Proteus mirabilis (MJA 2.6S) from saline-tolerant tilapia exhibits potent antagonistic activity against Vibrio spp., enhances immunity, controls NH3 levels and improves growth and survival in juvenile giant tiger shrimp, Penaeus monodon
Abstract
Of several isolates from saline-tolerant tilapia exhibiting anti-vibrio activity, an isolate that demonstrated inhibitory activity with the biggest zone of inhibition against the shrimp pathogens, Vibrio harveyi and V. parahaemolyticus were chosen and investigated in this study. The isolate showed significant inhibition against the shrimp pathogens, V. harveyi and V. parahaemolyticus in a competitive assay. Upon 16S rRNA sequencing, the isolate was identified as putative Proteus mirabilis (MJA 2.6S). P. mirabilis (MJA 2.6S) exhibited extracellular protease, cellulase and amylase activities. Pathogenicity tests showed that it was not pathogenic to the shrimp even at the highest concentration of 108 CFU ml−1. Further, P. mirabilis (MJA 2.6S) improved growth and survival, stimulated higher phenoloxidase and bactericidal activities in the shrimp host and protected it from V. harveyi experimental infection. In addition, the isolate P. mirabilis (MJA 2.6S) significantly reduced the ammonia level of the pond water samples in vitro. Taken together, the results clearly showed the strong probiotic potential of P. mirabilis (MJA 2.6S) in shrimp and hence further studies are needed to determine its wider application as a probiotic in P. monodon culture.
1 INTRODUCTION
Disease outbreaks are among the most challenging problems limiting the development and growth of the aquaculture industry. Chemotherapeutants such as antibiotics have been traditionally used by farmers as a means of fighting diseases. However, their indiscriminate use has resulted in detrimental outcomes such as bioaccumulation of residues in the farmed species, persistence of the drugs in the aquatic environment and the emergence of antibiotic-resistant strains of pathogens. To attain a more sustainable aquaculture operation, several alternative disease control approaches, e.g., the use of vaccines, immunostimulants and probiotics, have been promoted. Among these alternatives, the use of probiotics has been extensively adopted in shrimp farming in the Philippines. Evidence from numerous studies supports the use of probiotics as an alternative disease control strategy, and they are proving to be crucial in sustaining the viability of the aquaculture industry.
In the early years of shrimp probiotics research, the application of bacteria already being employed in the human health, waste bioremediation and veterinary fields is a common theme of many researches on shrimp production. Several commercially available probiotics have prospered, but their compositions are often unknown or poorly described and their use in shrimp has not been backed by adequate research. However, in recent years, many investigations have focused on the utilization of bacteria isolated from the host itself or from its rearing environment. Studies concerning bacterial disease control in shrimp aquaculture indicate that green water from saline-tolerant tilapia (Corre Jr. et al., 2000; Huervana et al., 2006; Lio-Po et al., 2002) and aqueous extracts obtained from the skin of tilapia (Caipang et al., 2011) are capable of inhibiting pathogenic Vibrio spp. Several studies have also demonstrated that the gastrointestinal tract of fish (Hagi & Hoshino, 2009; Hirimuthugoda et al., 2006; Lazado et al., 2010; Roy et al., 2008), aquatic habitats, and sediments (Agrawal & Gopal, 2013; Fry, 1987; Rao, 2006) harbour a diverse population of bacteria that could be used as potential probionts. Moreover, since saline-tolerant tilapia and the shrimp Penaeus monodon inhabit similar environments, the bacteria in this study were isolated from the gut of saline-tolerant tilapia, skin mucus and from pond sediments.
Owing to the shrimp's premium value, the Philippine shrimp aquaculture industry remains a major revenue earner for the country despite being beset with numerous problems (Fisheries Statistics of the Philippines 2017–2019, 2020). Use of endogenous probionts could mitigate environmental impacts such as introduction of “foreign” bacteria, emergence of resistant bacterial strains and disruption of the natural microflora. It is hoped that adoption of strategies to successfully combat bacterial diseases in shrimp farms will reduce shrimp mortality and increase production and profits thereby ensuring the sustainability of the industry.
Previously, we found that the endogenous Bacillus spp. (MJA 1.1 and MJA 2.1) demonstrated efficiency in inhibiting V. harveyi in vitro and in improving survival and resistance of P. monodon larvae to ammonia stress (Doroteo et al., 2018). However, the effects of the isolates on the immune parameters and on shrimp resistance to pathogen infection were not examined.
In contrast to well-studied probiotic genera such as Bacillus spp. and Lactobacillus spp., there are very few studies on the probiotic potential of Proteus spp. Proteus spp., to include P. mirabilis isolated from various aquatic organisms have shown significant probiotic properties with antagonistic action to many pathogens (Nguyen et al., 2014). Likewise, P. mirabilis demonstrated beneficial influence in fishes, shrimps and lobsters (Drzewiecka, 2016). Further, Proteus spp. have been reported to have abilities in bioremediation and environmental protection (Drzewiecka, 2016).
The present study builds on our previous results and dealt with the identification and evaluation of a new candidate probiont, Proteus mirabilis (MJA 2.6S) isolated from saline-tolerant tilapia with antagonistic activities against pathogenic V. harveyi and V. parahaemolyticus. We further determined the potential of the isolate as a bioremediation agent and measured the activities of their extracellular enzymes. More importantly, their impact on the immunity and resistance of P. monodon to V. harveyi infection were evaluated in vitro and in vivo.
2 MATERIALS AND METHODS
2.1 Bacterial isolation
Sevent nine (79) bacteria were isolated from the ten (10) saline-tolerant tilapia, Oreochromis niloticus weighing 50–100 g sampled from a privately owned brackishwater tilapia farm in Antique, Philippines. Tilapia was chosen because they live in similar environment as the shrimp. The fish were collected with a cast net and killed with a blow to the head. Bacteria were isolated from the fish skin mucus and gastrointestinal (GI) tract. The skin mucus was aseptically scraped using microbiological loops and plated on Nutrient Agar plus 2% NaCl (NA+). Also, the GI tract was aseptically removed and homogenized in sterile 1x PBS. The homogenate was serially diluted and plated onto NA+ plates. Distinct bacterial colonies were re-streaked onto fresh NA+ to obtain pure cultures. Isolates were stored in 1/3 strength NA+ (4°C) and in 20% glycerol (final concentration) for later use (−80°C). There were initially 79 pure isolates that were screened for anti-vibrio activity. Of the 51 isolates that showed antivibrio activity, 10 isolates with the biggest zones of inhibition and with both antivibrio harveyi and anti-V. parahaemolyticus activities were subjected to biochemical characterization. Isolates identified as belonging to different genera were then further characterized using molecular tools.
2.2 In vitro determination of antivibrio activity
2.2.1 Zone of inhibition
The antagonistic activity of the 79 isolates was determined employing the agar plate method, in which the mechanisms of action that the probiotic candidates exert on the bacterial pathogens such as competitive exclusion, and production of metabolites, among others, were determined. Briefly, 100 μl of the 18-24 h broth culture of either Vibrio harveyi (PN 9801) (de la Peña et al., 2001) or Vibrio parahaemolyticus was spread on NA+ plate. Four colonies of each endogenous isolate were separately grown on NA+ on the lawn of either V. harveyi or V. parahaemolyticus. Three (3) plates were prepared for each isolate. After a 24 h incubation period at room temperature, the antagonistic effect of the test isolate was observed by the development of the zone of inhibition, which appeared as a clearance zone around the bacterial colonies.
2.3 Molecular identification of the isolates
Ten bacterial isolates with the biggest zones of inhibition belonging to 4 genera based on biochemical characterization were submitted to First Base Laboratory in Malaysia through Asiagel Corporation Philippines for 16S rRNA sequencing. The sequences obtained were subjected to BLAST to determine the identity of the isolates based on available 16S rRNA sequences in the GenBank. One isolate identified as Proteus mirabilis (MJA 2.6S) based on its 16S rRNA sequence was further characterized in this study.
2.4 Competitive assay
P. mirabilis (MJA 2.6S), Vibrio harveyi (PN 9801) and V. parahaemolyticus were separately cultured in NA+ for 24 h and thereafter re-cultured in 500 ml nutrient broth plus 2% NaCl (NB+) for another 24 h with mild stirring. Each culture was centrifuged, washed and adjusted to 1.0 absorbance (109 CFU ml−1) at a wavelength of 600 nm. The count of each bacteria was further adjusted to 103 CFU ml−1. Five millilitre of P. mirabilis (MJA 2.6S) at 103 CFU ml−1 was mixed with the same volume and concentration of either Vibrio harveyi (PN 9801) or V. parahaemolyticus and grown for 72 h. The initial counts of V. harveyi (PN 9801) and V. parahemolyticus were recorded and thereafter monitored every 24 h for 3 days. Each treatment had three replicates.
2.5 Pathogenicity/toxicity test
P. mirabilis (MJA 2.6S) was tested for its pathogenicity or toxicity to the shrimp, P. monodon. Twenty-five shrimp postlarvae (PL25) were stocked in 3 L-capacity plastic containers with 1.5 L of UV-treated seawater containing 108 CFU ml−1 of the bacteria. A parallel control treatment was likewise set up as mentioned above containing only 25 shrimp PLs from the same batch with no P. mirabilis (MJA 2.6S) added. The tests were performed in three replicates. Mortality was observed for 7 days.
2.6 Preparation of probiotic-enhanced feed
The basal diet used in this study was the SEAFDEC/AQD formulated feed. Its proximate composition is presented in Table 1. The diet was prepared using half of the required amount of oil, the remaining half of which was later sprayed on the feeds as coating to prevent leaching of the incorporated bacteria. Two experimental diets were prepared (Diet 1—control or without the putative probiont; Diet 2—with Proteus mirabilis [MJA 2.6S]). The candidate probiotic bacteria, P. mirabilis (MJA 2.6S) was prepared by inoculating a flask containing Sterile NB+ medium with one loopful of the bacteria and cultured overnight before being centrifuged and media decanted and carefully drained leaving a pellet which was then weighed in a digital balance. Four (4) grams (wet weight) of the prepared isolate per kg feed (Laranja et al., 2014) was diluted in 2% NaCl and sprayed uniformly on the pelleted formulated feed. The feeds were then coated by spraying with the remaining amount of oil to minimize leaching of bacteria via hydrophobic interactions when introduced into the water (Nikoskelainen et al., 2001; Robertson et al., 2000) and air-dried for 1 h under a clean bench. While some leaching especially of water-soluble nutrients from the pelleted diet may occur, this does not seem to be a significant concern in the in-feed administration of probiotics (Kumar et al., 2016). The bacterial load of the P. mirabilis (MJA 2.6S) diet was determined to be 7.6 × 108 CFU g−1 diet. In aquaculture, probiotics inclusion usually ranges from 106 to 1010 CFU g−1 feed (Monica & Jayaraj, 2021).
| Ingredients | Amount (g/kg diet) |
|---|---|
| Fish meal (Danish) | 250 |
| Soybean meal | 250 |
| Shrimp head meal | 150 |
| Bread flour | 130 |
| Seaweed (Gracilaria sp.) | 50 |
| Cod liver oil | 25 |
| Soybean oil | 25 |
| Vitamin mix | 20 |
| Mineral mix | 10 |
| Dicalcium phosphate | 20 |
| Rice bran | 69.5 |
| BHT | 0.5 |
- Note: SEAFDEC AQD shrimp feed formulation – Crude Protein 42.1%, Crude Fat 6.8%, Crude Fibre 2.6%, Ash 13.8%, Moisture 1.0%, Nitrogen-Free Extract 30.2% (Dry matter basis).
2.7 Experiment 1 (Growth and survival)
2.8 Experiment 2 (Immune response test)
Twenty-five WSSV-free P. monodon with average weight of 0.51 ± 0.05 g were stocked in triplicate 30 L-capacity plastic tanks filled with 25 L UV-treated seawater. The shrimp were fed with the experimental diets for 8 weeks. At the end of the feeding trial, 12 shrimp were collected from each treatment (4 shrimp from each replicate) for the analysis of phenoloxidase and bactericidal activities. Collected samples were analysed immediately on the same day.
2.9 Collection of haemolymph
Shrimp was first “anaesthetized” by wrapping the shrimp in paper towel and placing it on top of an ice gel for a few seconds. Shrimp hemolymph was collected from the ventral sinus cavity using a syringe fitted with a 25 × 5/8″ gauge needle (Terumo), which had been flushed with anticoagulant solution (AC). The haemolymph was transferred to microcentrifuge tubes in aliquots required for the various analyses.
2.10 Phenoloxidase activity
The phenoloxidase assay was done using the method of Le Moullac et al. (1998) with modification. One hundred (100) μl of hemolymph mixed with anticoagulant was centrifuged at 3000 rpm for 5 min at 4°C to separate the hemocytes. One hundred (100) μl of the plasma was added with 200 μl of cold CAC to re-suspend the pellet. They were added with 20 μl of 0.01% zymosan in CAC buffer and incubated for 1 h then centrifuged at 3000 rpm for 5 min. at 4°C. Thereafter, 60 μl of the supernatant was placed in 96-well microplate, mixed with 20 μl of 0.4% L-DOPA, and incubated for 10 min. Then 220 μl CAC buffer was added into each well. The formation of dopachrome was monitored by measuring the absorbance every min for 4 min using OD 490 nm against a blank containing all the reagents minus the sample. A unit of enzyme activity is defined as an increase in absorbance of 0.001 min−1 mg protein−1.
2.11 Bactericidal activity
The plasma bactericidal activity was measured following the modified percent inhibition assay of Adams (1991). Briefly, a 24-h culture of Vibrio harveyi (PN 9801) was washed with 2% saline by centrifugation at 10,000 rpm for 15 min at 4°C, and the concentration was adjusted to 1 × 104 CFU ml−1. One hundred (100) μl of shrimp hemolymph was collected and centrifuged at 11,000 rpm for 10 min at 4°C. The collected plasma was filtered using a sterile filter (pore size 0.45 μm), 75 μl was mixed with an equal volume of bacterial suspension, and incubated for 24 h at room temperature. Initial absorbance was recorded and measured after the 24-h incubation period. The control was incubated with buffer only.
2.12 Experiment 3 (Challenge test—Experimental infection using V. harveyi PN 9801)
P. monodon with average weight of 1.21 ± 0.052 g and previously fed with the experimental diets for 8 weeks were experimentally infected with V. harveyi (PN 9801). The shrimp were immersed in 8.9 × 105 CFU ml−1 V. harveyi (PN 9801) concentration for 1.5 h and thereafter transferred to a separate plastic container at 15 shrimp per 30 L-capacity plastic tanks filled with 25 L UV-treated seawater with aeration. The water was not changed for 24 h but thereafter provided with the flow through water system. Each treatment was replicated three times. The shrimp were observed and mortality was recorded for 2 weeks.
2.13 Care of experimental animals
The experimental animals were handled following the Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council, 2011). The guidelines have been followed throughout the study such as during regular sampling and during collection of hemolymph at the end of every experiment. The study in general is compliant with the abovementioned guidelines.
2.14 Enzyme assay (in vitro)
2.14.1 Protease
The assay was performed following the method of Balaji et al. (2012). Briefly, P. mirabilis (MJA 2.6S) was inoculated on peptone gelatin agar and incubated at room temperature for 24 h. Thereafter, the agar plates were flooded with 15% mercuric chloride (HgCl2), and the appearance of transparent zone was measured. This was further confirmed in 1% skim milk agar with 1.5% NaCl. The assay was done in triplicate.
2.14.2 Cellulase
P. mirabilis (MJA 2.6S) was grown in carboxy methyl cellulose agar and incubated at room temperature for 24 h. Appearance of halo was further confirmed upon the addition of Grams' iodine (2.0 g KI and 1.0 g iodine in 200 ml distilled water) for 3 to 5 min (Kasana et al., 2008). The assay was conducted in triplicate.
2.14.3 Amylase
P. mirabilis (MJA 2.6S) was spottted on starch agar plates and incubated at room temperature for 24 h. Thereafter, the plates were flooded with 1% Lugol's iodine solution to check for amylase activity through the formation of a transparent zone (halo) surrounding the colony (Jacob & Gerstein, 1960). The assay was performed in triplicate.
2.15 Ammonia test
The bioremediation test was conducted based on the ability of the isolate to remove inorganic ammonia nitrogen from the shrimp pond water. Water from a shrimp pond was stocked at 1.5 L in 3 L-capacity plastic containers. The isolate was then added in each container at 106 CFU ml−1 in triplicate. Briefly, the 24-h broth culture was centrifuged to remove the culture medium and reconstituted with sterile NaCl. The concentration was adjusted to OD 1.0. The dilution ratio to obtain the standard concentration was used as the basis for determining the added probiotic bacterial density in the tank water. A control group was prepared similarly as with the treatment but with no isolate added. The total ammonia nitrogen was determined until day 6 following the phenol hypochlorite method by Strickland and Parsons (1972) with modifications using UV–Vis Spectrophotometer. Briefly, 25 ml of water sample was measured into a 125 ml flask. Successive addition of 1 ml phenol solution, 1 ml sodium nitroprusside solution and 2.5 ml of oxidizing reagents was done with thorough mixing after each addition. The samples were allowed to stand for 1 h at a temperature between 20°C and 24°C, and the absorbance was recorded against a distilled water blank in a UV–VIS spectrophotometer at a wavelength of 425 nm.
2.16 Statistical analysis
Results were analysed by one-way ANOVA using SYSTAT (SPSS, Chicago, USA). Differences among treatments were compared by Tukey's test. Values were considered significant at p ≤ 0.05. Data are reported as means ± standard deviation. The survival curves were estimated using the Kaplan–Meier (KM) method and compared statistically using the log rank test (Bland & Altman, 1998, 2004).
3 RESULTS
3.1 Zone of inhibition/in vitro antivibrio activity
Based on the initial screening, isolate A from the mucus of saline-tolerant tilapia exhibited large zones of inhibition against Vibrios. The zone of inhibition against V. harveyi (PN 9801) and V. parahaemolyticus was measured at 26 ± 4.3 mm and 14.5 ± 2.5 mm, respectively (Data not shown). The inhibition of the isolate was significantly higher in V. harveyi (PN 9801) than in V. parahaemolyticus.
3.2 Molecular identification and in vitro competitive test of the isolates
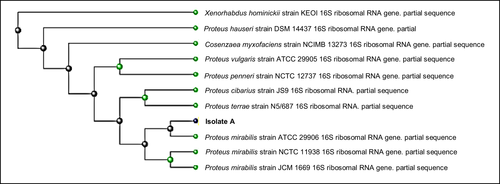
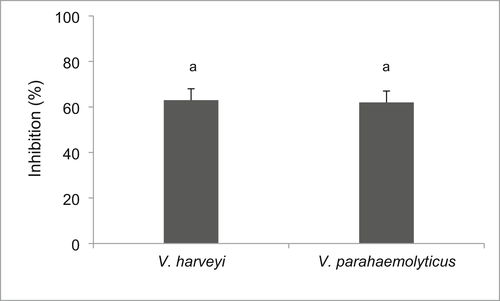
Based on the BLAST analysis of its 16 s RNA sequence, the isolate MJA 2.6S was identified as Proteus mirabilis with 99% identity (Figure 1). The in vitro tests demonstrated that the isolate possessed significant antivibrio activity against V. harveyi (PN 9801) and V. parahaemolyticus (Figure 2). The growth of the bacteria after 72 h of incubation was, respectively, reduced by 65% and 63% for V. harveyi (PN 9801) and V. parahaemolyticus in the presence of P. mirabilis (MJA 2.6S).
3.3 Pathogenicity test
P. mirabilis (MJA 2.6S) did not cause any manifestation of disease or toxicity that resulted in clinical signs or death of P. monodon larvae. All larvae survived after continuous exposure to the isolate at 108 CFU ml−1 for 7 days. The triplicated experiment was repeated 2 times (Table 2).
| Trial number | Survival (%) |
|---|---|
| 1 | 100 |
| 2 | 100 |
- a Values are average of 3 replicates.
3.4 Growth and survival
Inclusion of the bacterial isolate, P. mirabilis (MJA 2.6S) in P. monodon diet improved the growth and survival of the shrimp in the present study (Table 3). A remarkable increase in the growth of the shrimp fed the diet containing P. mirabilis (MJA 2.6S) was demonstrated with 217% weight gain compared to the control with only 153%. A significantly higher survival was attained in the group that received diet supplemented with P. mirabilis (MJA 2.6S) (61%) when compared with the control (37%).
| Treatment | Initial wt. (g) | Final wt. (g) | Weight gain (%) | SGR (%/day) | FCR | Survival (%) |
|---|---|---|---|---|---|---|
| Control | 0.40 ± 0.05 g | 1.03 ± 0.03 g | 153 ± 28 ga | 1.58 ± 0.05a | 3.18 ± 0.06b | 37 ± 8.9a |
| P. mirabilis | 0.40 ± 0.05 g | 1.13 ± 0.04 g | 217 ± 30 gb | 1.72 ± 0.07a | 2.96 ± 0.03a | 61 ± 10.4b |
- Note: Values represent means ± SD (n = 3 tanks). Means not sharing the same superscript letters are significantly different (p < 0.05).
3.5 Immune parameters
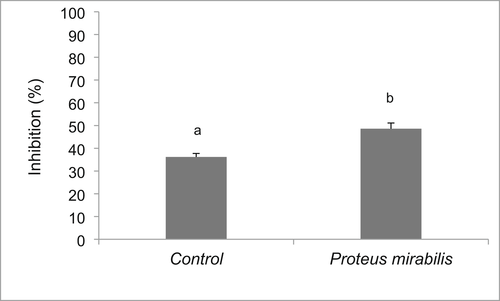
The phenoloxidase activity of P. monodon plasma increased significantly in the P. mirabilis (MJA 2.6S)-supplemented diet (0.0119 OD/min) compared to that in the control group (0.0054 OD/min) (Figure 3). Similarly, shrimp fed P. mirabilis (MJA 2.6S) diet exhibited higher plasma bactericidal activity (48%) compared to those in the control group (36%) (Figure 4).

3.6 Challenge test—Experimental infection using V. harveyi (PN 9801)
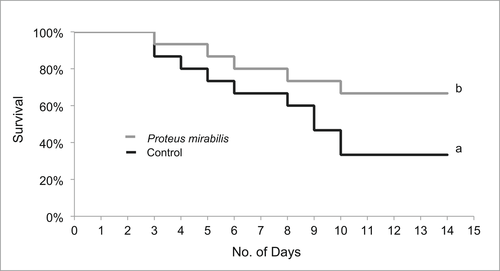
Consumption of P. mirabilis (MJA 2.6S)-enhanced diets for 8 weeks provided significant protection to the shrimp experimentally infected with V. harveyi (PN 9801) (Figure 5). Shrimp that consumed P. mirabilis (MJA 2.6S) survived better 10 days post infection with V. harveyi (67%) compared to the control group where only 33% of the shrimp survived.
3.7 Enzyme activities of the isolates (in vitro)
In the present study, P. mirabilis (MJA 2.6S) was positive for the extracellular enzymes. Enzyme activities of the putative probiont measured in vitro indicated that P. mirabilis (MJA 2.6S) produced 3 extracellular enzymes such as protease, cellulase and amylase (Table 4).
| Enzyme | Test result |
|---|---|
| Protease | + |
| Cellulase | + |
| Amylase | + |
3.8 Bioremediation potential of the isolates
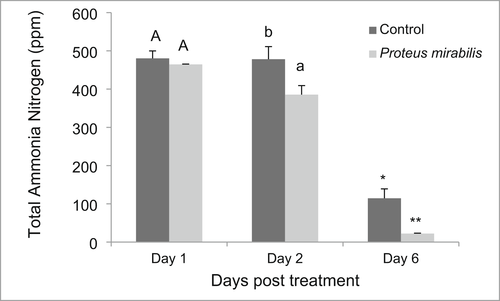
This study demonstrated that P. mirabilis (MJA 2.6S) was able to effectively reduce the ammonia level of the shrimp culture water (Figure 6). The results obtained showed that ammonia was reduced from about 3 ppm to levels that are safe for shrimp culture which is <1 ppm (Chien, 1992).
4 DISCUSSION
4.1 Growth and survival
Proteus mirabilis (MJA 2.6S) is a new isolate with interesting antivibrio activity both on V. harveyi (PN 9801) and V. parahaemolyticus and thus deserved to be further investigated. P. mirabilis (MJA 2.6S) in this study improved growth and survival in P. monodon larvae. The higher weight gain in the shrimp fed diet supplemented with P. mirabilis (MJA 2.6S) could be attributed to the extracellular enzymes produced by the isolate such as the protease, cellulase, and amylase that may have aided in the digestion of food making its nutrients more bioavailable to the shrimp. The P. mirabilis (MJA 2.6S) isolate produced all the three digestive enzymes. Besides aiding digestion, other factors could affect the efficacy of a probiotic (Gomez-Gil et al., 2000), which could influence growth and survival of the host species. These may include species composition, application level, frequency of application, and environmental conditions (Zhou et al., 2009). The length and mode of supplementation/application might as well affect the performance of the probiont. In this study, P. mirabilis (MJA 2.6S) was administered to the shrimp for 8 weeks by dietary supplementation. Changes in intestinal microbiota due to probiotic supplementation are often described to characterize the probiotic-influenced microbial composition and correlate it with treatment outcomes or to identify the factors that lead to the development of a particular microbiome (Rajeev et al., 2020). This analysis could have provided more insights but was not conducted due to the magnitude of the work. On the other hand, many other studies reported probiotic efficacy based on outcomes, e.g., growth, survival, immune response, disease resistance and bioremediating ability (Hasan & Banerjee, 2020), which were presumed to be due to the presence of the probiotics being the only factor that differed between the tested groups. Although this study did not confirm the presence of P. mirabilis (MJA 2.6S) in the shrimp gut, each dietary treatment was replicated three times to validate the results that were found to be consistent in all replicates of the diet groups with and without the P. mirabilis supplement. As shrimp growth and survival were improved remarkably by oral supplementation of the isolate, further investigation was conducted to unravel its beneficial properties and to explore its potential for commercial application.
4.2 Phenoloxidase (PO) and bactericidal activities
Probiotics are known to support the immune system, directly enhancing the innate immune response and stimulating local and systemic immunity against stress (Akhter et al., 2015; Mahdhi et al., 2011; Rengpipat et al., 1998). As in fish, the immune system of shrimp has evolved to tolerate commensals while fighting pathogens. Microbe-associated molecular patterns (MAMPs) of commensals that colonize the gut mildly stimulate the gut-associated lymphoid tissues into a level of alertness such that pathogens are prevented from proliferating (Gomez et al., 2013). The phenoloxidase is an important enzyme-linked mediator of crustacean immunity, which is considered as an important biomarker that can be used alone or in tandem with a challenge test for confirmation of immune enhancement in shrimp (Felix et al., 2004). In the present study, the significant enhancement of the phenoloxidase and bactericidal activities in shrimp supplemented with P. mirabilis (MJA 2.6S) was associated with improved protection and better survival of shrimp from the same group when experimentally challenged with V. harveyi (PN 9801). Also, P. mirabilis (MJA 2.6S) successfully inhibited the growth of the shrimp pathogens V. harveyi (PN 9801) and V. parahaemolyticus in vitro. This antibacterial effect might be due to the single or combined action of antibiotics, bacteriocins, siderohores, lysozymes, proteases and/or hydrogen peroxide and the alteration of pH values by the production of organic acids (Bruno & Montville, 1993; Lara-Flores, 2011; Sugita et al., 1997; Williams & Vickers, 1986).
4.3 Challenge test—Experimental infection using V. harveyi (PN 9801)
P. mirabilis (MJA 2.6S) in the present study gave significantly higher survival in P. monodon experimentally infected with V. harveyi (PN 9801). Previous studies have shown that antimicrobial activity of the bacterial strain B. subtilis against pathogenic Vibrio species, including V. parahaemolyticus and V. harveyi resulted in improved survival in shrimp, Litopenaeus vannamei (Balcázar & Rojas-Luna, 2007). Bacillus probionts also inhibited the growth and toxin production of V. harveyi in shrimp (Nakayama et al., 2009). In P. monodon postlarvae fed Bacillus-supplemented feed for 100 days, better survival (100%) was achieved compared to the control group (26%) when challenged with a pathogenic V. harveyi by immersion (Rengpipat et al., 1998). In rainbow trout, B. subtilis was found to be a potential agent for the control of bacterial diseases commonly affecting the species such as Streptococcosis (Kamgar & Ghane, 2012). With regard to Proteus spp., the bacteria (P. mirabilis, P. vulgaris, P. penneri and unidentified Proteus sp. strains) isolated from the gastrointestinal tract of various aquatic species such as black tiger shrimp, cobia, snubnosed pompano and ornate spiny lobster in Vietnam exhibited a surprisingly strong beneficial effect and demonstrated probiotic properties associated with bacteriocin production and other factors with antagonistic activity towards many pathogenic bacteria (Nguyen et al., 2014). Thus, the higher survival of shrimp upon experimental challenge in this study could be attributed to the enhanced immunity as shown by high phenoloxidase activity and to the production of various bactericidal substances mentioned above as revealed by the significant inhibitory effects in in vitro assays that together limited the proliferation and infectivity of the challenge bacteria.
4.4 Bioremediation potential of the isolates
One of the effective approaches in water quality improvement is through bioremediation or the application of microbes/enzymes. In bioremediation processes, microorganisms use the metabolic wastes as nutrient or energy sources (Agarwal, 1998; Tang et al., 2007). Probiotics have been documented to improve water quality in aquaculture. The P. mirabilis (MJA 2.6S) isolate in the present study was able to significantly lower the ammonia levels of pond water indicating its potential role as bioremediator. This result is in agreement with previous studies using beneficial bacteria as bioremediators. For example, Bacillus spp. lowered the concentrations of ammonia and nitrite in P. monodon culture ponds (Porubcan, 1991). Also, Bacillus isolates from Cyprinus carpio were able to reduce the concentrations of ammonia, nitrate, and phosphate in ornamental fish culture by as much as 74% (Lalloo et al., 2007). As aerobic heterotrophs Bacillus spp. can utilize ammonia and are good bacteria for nitrogen removal (Kim et al., 2005; Yang et al., 2011). A commercial probiotics containing 4 Bacillus species reduced the levels of nitrogenous pollutants in ponds like ammonia, nitrate and nitrite to very low concentrations compared to the untreated one (Barman et al., 2015). Furthermore, commercial products of Bacillus sp., Saccharomyces cerevisiae, Nitrosomonas sp. and Nitrobacter sp. lowered the levels of inorganic nitrogen and phosphate in fish and shrimp culture water (Li et al., 2006; Taoka et al., 2006). In the present study, the effect of P. mirabilis (MJA 2.6S) on ammonia was quite remarkable, reducing the level of ammonia from 3 to <1 ppm, which is the safe level for shrimp culture (Chien, 1992). The presence of the isolate MJA 2.6S in the culture water could mitigate the production and accumulation of toxic gases, particularly ammonia.
Proteus spp. are reported to be potential bioremediation agents for the environment due to their ability to tolerate or utilize polluting nitrogenous compounds (Drzewiecka, 2016). P. mirabilis strain isolated from coastal seawater in China effectively removed ammonia (NH4+) ions and reduced the nitrite (NO2−) and nitrate (NO3−) to gaseous nitrogen (N2) (Zhang et al., 2014). The bacterial strain was characterized as a heterotrophic nitrifier. There are reports that Proteus spp. are also capable of degrading hydocarbons (Survery et al., 2004). Proteus spp. especially P. mirabilis were abundantly found in human intestines as they form part of the natural microflora of a certain percentage of the human population (Różalski & Stączek, 2010). Commonly, P. mirabilis is harmless to humans (Gong et al., 2019), although some novel strains pose risks to human health (Rozalski et al., 1997; Shi et al., 2014; Shi et al., 2016; Wang et al., 2010). Therefore, although the P. mirabilis (MJA 2.6S) strain in the present study exhibited beneficial effects on P. monodon, it is subject to further verification with regard to its safety and impact on human health when used as a probiont for shrimp.
Significant losses in the shrimp industry are caused by poor water quality resulting from waste accumulation due to aggressive feeding rates and high protein composition of feeds in intensive aquaculture systems (Boyd, 1985; Shimeno et al., 1997). During feeding, an estimated 30%–40% of the feed given is being wasted in the pond during the grow-out culture period (Barman et al., 2015). This feed waste and its derivatives pose a real pollution threat and can predispose the organism to infection by pathogens commonly found in the shrimp culture environment such as the various Vibrio spp. (e.g., V. harveyi, V. vulnificus, V. alginolyticus, V. splendidus and V. parahaemolyticus). Enzymes produced by probiotic bacteria can digest waste feed particles and mitigate the toxic effects of pollutants in the water and sediments. Protease enzymes help in the utilization of animal and plant proteins (Barman et al., 2015), whereas amylase in the pond functions in the utilization of starch and complex polysaccharides, for the release of phosphorus from phytate as well as of bound minerals and amino acids, leading to maximum utilization of nutrients.
In conclusion, Proteus mirabilis (MJA 2.6S) isolated in our study exhibited properties during in vitro and in vivo characterization that would warrant its use as a probiont in P. monodon culture. Towards this goal, the possible synergistic effects of the isolate when used together with other saline-tolerant tilapia isolates from this study, as well as its safety to human health merit further investigation.
AUTHOR CONTRIBUTIONS
All authors listed above have contributed significantly to this paper.
ACKNOWLEDGEMENTS
This study was fully funded by the University of the Philippines Visayas (UPV) In-house Research Grant. Also, the authors are grateful to the Institute of Aquaculture, College of Fisheries and Ocean Sciences for all the support extended to the project.
FUNDING INFORMATION
This study was fully funded by the University of the Philippines Visayas (UPV) In-house Research Grant.
CONFLICT OF INTEREST
None.
CODE AVAILABILITY
Not Applicable.
Open Research
DATA AVAILABILITY STATEMENT
The raw data in this paper are not publicly available. Request should be addressed to the corresponding author.




