Dietary lipid requirement of juvenile white-leg shrimp, Penaeus vannamei (Boone, 1931) reared in inland ground saline water of 15 ppt
Abstract
A 60-day feeding trial was conducted to evaluate the optimum dietary lipid level in terms of growth performance, digestive enzymes and hemato-biochemical parameters of white-leg shrimp, Penaeus vannamei juveniles in inland ground saline water (IGSW) of 15 ppt salinity. The shrimps (avg. wt., 4.04 ± 0.03 g) were randomly distributed in six groups in triplicates (15 shrimps/tank, 290 L), viz., TCL40 (40 g/kg lipid), TCL60 (60 g/kg lipid), TCL80 (80 g/kg lipid), TCL100 (100 g/kg lipid), TCL120 (120 g/kg lipid) and TCL140 (140 g/kg lipid). Six semi-purified hetero-lipidic (40-140 g lipid/kg), hetero-caloric (376–426 Kcal DE/100 g) and iso-nitrogenous (400 g crude protein/kg) diets were prepared and fed to respective groups four times daily. Weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency ratio (PER) showed higher quadratic relations (R2 = 0.97, 0.98, 0.94 and 0.87, respectively) to dietary lipid levels (p < 0.05) with enhancing dietary lipid up to 60 g/kg. Whole body moisture and lipid contents exhibited a high linear (R2 = 0.87 and 0.98) and quadratic (R2 = 0.88 and 0.98) (p < 0.05) inverse relationship with enhancing dietary lipid levels up to 140 g/kg. Hemolymph hemocyanin, serum total protein and glucose level did not differ significantly (p > 0.05), but the TCL60 group showed significantly (p < 0.05) higher serum cholesterol and triglyceride levels. Serum osmolality, osmoregulatory capacity, and branchial Na+/K+-ATPase activity was similar (p > 0.05) among the groups. Hepatopancreatic lipase and amylase activity significantly (p < 0.05) increased, but trypsin activity did not vary significantly (p > 0.05) among the groups. Second-degree polynomial analysis with WG, SGR and FCR indicated that a 55.80–58.80 g lipid/kg diet is optimum for P. vannamei juveniles in IGSW of 15 ppt salinity.
1 INTRODUCTION
The fastest-growing aquaculture industry continues to grow among all the agri and allied sectors and contributes a substantial share of the global food basket to alleviate global hunger (FAO, 2020). The stagnation of marine capture fisheries drives the demand for fish from the culture-based fisheries sector to an increased extent (FAO, 2018). Further, horizontal expansion of land-based aquaculture creates a major conflict with the growing demand for food and land resources for the human population. Therefore, by 2030, the huge targeted fish production can only be achieved using new or alternative aqua farming practices, i.e., adopting unutilized or underutilized natural resources for commercial aqua farming. More than 385 million hectares of land are salt-affected and are expected to rise by 50% by 2050 (Jamil et al., 2011; Partridge et al., 2008). These vast natural resources are not suitable for agricultural activities (Bui et al., 2017). Exploring the degraded soils and under-utilized inland ground saline water (IGSW, salinity 3–25 ppt in India) for aquaculture practices can provide employment opportunities and livelihood generation through enhanced fish and shrimp production (Talukdar et al., 2021). However, in contrast to brackish water and sea water, ionic imbalance (variable concentration of Mg2+ with high Ca2+ and low K+ ions) (Aklakur, 2017) of IGSW can cause a pronounced effect on the growth and physiological homeostasis of the cultured animals (Antony et al., 2020; Saoud et al., 2003; Singha et al., 2020; Talukdar et al., 2020). Available calcium to magnesium ratio (Ca: Mg) in IGSW is higher than in brackish water and sea water, resulting in poor growth performance and less farmed shrimp production (Talukdar et al., 2021). Besides, K+ is essential for osmoregulatory adaptations by activation of Na+/K+-ATPase in the body of shrimp (Roy et al., 2007). Therefore, potassium supplementation through diet and water fortification can be a practical approach for successful shrimp culture in inland ground water of different ambient salinities (Antony et al., 2015; Davis et al., 2005; Jahan et al., 2018; Raizada et al., 2015; Saoud et al., 2007).
Throughout the globe, the farming of high prized euryhaline crustacean, Pacific white-leg shrimp, Penaeus vannamei is very popular due to its faster growth rate, higher disease resistance and better survival with promising economic return (Cuzon et al., 2004; Flores et al., 2007; Hamidoghli et al., 2018). Moreover, comparatively lower dietary crude protein requirements than black tiger shrimp, Penaeus monodon and tolerance of widespread salinity (1–50 ppt, recommended salinity 15–30 ppt) make P. vannamei more suitable species for IGSW (Lightner et al., 2009; Samocha et al., 2002; Yun et al., 2016; Zhou et al., 2014). Huong et al. (2010) and Li et al. (2017) reported that P. vannamei could adjust ambient salinity changes through osmoregulation comprising amino acid balance pools, regulating the volume of cells and activation of enzymes of ion transporter system. However, drastic change in water salinity greatly influences the growth performance, survival rate, physio-metabolic responses and finally, resistance to diseases, resulting in the diversion of a higher amount of energy to compensate the metabolic needs (Diaz et al., 2001; Tseng & Hwang, 2008; Wang et al., 2014). Thus, nutritional intervention through diet could be a cost-effective and useful practice to uphold the basic physio-metabolic responses for improving the growth performance of shrimp in IGSW (Li et al., 2008; Ur-Rahman et al., 2005; Wang et al., 2015; Xu et al., 2016).
Dietary lipid is a concentrated source of digestible energy. It serves as a basic precursor of EFAs (essential fatty acids), phospholipids, glycolipids and sterols mostly required for the growth, physio-metabolic and immune responses of shrimp (González-Félix, Gatlin III, et al., 2002; González-Félix, Lawrence, et al., 2002; Xie et al., 2019; Zhang et al., 2013). Besides, it also carries essential fat-soluble vitamins (He et al., 1992), maintaining cellular and sub cellular membrane structure, function and integrity (Bou et al., 2017), synthesis of moulting hormones (Arts & Kohler, 2009; González-Félix et al., 2010) and osmoregulatory adaptations (Jannathulla et al., 2019). Dietary lipid also has the protein-sparing effect of reducing the nitrogenous wastes from protein catabolism in the rearing water (Luo et al., 2005).
Several authors have investigated and reported that the dietary lipid requirements of P. vannamei ranged between 50 and140 g/kg (Goda, 2008; González-Félix, Lawrence, et al., 2002; Tzeng et al., 2004). Dietary lipid requirement changes with the salinity, different life stages and the culture system (Chen et al., 2014; González-Félix, Lawrence, et al., 2002; Hamidoghli et al., 2020; Jannathulla et al., 2019; Toledo et al., 2016; Xie et al., 2019; Zhu et al., 2010). Xu et al. (2018) reported that 90 g/kg dietary lipid is optimum for P. vannamei juveniles regardless of salinity (3 and 25 ppt) in terms of growth performance and nutrient utilization, but feeding higher lipid levels at 120 g/kg adversely affects the growth and oxidative damage of the hepatopancreas of the animal. Jannathulla et al. (2019) described higher growth and better survival of shrimp P. vannamei due to feeding of 60 g lipid/kg with12 g/kg essential fatty acids at 25 ppt ambient salinity. However, Xie et al. (2019) described relatively higher dietary lipid requirement (118–124 g lipid/kg diet) of white-leg shrimp post-larvae to improve the growth, survivability, immune response and stress resistance at 28 ppt water salinity. Similarly, Zhang et al. (2013) recommended that a 100–120 g lipid/kg dietary level is optimum for P. vannamei juveniles in terms of growth and immunity reared at 7 ppt salinity. Hamidoghli et al. (2020) and Toledo et al. (2016) reported that P. vannamei juveniles require 56–60 g/kg and 8.5–9.5 g/kg dietary lipids, respectively, in biofloc based culture system. However, earlier studies (González-Félix, Gatlin III, et al., 2002; González-Félix, Lawrence, et al., 2002) demonstrated that dietary 30–90 g lipid/kg is optimum for better growth and survival of white-leg shrimp juveniles.
With this backdrop, the current experiment was designed and aimed to evaluate the dietary lipid requirement based on the growth performance, nutrient utilization, digestive enzymes and hemato-biochemical parameters of juvenile white-leg shrimp, P. vannamei, cultured in 15 ppt IGSW. This is the first report of its kind for white-leg shrimp reared in IGSW. We also optimized the protein requirement for the P. vannamei diet specific to IGSW (Jana et al., 2021). The outcome of the current study will serve as the baseline information for the global aqua feed industry for precise shrimp feed formulation, especially for the vibrant commercial inland saline water shrimp culture.
2 MATERIALS AND METHODS
2.1 Ethical declarations
The current study strictly adhered to animal welfare norms for scientific investigations after approval from the Animal Ethics Committee of ICAR-Central Institute of Fisheries Education (CIFE), India and CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment & Forests (Animal Welfare Division), Government of India.
2.2 Lab animal
White-leg shrimp juveniles (one thousand fifteen hundred) were carefully packed in polythene triple-layered bags containing well-oxygenated 15 ppt IGSW and immediately transported from Anwal Shrimp farm, Rohtak, Haryana, to the Experimental Laboratory for Shrimp of ICAR-CIFE, Rohtak centre. For acclimatization, shrimps were immediately shifted to circular tanks (10.03 m2 × 0.87 m, 1000 L water capacity, 800 L water volume) filled with IGSW of 15 ppt under natural photoperiod with vigorous aeration. During 15 days acclimatization period, shrimps were fed four times a day with a commercial diet containing 400 g/kg crude protein and 50 g/kg dietary lipid.
2.3 Formulation and preparation of diets
Six iso-nitrogenous (400 g/kg dietary protein), hetero-lipidic (40 to 140 g/kg dietary lipid), and hetero-energetic (376 to 426 Kcal DE/100 g) semi-purified experimental diets were formulated (Table 1) with varying lipid levels viz., TCL40 (40 g/kg lipid), TCL60 (60 g/kg lipid), TCL80 (80 g/kg lipid), TCL100 (100 g/kg lipid), TCL120 (120 g/kg lipid) and TCL140 (140 g/kg lipid). All the finely milled ingredients were properly weighed according to the formulation and kept in separate plastic airtight containers. Except for oils and additives, all the ingredients were uniformly mixed in a mechanical mixer. The dough was prepared and steam cooked for 25 min. After cooling at room temperature, additives and oils were homogenously mixed with the dough and pellets (2 mm dia) were prepared using a pelletizer (S.B. Panchual & Co. Ltd, India). The feed pellets were air-dried at room temperature afterwards in a hot air oven at 42°C till to achieve around 10% moisture level. The dried pellets were properly packed, sealed, labelled, and kept at 4°C until further use.
| Diets (experimental groups)a | ||||||
|---|---|---|---|---|---|---|
| TCL40 | TCL60 | TCL80 | TCL100 | TCL120 | TCL140 | |
| Ingredients composition (g/kg) | ||||||
| Fish Meal | 200 | 200 | 200 | 200 | 200 | 200 |
| Soybean meal | 100 | 100 | 100 | 100 | 100 | 100 |
| Casein | 208 | 208 | 208 | 208 | 208 | 208 |
| Gelatin | 52 | 52 | 52 | 52 | 52 | 52 |
| Starch | 202.5 | 182.5 | 162.5 | 142.5 | 122.5 | 102.5 |
| Dextrin | 150 | 150 | 150 | 150 | 150 | 150 |
| Cellulose | 19.4 | 19.4 | 19.4 | 19.4 | 19.4 | 19.4 |
| Fish oil | 15 | 35 | 55 | 75 | 95 | 115 |
| CMC | 25 | 25 | 25 | 25 | 25 | 25 |
| Vit. min. mixb | 15 | 15 | 15 | 15 | 15 | 15 |
| Soy lecithin | 5 | 5 | 5 | 5 | 5 | 5 |
| Cholesterol | 2 | 2 | 2 | 2 | 2 | 2 |
| BHT | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Betaine hydrochloride | 5 | 5 | 5 | 5 | 5 | 5 |
| Choline chloride | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Stay C 35c | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Vit. E | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition (g/kg dry matter basis) | ||||||
| Dry matter | 911.2 | 912.6 | 909.8 | 910.5 | 913.2 | 911.6 |
| Crude protein | 401.5 | 400.9 | 401.0 | 401.3 | 400.3 | 401.7 |
| Ether extract | 40.8 | 60.5 | 81.3 | 102.1 | 121.5 | 141.9 |
| Crude fibre | 29.2 | 29.1 | 29.4 | 29.5 | 29.6 | 30.1 |
| Nitrogen free extract | 447.0 | 427.7 | 407.7 | 384.6 | 366.9 | 345.4 |
| Total ash | 81.4 | 81.6 | 80.5 | 82.3 | 81.7 | 80.9 |
| GE (Kcal/100 g) | 449.98 | 460.03 | 471.36 | 481.44 | 491.72 | 502.84 |
| DEd (Kcal/100 g) | 376.11 | 385.86 | 396.58 | 405.87 | 416.10 | 426.57 |
| P/Ee (mg CP/Kcal DE) | 106.75 | 103.90 | 101.11 | 98.87 | 96.20 | 94.17 |
- Note: Proximate composition is expressed as mean (n = 3).
- Abbreviations: BHT, butylated hydroxytoluene; CMC, carboxymethyl cellulose; GE, gross energy.
- a TCL40 (40 g/kg dietary lipid), TCL60 (60 g/kg dietary lipid), TCL80 (80 g/kg dietary lipid), TCL100 (100 g/kg dietary lipid), TCL120 (120 g/kg dietary lipid), TCL140 (140 g/kg dietary lipid).
- b Composition of vitamin-mineral mix (PRE-EMIX PLUS) (quantity/kg): Vitamin A, 5,500,000 IU; Vitamin D3, 1,100,000 IU; Vitamin B2, 2000 mg; Vitamin E, 750 mg; Vitamin K, 1000 mg; Vitamin B6, 1000 mg; Vitamin B12, 6 mg; Calcium Pantothenate, 2500 mg; Nicotinamide, 10 g; Choline Chloride, 150 g; Mn, 27,000 mg; I, 1000 mg; Fe, 7500 mg; Zn, 5000 mg; Cu, 2000 mg; Co, 450 L-lysine, 10 g; dl- Methionine, 10 g; Selenium 125 mg; Vitamin C,2500 mg.
- c Stay C 35, protected vitamin C.
- d DE, digestible energy (Kcal/100 g) = {4 × CP (g/100 g) + 9 × EE (g/100 g) + 4 × NFE (g/100 g)} (Halver, 1976).
- e P/E, protein to energy ratio (mg CP/Kcal DE) = {(CP% × 1000)/DE}.
2.4 Collection, filling and storage of IGSW
IGSW of identical salinity was taken out and passed through a fixed filter bag (80 μm mesh size). Subsequently, the filtered water was filled into rectangular tanks (3.1 × 2.1 × 1.1 m3, 10,000 L capacity) and kept for a week. After that, IGSW was pumped into circular FRP storage tanks (10.03 m2 × 0.87 m, 1000 L water capacity). These storage tanks were fitted with round a clock aeration facility and fortified with commercially available muriate of potash (KCl) as and when required. The requisite was calculated using the formula: K+ requirement for fortification = {(Experimental salinity level × 10.7) − IGSW available K+} (Davis et al., 2005).
2.5 Setting up of experiment and feeding trial
For setting up of the trial, 18 circular tanks (8.82 m2 × 51 m, 350 L capacity and 290 L water volume) were thoroughly washed with potassium permanganate (KMnO4) solution (5 g/L) followed using freshwater and dried under direct sunlight. The tanks were then filled with fortified IGSW with continuous aeration. Before stocking, shrimps were starved overnight, and a digital weighing balance recorded carefully initial weight. Well acclimatized two hundred and seventy shrimps (avg. wt., 4.04 ± 0.03 g) were randomly distributed (15 shrimps/tank) among six experimental groups in triplicates following a completely randomized design (CRD). Respective diets were used to feed the shrimp four times a day (7:30, 13:30, 18:30 and 23:30 h). Faecal matter from each tank was siphoned out prior to next day's feeding, and an equal volume of water was replenished from the storage tank. About 20–25% water from each tank was exchanged with IGSW of 15 ppt salinity from the storage facility at the interval of 3 days during the experimental period. Fortnightly, the total biomass of the shrimps from each tank was taken to calculate the daily ration size. Mortality of shrimps was recorded to calculate the survival percentage.
Water quality parameters like temperature, pH and dissolved oxygen (DO) were estimated using a digital thermometer (Oxyguard International, India), digital pH meter (Manti Lab India, Pvt. Ltd.) and portable DO meter (Manti Lab India, Pvt. Ltd.), respectively, thrice a day. At every 3 days intervals, Physico-chemical parameters of water like salinity, free carbon-di-oxide (CO2), total alkalinity, total hardness, ammonia-N (TA-N), nitrite-N (NO3-N), nitrate-N (NO2-N), calcium, magnesium were analysed as per the method described using APHA (1998). A digital automated flame photometer (1835, Esico India Pvt. Ltd.) was used to determine K+ concentration in IGSW. Experimental IGSW quality parameters like temperature ranged between 26.03 ± 0.3 to 27.15 ± 0.56°C, pH 8.35 ± 0.11 to 8.47 ± 0.18, salinity 15.15 ± 0.18 to 15.36 ± 0.17 g/L, dissolved oxygen 6.81 ± 0.42 to 7.09 ± 0.30 mg/L, total alkalinity 297.55 ± 6.71 to 309.59 ± 6.52 mg/L, total hardness 2948.00 ± 9.61 to 2975.00 ± 17.73 mg/L with no detectable free CO2. The TA-N, NO2-N and NO3-N were found in the range of 0.17 ± 0.03 to 0.22 ± 0.03 mg/L, 0.12 ± 0.01 to 0.14 ± 0.01 mg/L, 0.24 ± 0.02 to 0.30 ± 0.03 mg/L, respectively, during the experimental period. The concentrations of calcium, magnesium and potassium were ranged from 241.34 ± 3.97 to 250.98 ± 3.61 mg/L, 533.79 ± 3.02 to 541.72 ± 3.46 mg/L, and 100.53 ± 0.67 to 101.46 ± 0.93 mg/L, respectively (Table 2).
| Parameters | aDiets (experimental groups) | ||||||
|---|---|---|---|---|---|---|---|
| TCL40 | TCL60 | TCL80 | TCL100 | TCL120 | TCL140 | p-value | |
| Temperature (°C) | 26.17 ± 0.46 | 26.03 ± 0.34 | 26.79 ± 0.26 | 27.15 ± 0.56 | 26.59 ± 0.23 | 27.07 ± 0.21 | 0.761 |
| pH | 8.44 ± 0.15 | 8.41 ± 0.13 | 8.47 ± 0.18 | 8.36 ± 0.09 | 8.41 ± 0.17 | 8.35 ± 0.11 | 0.653 |
| Salinity (g/L) | 15.15 ± 0.18 | 15.21 ± 0.23 | 15.27 ± 0.09 | 15.31 ± 0.19 | 15.36 ± 0.17 | 15.34 ± 0.12 | 0.723 |
| DO (mg/L) | 6.81 ± 0.42 | 6.92 ± 0.46 | 6.99 ± 0.24 | 6.94 ± 0.43 | 7.05 ± 0.52 | 7.09 ± 0.30 | 0.638 |
| Free CO2 (mg/L) | Nil | Nil | Nil | Nil | Nil | Nil | - |
| Alkalinity (mg/L) | 299.74 ± 3.89 | 297.55 ± 6.71 | 305.98 ± 4.65 | 302.36 ± 4.21 | 305.39 ± 5.46 | 309.59 ± 6.52 | 0.823 |
| Hardness (mg/L) | 2959.00 ± 14.64 | 2966.00 ± 15.62 | 2951.50 ± 11.27 | 2975.00 ± 17.73 | 2948.00 ± 9.61 | 2956.50 ± 10.12 | 0.757 |
| TA-N (mg/L) | 0.22 ± 0.03 | 0.19 ± 0.04 | 0.20 ± 0.03 | 0.19 ± 0.04 | 0.17 ± 0.03 | 0.18 ± 0.02 | 0.569 |
| NO2-N (mg/L) | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.03 | 0.618 |
| NO3-N (mg/L) | 0.30 ± 0.03 | 0.28 ± 0.02 | 0.29 ± 0.04 | 0.24 ± 0.02 | 0.27 ± 0.02 | 0.28 ± 0.03 | 0.702 |
| Ca2+ (mg/L) | 241.34 ± 3.97 | 243.86 ± 2.68 | 247.80 ± 3.42 | 248.67 ± 2.73 | 250.98 ± 3.61 | 242.05 ± 4.74 | 0.839 |
| Mg2+ (mg/L) | 533.79 ± 3.02 | 535.62 ± 2.95 | 538.74 ± 2.79 | 539.78 ± 3.99 | 541.72 ± 3.46 | 534.09 ± 2.16 | 0.694 |
| K+ (mg/L) | 100.61 ± 1.24 | 100.53 ± 0.67 | 100.58 ± 0.48 | 101.21 ± 0.74 | 101.46 ± 0.93 | 101.01 ± 1.11 | 0.688 |
- Notes: Data are expressed as mean ± SE (n = 6); Mean values in the same row with different superscripts differ significantly (p < 0.05).
- Abbreviations: CO2, carbon di-oxide; DO, dissolved oxygen; NO2-N, nitrite nitrogen; NO3-N, nitrate nitrogen; TA-N, total ammonia nitrogen.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
2.6 Sampling
Prior to commencement of the experiment, 25 shrimps were randomly collected for initial body composition analysis. After completion of the trial, shrimps were starved overnight to measure final body weight. Randomly three shrimps from each replicate (nine shrimps from a treatment) were taken out for the final proximate composition analysis. Five shrimps from each tank were anaesthetized with chilled iced water and hemolymph was drawn from the shrimp's heart and pooled (Antony et al., 2015). For collection of serum, hemolymph samples were kept in a freezer (4°C) for 20 min followed using crushing and centrifugation at 3500g for 12 min (Tantulo & Fotedar, 2006). Again, from three shrimps of each treatment (one from each replicate), hemolymph was collected and mixed with ice-cold SIC-EDTA anticoagulant in a 1:2 ratio for estimation of hemocyanin content (Vargas-Albores et al., 1993). From these eight shrimps carefully hepatopancreas (weight was recorded for calculation of HPSI) and intestine were dissected out and quickly homogenized (5% homogenate) in iced phosphate buffer solution (pH 7.4) for assay of digestive enzymes. Gills were homogenized (10% homogenate) in iced SEI buffer (pH 7.3) using an automated homogenizer (Miccra, D9, Remi India) under iced conditions (Jana et al., 2021; Saraswathy et al., 2021) for Na+/K+-ATPase assay. The tissue homogenates were then centrifuged at 7000g for 15 min in a refrigerated centrifuge, and the aliquot supernatant was collected in sample vials. All the samples were properly labelled and stored at −40°C until further use.
2.7 Proximate composition of the diets and tissues
The lipid levels of the experimental diets viz., TCL40, TCL60, TCL80, TCL100, TCL120 and TCL140 were 40.8, 60.5, 81.3, 102.1, 121.5 and 141.9 g/kg, respectively (Table 1). Dietary crude protein (CP), crude fibre (CF), total ash (TA) and nitrogen-free extract (NFE) ranges between 400.3 to 401.7, 29.1 to 30.1, 80.5 to 82.3 and 345.4 to 447 g/kg, respectively, in different experimental diets. The values of gross energy (GE), digestible energy (DE) and protein to energy ratio (P: E) range between 449.98 to 502.84 Kcal/100 g, 376.11 to 426.57 Kcal/100 g and 94.17 to 106.75 mg CP/Kcal DE, respectively (Table 1). Thus, the semi-purified experimental diets were iso-nitrogenous, hetero-lipidic and hetero-energetic.
2.8 Growth metrics, nutrient utilization and survival
2.9 Hemato-biochemical analysis
Hemolymph hemocyanin was estimated according to Chen and Cheng (1993a) and Chen and Cheng (1993b). In a quartz cuvette, 10 μl hemolymph was mixed with 990 μl demineralized water and reading was taken in a double beam spectrophotometer (Shimadzu 1800, Shimadzu Corporation, Pvt. Ltd., China) at an optical density of 335 nm. For calculation of the final concentration of hemocyanin, an extinction coefficient of E = 17.26 was used based on the 74 KDa functional subunit of shrimp.
2.10 Enzyme assay
2.10.1 Quantification of tissue protein
Protein quantification in different tissue homogenates was performed according to Lowry et al. (1951). The values of tissue protein obtained were used for calculating the activities of tissue specific-enzymes.
2.10.2 Activities of Na+/K+-ATPase
Na+/K+-ATPase specific activities (micromoles of ADP released/h/mg protein at 37°C) in the gill lamellae were measured according to the method of McCormick (1993).
2.10.3 Activities of digestive enzymes
Trypsin specific activities (units/min/mg protein at 37°C) were estimated using following Na-benzoyl-DL-arginine-p-nitroanilide (BAPNA) substrate method (Garcia-Carreno et al., 1994). The activities of amylases (micromole of maltose released/ mg protein/ min at 37°C) were determined as described by Rick and Stegbauer (1974). Lipase specific activities (units/min/mg protein at 37°C) were assayed according to Cherry and Crandall Jr. (1932).
2.11 Statistical analysis
Results were analysed through one-way analysis of variance (ANOVA) using SPSS (22 for windows) software. Overall, linear and quadratic trends of the parameters dietary lipid levels were confirmed using employing the orthogonal-polynomial contrast analysis. Duncan multiple range tests (DMRT) along with post-hoc analysis were applied to observe the significant differences among the means between the treatments at a 5% probability level (p < 0.05). Second-degree polynomial analysis was executed to optimize the dietary lipid requirement of white-leg shrimp, P. vannamei reared in IGSW of 15 ppt salinity.
3 RESULTS
3.1 Growth metrics, nutrient utilization and survivability of shrimp
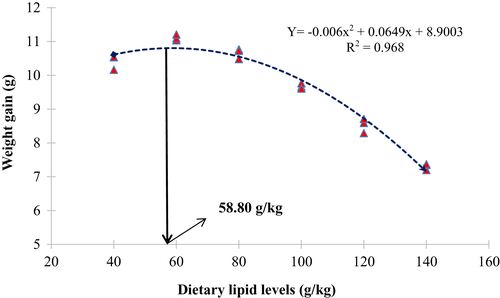
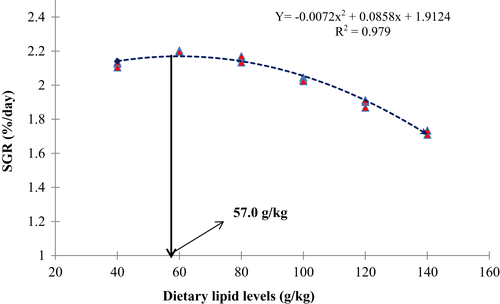
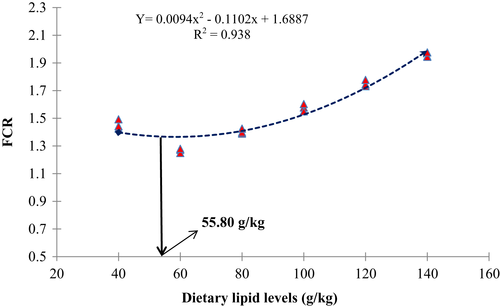
Growth metrics and nutrient utilization parameters of juvenile white-leg shrimp, P. vannamei varied significantly (p < 0.05) owing to varying dietary lipid levels (Table 3). The highest (p < 0.05) weight gain (WG) and specific growth rate (SGR) were observed in the TCL60 group, followed by TCL80 and TCL40 group, whereas the TCL140 group exhibited the lowest (p < 0.05) value. Similarly, TCL60 and TCL140 groups exhibited the lowest and highest (p < 0.05) feed conversion ratio (FCR), respectively. However, a decreasing trend was observed for protein efficiency ratio (PER) with the increased lipid levels in the diet. Hepatopancreas-somatic index (HPSI) and survival rate of shrimp were found to be similar (p > 0.05) among the treatment groups. Regression analysis revealed variable linear and quadratic correlation of WG (linear R2 = 0.80 and quadratic R2 = 0.97), SGR (linear R2 = 0.79 and quadratic R2 = 0.98) and FCR (linear R2 = 0.77 and quadratic R2 = 0.94), PER (linear R2 = 0.73 and quadratic R2 = 0.87) in relation to the dietary lipid levels. However, dietary lipid levels exhibited poor linear and quadratic relations with survival rate (linear R2 = 0.09 and quadratic R2 = 0.11) and HPSI (linear R2 = 0.09 and quadratic R2 = 0.11) of the treatment groups. Based on second-degree polynomial analysis of WG, SGR and FCR with the dietary lipid levels, optimum values of dietary lipid were found to be 58.80, 57.0 and 55.80 g/kg, respectively, for white-leg shrimp, P. vannamei reared in IGSW (Figures 1-3).
| Diets (experimental groups)a | Parameters | Survival (%) | HPSI (%) | ||||
|---|---|---|---|---|---|---|---|
| WG (g) | SGR (%/day) | FCR | PER | ||||
| TCL40 | 10.41d | 2.12d | 1.45c | 1.72d | 91.11 | 1.76 | |
| TCL60 | 11.11f | 2.20f | 1.27a | 1.97f | 93.33 | 1.72 | |
| TCL80 | 10.66e | 2.15e | 1.41b | 1.77e | 84.45 | 1.73 | |
| TCL100 | 9.68c | 2.03c | 1.58d | 1.58c | 88.89 | 1.72 | |
| TCL120 | 8.53b | 1.89b | 1.75e | 1.43b | 88.89 | 1.73 | |
| TCL140 | 7.31a | 1.73a | 1.96f | 1.27a | 86.67 | 1.71 | |
| SEM | 0.32 | 0.04 | 0.06 | 0.06 | 1.20 | 0.01 | |
| Contrast analysis, p value | |||||||
| Overall | <0.001 | <0.001 | <0.001 | <0.001 | 0.366 | 0.692 | |
| Linear | <0.001 | <0.001 | <0.001 | <0.001 | 0.219 | 0.275 | |
| Quadratic | <0.001 | <0.001 | <0.001 | <0.001 | 0.622 | 0.572 | |
| Regression equation, R2 value | |||||||
| Linear | Equationb | y = −0.3464x + 12.041 | y = −0.0431x + 2.3227 | y = 0.0591x + 1.1547 | y = −0.0575x + 2.0256 | y = −0.4444x + 92 | y = −0.0029x + 1.7464 |
| R2 | 0.80 | 0.79 | 0.77 | 0.73 | 0.09 | 0.09 | |
| Quadratic | Equationb | y = −0.0553x2 + 0.4277x + 9.9769 | y = −0.0072x2 + 0.0572x + 2.0554 | y = 0.0094x2–0.0726x + 1.5059 | y = −0.0084x2 + 0.0605x + 1.7109 | y = 0.0595x2–1.2778x + 94.222 | y = 0.0005x2–0.01x + 1.7653 |
| R2 | 0.97 | 0.98 | 0.94 | 0.87 | 0.11 | 0.11 | |
- Notes: Data are expressed as mean (n = 3); Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Abbreviations: FCR, feed conversion ratio; HPSI, hepatopancreatic-somatic index; PER, protein efficiency ratio; SEM, average standard error of means; SGR, specific growth rate; WG, weight gain.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
- b In equation, ‘x’ and ‘y’ represents dietary lipid levels and the respective parameters.
3.2 Proximate composition of the whole body of shrimp
Proximate analysis of whole shrimp body showed that moisture and lipid contents exhibited a significant (p < 0.05) inverse relationship with the increasing dietary lipid levels (Table 4). The lowest and highest (p < 0.05) moisture and lipid contents in shrimp's whole body were observed in TCL140, TCL40 and TCL40, TCL140 groups, respectively. However, whole shrimp body protein and ash content did not vary significantly (p > 0.05) among the treatment groups with the increasing levels of dietary lipid. Regression analysis of shrimp whole body moisture (linear R2 = 0.87 and quadratic R2 = 0.88) and crude lipid (linear R2 = 0.98 and quadratic R2 = 0.98) showed a linear and quadratic relation, whereas shrimp whole body protein and total ash showed no defined correlation with respect to crude lipid level of the experimental diets.
| Diets (experimental groups)a | Proximate composition (g/kg) | ||||
|---|---|---|---|---|---|
| Moisture | Crude protein | Crude lipid | Total ash | ||
| IBC | 748.67d | 175.72 | 12.55a | 32.14 | |
| FBC | |||||
| TCL40 | 748.50d | 175.83 | 12.60a | 32.01 | |
| TCL60 | 741.73c | 175.10 | 18.97b | 33.13 | |
| TCL80 | 732.90b | 176.60 | 37.50c | 32.20 | |
| TCL100 | 733.37b | 175.10 | 44.67d | 31.90 | |
| TCL120 | 724.63a | 174.73 | 56.30e | 31.40 | |
| TCL140 | 720.03a | 175.53 | 63.20f | 31.93 | |
| SEM | 1.88 | 0.37 | 4.45 | 0.52 | |
| Contrast analysis, p value | |||||
| Overall | <0.001 | 0.808 | <0.001 | 0.976 | |
| Linear | <0.001 | 0.632 | <0.001 | 0.647 | |
| Quadratic | 0.586 | 0.983 | 0.002 | 0.927 | |
| Regression equation, R2 value | |||||
| Linear | Equationb | y = −2.7594x + 752.84 | y = −0.0586x + 175.89 | y = 5.3167x + 1.6556 | y = −0.0833x + 32.678 |
| R2 | 0.87 | 0.02 | 0.98 | 0.02 | |
| Quadratic | Equationb | y = 0.0503x2–3.4634x + 754.72 | y = 0.0009x2 − 0.0711x + 175.93 | y = −0.1113x2 + 6.875x - 2.5 | y = −0.0057x2 − 0.0042x + 32.467 |
| R2 | 0.88 | 0.02 | 0.98 | 0.02 | |
- Notes: Data are expressed as mean (n = 3); Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Abbreviations: FBC, final whole body composition; IBC, initial whole body composition; SEM, average standard error of means.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
- b In equation, ‘x’ and ‘y’ represents dietary lipid levels and the respective parameters.
3.3 Hemolymph hemocyanin and serum biochemical indices
Total triglyceride and cholesterol contents of serum significantly (p < 0.05) increased and decreased, respectively, in a similar fashion to the dietary lipid levels (Table 5). However, hemolymph hemocyanin and concentrations of glucose and total protein in the serum did not show any significant (p > 0.05) variations between the groups. On the other hand, there was a significant (p < 0.05) increment in serum triglyceride and cholesterol contents while enhancing the lipid levels in the diet up to the TCL60 group (60 g lipid/kg), and then gradually decreased with the lowest (p < 0.05) value observed in TCL140 (140 g lipid/kg diet) group. Regression analysis of serum total protein, hemocyanin and glucose contents did not show any defined correlation while serum cholesterol (quadratic R2 = 0.60) and triglyceride (linear R2 = 0.68 and quadratic R2 = 0.79) exhibited a high correlation with the dietary lipid levels.
| Diets (experimental groups)a | Total Proteinb (g/dL) | Hemocyanin (Hc)c (mmol/L) | Glucosec (mg/dL) | Cholesterolc (mg/dL) | Triglyceridec (mg/dL) | |
|---|---|---|---|---|---|---|
| TCL40 | 26.65 | 1.75 | 18.45 | 166.87a | 189.68c | |
| TCL60 | 24.61 | 1.74 | 20.60 | 178.93c | 209.45e | |
| TCL80 | 25.78 | 1.73 | 22.29 | 173.71c | 199.64d | |
| TCL100 | 26.11 | 1.73 | 19.78 | 162.95b | 176.59b | |
| TCL120 | 25.88 | 1.71 | 21.26 | 156.62a | 163.15a | |
| TCL140 | 26.35 | 1.75 | 20.98 | 151.60a | 158.26a | |
| SEM | 0.26 | 0.01 | 0.72 | 2.57 | 4.58 | |
| Contrast analysis, p value | ||||||
| Overall | 0.281 | 0.910 | 0.787 | <0.001 | <0.001 | |
| Linear | 0.599 | 0.677 | 0.463 | <0.001 | <0.001 | |
| Quadratic | 0.224 | 0.389 | 0.471 | <0.001 | <0.001 | |
| Regression equation, R2value | ||||||
| Linear | Equationd | y = 0.038x + 25.631 | y = −0.0013x + 1.7429 | y = 0.173x + 19.348 | y = −1.2003x + 171.18 | y = −4.558x + 214.7 |
| R2 | 0.02 | 0.01 | 0.04 | 0.14 | 0.68 | |
| Quadratic | Equationd | y = 0.0309x2–0.3949x + 26.785 | y = 0.0009x2 − 0.0142x + 1.7773 | y = −0.0581x2 + 0.9869x + 17.178 | y = −0.7137x2 + 8.7912x + 144.54 | y = −0.6151x2 + 4.0541x + 191.74 |
| R2 | 0.10 | 0.07 | 0.08 | 0.60 | 0.79 | |
- Notes: Data are expressed as mean (n = 3); Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Abbreviation: SEM, average standard error of means.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
- b Using hemolymph.
- c Using serum.
- d In equation, ‘x’ and ‘y’ represents dietary lipid levels and the respective parameters.
3.4 Na+/K+-ATPase enzyme activity, osmolality and osmoregulatory capacity (OC) of shrimp
Na+/K+-ATPase enzyme-specific activity in gill, osmoregulatory capacity and osmolality of cultured water and shrimp serum exhibited no significant (p > 0.05) relationship with respect to different lipid levels of the diet (Table 6). Very poor linear and quadratic correlation of water and shrimp serum osmolality, Na+/K+-ATPase activities in gill and osmoregulatory capacity were observed in relation to dietary lipid levels.
| Diets (experimental groups)a | Serum osmolarityb | Water osmolarityb | Osmoregulatory capacityb (OC) | Na+/K+-ATPasec | |
|---|---|---|---|---|---|
| TCL40 | 687.32 | 251.64 | 435.68 | 13.67 | |
| TCL60 | 687.45 | 248.91 | 438.54 | 13.17 | |
| TCL80 | 686.72 | 251.10 | 435.62 | 13.85 | |
| TCL100 | 684.62 | 248.18 | 436.45 | 13.38 | |
| TCL120 | 688.12 | 252.06 | 436.06 | 13.06 | |
| TCL140 | 688.33 | 248.58 | 439.76 | 12.96 | |
| SEM | 1.34 | 0.94 | 1.30 | 0.20 | |
| Contrast analysis, p value | |||||
| Overall | 0.984 | 0.792 | 0.944 | 0.827 | |
| Linear | 0.879 | 0.682 | 0.658 | 0.359 | |
| Quadratic | 0.63 | 0.897 | 0.674 | 0.692 | |
| Regression equation, R2 value | |||||
| Linear | Equationd | y = 0.0712x + 686.6 | y = −0.1254x + 250.95 | y = 0.1966x + 435.64 | y = −0.0618x + 13.78 |
| R2 | 0.00 | 0.01 | 0.02 | 0.07 | |
| Quadratic | Equationd | y = 0.0774x2 − 1.0126x + 689.49 | y = 0.0135x2 − 0.3143x + 251.46 | y = 0.0639x2 − 0.6982x + 438.03 | y = −0.009x2 + 0.0642x + 13.444 |
| R2 | 0.02 | 0.01 | 0.03 | 0.08 | |
- Notes: Data are expressed as mean (n = 3); Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Abbreviation: SEM, average standard error of means.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
- b Water osmolality, serum osmolality and osmoregulatory capacity (OC) are expressed as mOsmol/kg.
- c Branchial Na+/K+-ATPase activity is expressed as micromoles of ADP released/hour/mg protein at 37°C.
- d In equation, ‘x’ and ‘y’ represents dietary lipid levels and the respective parameters.
3.5 Hepatopancreatic and intestinal digestive enzymes activities
Hepatopancreatic lipase and amylase specific activities showed significant (p < 0.05) differences among the groups in relation to varying dietary lipid levels, with the highest (p < 0.05) activities were observed inTCL80 (80 g lipid/kg diet) and TCL120 (120 g lipid/kg diet) groups, respectively, and beyond that level, the activity of these enzymes gradually decreased (Table 7). Regression analysis revealed that dietary lipid levels exhibited poor linear and quadratic relations with hepatopancreatic and intestinal trypsin, intestinal amylase and intestinal lipase enzyme activities. However, the activities of hepatopancreatic amylase (linear R2 = 0.35 and quadratic R2 = 0.51) and lipase (quadratic R2 = 0.90) enzymes exhibited variable linear and quadratic relations with graded levels of dietary lipid.
| Diets (experimental groups)a | Digestive enzymes | ||||||
|---|---|---|---|---|---|---|---|
| Trypsinb | Amylasec | Lipased | |||||
| Hepatopancreas | Intestine | Hepatopancreas | Intestine | Hepatopancreas | Intestine | ||
| TCL40 | 9.48 | 9.08 | 1.93ab | 1.69 | 2.36b | 8.67 | |
| TCL60 | 9.55 | 9.11 | 1.84a | 1.78 | 2.57c | 8.69 | |
| TCL80 | 9.54 | 9.11 | 2.44cd | 1.66 | 2.75d | 8.67 | |
| TCL100 | 9.48 | 9.15 | 2.56e | 1.75 | 2.67cd | 8.69 | |
| TCL120 | 9.55 | 9.08 | 2.62ef | 1.68 | 2.44b | 8.68 | |
| TCL140 | 9.54 | 9.08 | 2.32c | 1.68 | 2.21a | 8.71 | |
| SEM | 0.02 | 0.02 | 0.09 | 0.02 | 0.05 | 0.03 | |
| Contrast analysis, p value | |||||||
| Overall | 0.861 | 0.980 | 0.009 | 0.204 | <0.001 | 0.999 | |
| Linear | 0.620 | 0.903 | 0.003 | 0.368 | 0.002 | 0.764 | |
| Quadratic | 0.891 | 0.541 | 0.031 | 0.410 | <0.001 | 0.900 | |
| Regression equation, R2 value | |||||||
| Linear | Equatione | y = 0.0035x + 9.4996 | y = −0.001x + 9.1084 | y = 0.0623x + 1.8484 | y = −0.004x + 1.7347 | y = −0.0168x + 2.6173 | y = 0.0027x + 8.6649 |
| R2 | 0.02 | 0.00 | 0.35 | 0.04 | 0.09 | 0.01 | |
| Quadratic | Equatione | y = −0.0003x2 + 0.0081x + 9.4873 | y = −0.0018x2 + 0.0244x + 9.0407 | y = −0.0143x2 + 0.2627x + 1.314 | y = −0.0013x2 + 0.0135x + 1.688 | y = −0.0172x2 + 0.2239x + 1.9757 | y = 0.0004x2–0.0027x + 8.6793 |
| R2 | 0.02 | 0.03 | 0.51 | 0.08 | 0.90 | 0.01 | |
- Notes: Data are expressed as mean (n = 3); Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Mean values in the same column with different superscripts differ significantly (p < 0.05).
- Abbreviation: SEM, average standard error of means.
- a TCL40-TCL140, 40–140 g/kg dietary crude lipid.
- b Trypsin activity is expressed as units/min/mg protein at 37°C.
- c Amylase activity is expressed as micromole maltose released/min/mg protein at 37°C.
- d Lipase activity is expressed as units/min/mg protein at 37°C.
- e In equation, ‘x’ and ‘y’ represents dietary lipid levels and the respective parameters.
4 DISCUSSION
IGSW quality parameters of the present study like temperature, pH, DO, total alkalinity, total hardness, Ca2+, Mg2+ and K+ ions concentration were found within the recommended levels described by Talukdar et al. (2021) and Jahan et al. (2018) required for maximum growth performance of white-leg shrimp, P. vannamei in IGSW. The presence of higher levels of nitrogenous compounds in water like TA-N is highly toxic and lethal to shrimp (Burford & Lorenzen, 2004), causing severe stress, poor growth and even death of the cultured animal (Carbajal-Hernández et al., 2012). However, the level of toxicity also depends upon the size of the cultured animal, DO, temperature, pH, salinity and duration of exposure (Magallón Barajas et al., 2006). The level of TA-N was within the acceptable limit for P. vannamei culture (Talukdar et al., 2021). Nevertheless, TA-N is more lethal to shrimp than NO3-N, and NO2-N concentration in the surrounding water, whereas 100 mg/L of NO2-N in water is reported to be deadly for shrimps (Van Rijn et al., 2006).
Previous studies on the dietary lipid requirement of P. vannamei explained that culture salinity and different growth stages could influence the dietary lipid requirement (Chen et al., 2014; González-Félix, Lawrence, et al., 2002; Hamidoghli et al., 2020; Jannathulla et al., 2019; Xie et al., 2019; Xu et al., 2018; Zhang et al., 2013; Zhu et al., 2010). In the current study, higher quadratic values of WG and SGR (R2 = 0.97 and 0.98, respectively) were observed with the dietary lipid level up to 60 g/kg (TCL60) diet beyond this level growth reduced significantly. This agrees with the findings of González-Félix, Lawrence, et al. (2002) and González-Félix, Gatlin III, et al. (2002), who concluded that 30–90 g lipid/kg is optimum for higher survival, growth and production of juvenile P. vannamei at 25 ppt ambient salinity but growth performance gradually decreases at higher levels of dietary lipid. On the other hand, Hamidoghli et al. (2020) reported that P. vannamei juveniles require 56–60 g lipid/kg when reared in the biofloc system. The lowest FCR was recorded in the TCL60 group, but further enhancing the lipid levels beyond 60 g/kg significantly increased the value, suggesting efficient utilization of lipid using white-leg shrimp at this level to satisfy the energy requirement for higher growth, which is in agreement with Hamidoghli et al. (2020) and Jannathulla et al. (2019). In the current investigation, growth metrics and feed utilization using shrimp significantly reduced in higher lipid fed groups which might be attributed to increased metabolic stress on the cultured animal (Jannathulla et al., 2019). In contrast some authors (Xie et al., 2019; Xu et al., 2012; Zhang et al., 2013) recommended more than 100 g lipid/kg for optimum growth, survival, immune response and stress resistance of juvenile and post-larvae of whiteleg shrimp, P. vannamei under different culture salinities. In our present study, poor linear and quadratic values for survival and HPSI demonstrate that dietary lipids did not influence the survival rate and HPSI of P. vannamei juveniles reared in IGSW.
Contrast analysis of shrimp whole body composition indicated that feeding varying levels of dietary lipid has a significant effect on lipid and moisture contents in the body of shrimp. Shrimp whole-body lipid and moisture contents increased and decreased, respectively, with the dietary lipid level up to 140 g/kg. At the same time, shrimp whole-body ash and protein contents did not vary significantly among the treatment groups. Therefore, a diet with 400 g CP/kg and 60 g lipid/kg corresponding with 103.90 mg protein/Kcal DE P: E ratio could satisfy the protein and energy needs with higher body protein accretion at 15 ppt salinity. Moreover, at higher salinity regimes, energy requirement increases for osmoregulatory responses. Therefore energy satiation could be fulfilled using the protein-sparing action of non-protein nutrients to increase the somatic growth performance of shrimp. A similar observation was also made by Zhang et al. (2013), Xu et al. (2018) and Jannathulla et al. (2019) in P. vannamei juveniles. On the other hand, in contrast to our observation, Xie et al. (2019) and Hamidoghli et al. (2020) demonstrated significant variation in white shrimp's whole-body protein and ash contents due to feeding of graded levels of lipid.
Chen and Cheng (1995) and Adachi et al. (2003) described that subsequently after salinity adaptation, body amino acid pool derived amino acids are directly directed towards the synthesis of new tissues, osmoregulation, energy needs and immuno modulation. Pascual et al. (2003) also stated that the concentration of free amino acids in the hemolymph is very less as shrimps largely convert newly synthesized protein to hemocyanin (65%–95% of the hemolymph protein) after acclimatized to the ambient water salinity. In the present study, hemolymph hemocyanin and serum total protein contents did not differ significantly by feeding with different lipid levels. Depending upon the physiological status of shrimp, amino acids derived from lower dietary protein levels can be efficiently used either for osmoregulatory responses to metabolic energy needs. However, a diet with optimum levels of protein along with required levels of lipid could support the protein-sparing effect where dietary non-protein sources will supply the energy to meet the physiological energy demand, and a major portion of the amino acids will be directed towards the production and deposition of protein in the tissue of shrimp (Cuzon et al., 2004; Dall & Smith, 1986; Hamidoghli et al., 2018). Due to this reason, a diet with 400 g CP/kg with 60 g lipid/kg (TCL60) is attributed to maximum growth performance in shrimp. However, an excess dietary lipid and protein can cause a metabolic load on the shrimp resulting in growth retardation. This is in agreement with the observation by several authors (Hamidoghli et al., 2020; Xu et al., 2018) in P. vannamei.
Shan et al. (2019) described that a higher concentration of glucose in serum resulted in stress in shrimp associated with higher energy needs. In the present study, no significant difference was found in the serum glucose levels of shrimp while increasing the lipid levels of the diet. Shrimps have a restricted capability to convert glucose via glycolytic or glycogenesis pathways (Kumar et al., 2018; Rosas et al., 2000). Furthermore, shrimps could able to store very less quantity of carbohydrates in their body due to saturation of glycogenesis pathway at carbohydrate level 230 g/kg diet (Rosas et al., 2000). Concentrations of triglyceride and cholesterol in the serum are the key biomarkers of growth response in respect of diet design and formulations (Adhikari et al., 2004; Maheswaran et al., 2008). In the present study, total triglyceride and cholesterol contents in the serum increased significantly up to 60 g lipid/kg and then decreased significantly at higher lipid levels. Several authors well support this finding (Chen et al., 2014; Hamidoghli et al., 2020; Jannathulla et al., 2019; Xu et al., 2018) who described that dietary lipid at higher levels might likely reduce feed intake and poor feed utilization using shrimp P. vannamei resulting in lower total triglyceride and cholesterol contents in shrimp.
Among all the cultivable shrimp species, P. vannamei can easily adopt and withstand a wider ambient salinity ranges from 1 to 50 g/L, but optimum salinity is reported to be 15–30 ppt for better growth, survival rate and health status (Dall & Smith, 1981; Lightner et al., 2009; Pante, 1990). But deviation from the recommended culture salinity range results in breakdown of a significant amount of macro nutrients to meet the higher energy demand associated with the salinity induced osmoregulatory stress (Talukdar et al., 2021). These physiological adaptations lead to the reduced growth performance of shrimp and thus increase the aquaculture production cost. In the current study, no significant variation was observed in osmoregulatory capacity and osmolality of serum of the experimental shrimp. This may be because of hyper osmoregulation as a function of serum osmolality regulated through water salinity lesser than the iso-osmotic point (Castille Jr & Lawrence, 1981). In corroboration to our findings, Roy et al. (2007) stated that serum osmolality did not influence either dietary protein or lipid levels as it is a major function of water salinity. Thus, lower lipid levels in the diet may support the energy satiation for osmoregulatory adaptation as shrimp priorly ensures its physiological homeostasis due to deviation from the recommended optimum salinity range resulting in growth retardation of the shrimp.
In all the crustaceans, including shrimp, branchial Na+/K+-ATPase is directly involved in the osmoregulatory process and regulates the movement of ions throughout the body and the activation of the enzyme is directly related to the potassium ion concentration (Mantel & Farmer, 1983; Perry & Fryer, 1997). In our current study, no significant variation was found in Na+/K+-ATPase enzyme activities in the gill of the shrimp. This is attributed to the similar concentration of K+ ions in the rearing water after fortification with potash. In support of our findings, Hurtado et al. (2007) and Jana et al. (2021) also documented that dietary lipid or protein or the ambient water salinity did not influence the Na+/K+-ATPase enzyme activities in the gill of shrimp.
Proteolytic enzymes like trypsin and chymotrypsin play a major role in protein digestion as pepsin is absent in shrimps (Hernández & Murueta, 2009). However, Gaxiola et al. (2005) specified that requirement of dietary nutrients and availability of substrates in the digestive tract could alter the activation and function of digestive enzymes in shrimps. Hepatopancreatic amylase and lipase activities exhibited a significantly decreasing and increasing relationship, respectively, with the increasing dietary lipid levels which can be corroborated with the report of Lista and Velásquez (2003) who found that shrimps generally require lower levels of dietary lipid and higher levels beyond 100 g lipid/kg could not be sufficiently utilized. Similarly, Hamidoghli et al. (2020) concluded that feeding a lower dietary lipid level 45–90 g/kg diet could support the higher lipase activities in the hepatopancreas of shrimp and beyond that level the activity decreases gradually. Cuzon et al. (2004) and Li et al. (2008) also described the induced digestive enzyme activities for enhanced energy-dense nutrients to satiate the energy burden produced over iso-osmotic deviation stress. However, hepatopancreatic and intestinal trypsin activities did not differ significantly, which may be attributed to feeding similar dietary protein levels to different treatments.
5 CONCLUSION
From the current study, it can be recommended that a 55.80 to 58.80 g lipid/kg diet could be optimum for higher growth and production of white-leg shrimp, P. vannamei in 15 ppt IGSW. Feeding dietary lipid beyond this level reduced the growth performance. Nevertheless, the feed used for P. vannamei culture in the inland saline water is not yet standardized and the farmers are relying on regular commercial feeds available in the market. The outcome of the present study will serve as baseline information for the aqua feed industry to formulate an appropriately nutritious diet for sustainable shrimp culture in IGSW.
AUTHOR CONTRIBUTIONS
Prasanta Jana investigated the study, validated data, implemented software programs and prepared the original draft. Narottam Prasad Sahu conceptualized the study, supervised the study, and reviewed and edited the manuscript. Parimal Sardar designed methodology, involved in visualization and validation, and reviewed and edited the manuscript. Tincy Varghese and Ashutosh Dharmendra Deo performed data curation and validation. Nazeemashahul Shamna was involved in visualization and validation, and edited the manuscript. Vungurala Harikrishna supervised the study and edited the manuscript. Mritunjoy Paul performed investigation, formal analysis, software implementation and data curation. Nisha Chuphal performed formal analysis and data curation. Gopal Krishna reviewed and edited the manuscript.
ACKNOWLEDGEMENTS
The first author is grateful to the Indian Council of Agricultural Research for providing research grant for the PhD programme (2017-2020). The authors are thankful to Director of the Indian Council of Agricultural Research-Central Institute of Fisheries Education (ICAR-CIFE) for providing the necessary infrastructural facilities, and the World Bank, Govt. of India and ICAR funded National Agricultural Higher Education Project (NAHEP) are duly acknowledged for financial support to complete this research.
ETHICAL ISSUES
The authors hereby declare that there were no ethical issues while conducting this research work.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.




