (Ma et al. 2012). Therefore, in addition to ovarian function and oocyte quality, oviduct condition seems to contribute to the inhibited embryogenesis observed in obese donors.
Mature bovine adipocytes vary in diameter, which can be greater or less than 100 μm (Kadokawa et al. 2007). This variation occurs because mature adipocytes are capable of division, during which they evenly distribute lipid droplets between the two daughter cells (Nagayama et al. 2007). We cannot deny the possibility that cryostatic cutting affects the area of ORO-positive cells observed under the microscope in a random manner; even regions of cells of the same actual area may be cut near the center in some instances and near the edge in others, causing inconsistencies in area estimation. For these two reasons, we did not measure the area of ORO-positive cells; instead, we counted the number of ORO-positive cells.
This study demonstrates that the obese cows had larger numbers of ORO-positive cells in the longitudinal muscularis of the oviduct isthmus than did control cows. Further study is therefore necessary to determine the effects of these ectopic fat cells on reproduction in obese cows.




