Short-term exposure of 17α-ethynylestradiol impairs water homeostasis by inhibiting osmoregulatory and cortisol regulating genes in adult zebrafish
Cheng-Ting Xie and Xin Tu contributed equally to this work.
Abstract
The aim was to study the effect of 17α-ethynylestradiol (EE2) on osmoregulation of fish, adult zebrafish (Danio rerio) were exposed to 10 ng/L and 100 ng/L of EE2 for 7 days. After exposure, EE2 induced body oedema, trichangiectasis in the skin and protrusion of the proptosis and significantly elevated the condition factor, water weight and water content, decreased the RBC count and HCT of the fish. In addition, various histopathological damages in the gill and kidney were observed. We therefore speculate that EE2 exposure may disrupt water homeostasis in zebrafish. Further research found that the mRNA levels of crh, crhr and acth were remarkably down-regulated in the brain, in accordance with the decreased level of plasma cortisol. Moreover, remarkably increased aqp3a and aqp1a1 mRNA levels in the gill were observed, suggesting elevated water intake in the gill. Meanwhile, decreased expression of atp1a1a.1 and the activity of Na+, K+-ATPase in the gill, which might have resulted from lower plasma cortisol levels, were also present, in consistence with the lesions of histocyte. Moreover, suppressed expression of atp1a1a.4 and the activity of Na+, K+-ATPase in the kidney were probably resulted from decreased plasma cortisol levels and low expression of avp in the brain.
1 INTRODUCTION
Synthetic oestrogen, 17α-ethynylestradiol (EE2), the main component of the contraceptive pill, has raised concern for its strong estrogenic effects (Hua et al., 2016; Sridevi et al., 2015). EE2 is widespread in surface waters with reported concentrations from below 1 ng/L up to 300 ng/L (Kolpin et al., 2002; Hannah et al., 2009; Laurenson et al., 2014).
Due to its estrogenic effects and development toxicity, many studies have focused on the impairment caused by EE2 in embryos (Santos et al., 2014; Ortiz-Zarragoitia et al., 2006) and fry (Nikoleris et al., 2016) or on the impairment of the reproductive system, behaviour and sexual morphology of adult fish (Young et al., 2017; Hua et al., 2016; Sridevi et al., 2015; Kidd et al., 2007; Santos et al., 2007; Forette et al., 2015; Baatrup & Henriksen, 2015; Volkova et al., 2015). However, whether EE2 is capable of disrupting balanced osmoregulation of fish remains unclear.
Living in freshwater (FW) and seawater (SW), fish need to maintain the balance of water and ions in intracellular and extracellular fluids (Madsen et al., 2015). In FW fish, water from the environment enters the body mainly through the gill and they must secrete excess water through the kidney in the form of hypotonic urine to maintain the hypertonic environment in the body (Kato et al., 2011).
Previous data suggested that water passes hydrophobic epithelia probably through either transcellular pathways (permeable cellular membranes apically and basolaterally) and/or paracellular pathways (intercellular junction complexes) (Madsen et al., 2015). Furthermore, transcellular water transport can be greatly increased through simple diffusion of lipid bilayers or aquaporins (AQPs) in the plumbing system of the plasma membrane (Madsen et al., 2015; Agre et al., 1998).
Recently, Tingaud-Sequeira et al. (2010) and Madsen et al. (2015) demonstrated that zebrafish (Danio rerio) retain up to 18 AQP genes, of which AQP1a1 and AQP3 located in gill are mainly involved in zebrafish osmoregulation (Horng et al., 2015; Chen et al., 2021; Tingaud-Sequeira et al., 2010; Madsen et al., 2015). However, the specific location of AQP1a1 and AQP3 in zebrafish gill remains unclear. Recent studies have shown that AQP1a is located on the basolateral membranes of ionocytes on the skin of zebrafish, and aqp1a1 gene knockout significantly reduced the water intake of zebrafish larvae by 30% (Horng et al., 2015; Kwong et al., 2013). Meanwhile, AQP3 was located on the basolateral membranes of ionocytes in tilapia (Oreochromis mossambicus) gills. Increased expression of this gene promoted water permeability in tilapia (Breves et al., 2016; Watanabe et al., 2005). This evidence proved the expression of aqp1a1 and aqp3 may play pivotal roles in the regulation of water intake in zebrafish gills.
A large number of studies have revealed the regulatory role of cortisol on aqp expression in fish (Breves et al., 2016; Cutler & Cramb, 2002). The mRNA level of aqp3 in gill was found to be inhibited after injection of cortisol in eel (Anguilla anguilla) when adapted to FW (Cutler & Cramb, 2002), indicating that plasma level of cortisol is inversely correlated with aqp3 gene expression in the gill. Breves et al. (2016) also proved in tilapia that cortisol can directly inhibit the mRNA level of aqps in gill and impair osmoregulatory balance of fish (Breves et al., 2016). On the other hand, cortisol was also found to regulate the activity of Na+, K+-ATPase in the osmoregulatory organs of fish, such as gill and kidney, thereby controlling the cellular permeability and cell volume (McCormick et al., 1991; Madsen et al., 1995). Generally, cortisol synthesis is controlled by the hypothalamus-pituitary-interrenal (HPI) axis in fish (Tu et al., 2020). In vertebrates, hypothalamic magnocellular nuclei secrete corticotropin releasing hormone (CRH), which promotes the release of pituitary adenocorticotropic hormone (ACTH), in turn responsible for the secretion of cortisol in interrenal glands (McCormick et al., 2008; Donato et al., 2014). Consequently, altered expression of the genes encoding these proteins and the plasma level of cortisol may thus interfere with the expression of aqps in the gill and the activity of Na+, K + -ATPase in the gill and kidney, thereby disrupting the osmolarity of fish.
In addition, water homeostasis is also regulated by arginine vasopressin (AVP, also known as ADH) secreted from the pituitary (Boone & Deen, 2008). AVP is released into the circulation due to hypovolemia and/or hyperosmolarity and promotes the reabsorption of water in the kidney (Zeynalov et al., 2020; Li et al., 2012; Sholokh & Klussmann, 2021). However, FW fish secrete hypotonic urine to discharge extra water absorbed through the gill and skin (Nebel et al., 2005). Consequently, FW fish remain an extremely low level of water reabsorption in the kidney. Recent studies have shown the regulatory role of AVP on the activity of Na+, K + -ATPase in the kidneys of rat (Rattus norvegicus) either directly through protein kinase A or indirectly through hyperosmolarity (Martin et al., 1999; Sakuma et al., 2005). Charlton and Baylis (1990) also found a positive correlation between AVP levels and the activity of Na+, K+-ATPase in rat kidneys. These data suggested that AVP may take part in the regulation of Na+, K+-ATPase activity in animal kidneys to maintain osmotic homeostasis (Charlton & Baylis, 1990).
To understand the osmotic toxicity of EE2 on fish, female and male adult zebrafish were exposed to two nominal concentrations of EE2 (10 and 100 ng/L) for 7 days. After exposure, the concentration of EE2 in the brain and gill was evaluated. The effect of EE2 on osmoregulation was evaluated by phenotypic factors (morphology, condition factor, water content and water weight), haematocrit and red blood cell (RBC) count, as well as the histology of the gill and kidney. To elucidate the possible mechanisms involved, plasma levels of cortisol, and mRNA levels of crh, crhr, acth and avp in the brain, and aqps in gill were investigated by quantitative RT–PCR (qRT–PCR). In addition, the mRNA levels of atp1a1a.1 and atp1a1a.4 and the activity of Na+, K+-ATPase were further estimated in the gill and kidney respectively.
2 MATERIALS AND METHODS
2.1 Chemicals and animals
17α-ethynylestradiol (EE2, E4876-100 mg, purity ≥98%) was purchased from Sigma-Aldrich, USA.
Female and male adult zebrafish (4 months old, AB strain) were purchased from Nanjing EzeRinka Biotechnology Co., Ltd. During domestication, 252 fish were reared in a 500 L glass tank at 27 ± 1°C. The fish were reared with tap water after aeration and dechlorination with a photoperiod (light/dark cycle) of 14 h: 10 h. The fish were fed three times a day (9:00 a.m., 15:00 p.m. and 21:00 p.m.) with dry flake food (Shengsuo, Shandong, China). Animal experiments were approved by the Committee of Laboratory Animal Experimentation at Chongqing Normal University, and carried out according to the Guideline for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
2.2 Experimental exposure
After a 2-week acclimation, female and male zebrafish (126 each) adults (female, 0.54 ± 0.08 g; male, 0.45 ± 0.06 g) were randomly selected into three equal groups for each sex, with triplicate tanks per group (14 fish in each tank). The exposure concentrations in this experiment referred to other reference reports and environmental relevance (Hua et al., 2016; Sridevi et al., 2015; Laurenson et al., 2014). In our pilot study, 7-day exposure to EE2 at a concentration of 100 ng/L was sufficient to cause obvious morphological alterations (such as vessel dilation in the skin, body oedema and proptosis) in zebrafish females without inducing mortality. Consequently, zebrafish were exposed to two nominal concentrations of EE2 (10 and 100 ng/L) for 7 days in semistatic exposure regimes. The control group only used the solvent DMSO, and the concentration of DMSO in each group was 0.05%. During exposure, the environment of the experimental fish was consistent with that of the domestication period, and the water was renewed once a day. The water in each tank was sampled each day in the morning before water renewal to measure the actual concentration of EE2. After exposure, the measured values for each tank were calculated, and the actual concentration of EE2 in each tank was 7.1 ± 0.4 ng/L (EE2-low, female), 7.6 ± 0.3 ng/L (EE2-low, male), 92.2 ± 0.7 ng/L (EE2-high, female) and 92.8 ± 0.4 ng/L (EE2-high, male) (N = 3). Water quality was tested weekly and the parameters were as follows: dissolved oxygen, 6.2–7.5 mg/L; water temperature, 26–28°C; pH, 7.2–7.9; hardness, 134.3–139.8 mg/L as CaCO3.
2.3 Experimental sampling
After exposure, MS-222 at a final concentration of 0.1% was carefully injected from the corner of the tank using a glass capillary to avoid disturbing the fish. The body weight and body length of all 14 fish in each tank were then measured, and the condition factor was then calculated (condition factor = body weight/body length × body length × body length) (N = 14) (Duarte et al., 2018). For measurements of water weight and water content, 3 fish in each tank were sacrificed and dried for 1 h at 105°C and then weighted. Water weight (=weight before drying−weight after drying) and water content (=water weight/body weight × 100%) (N = 9 for each group) were calculated thereafter (López-Pérez et al., 2020). Eight fish were tail-docked for blood sampling as described by Babaei et al. (2013). Whole blood from each fish was collected into 0.5 ml anti-coagulated centrifuge tubes using a heparinized capillary tube (5 μl of 10 μg/ml heparin was used) as previously described (Xiao et al., 2018). For RBC counts, the blood of 3 fish in each tank was used according to the method mentioned previously (Hossain et al., 2021). The mean RBC concentration of the 3 fish in each tank was set as one biological sample (N = 1). To measure haematocrit (HCT), the blood of 5 fish (5 μl for each fish) in each tank was sampled (N = 15 for each group) and centrifuged at 5000 × g for 20 min at 4°C, and HCT was then calculated (HCT = [5 μl−supernatant volume]/5 μl) (Deebani et al., 2019). Plasma isolated from blood samples of 3 fish per tank mentioned above was pooled as one biological sample (N = 1) and used for the estimation of cortisol. Gills (from one side of each fish) or brains of 3 fish in each tank were pooled as one biological sample (N = 1) for measuring the EE2 concentration. For histological study, the gills and kidneys of 3 fish in each tank were fixed in Bouin's solution overnight and stored in 75% ethanol before use. To estimate the activity of Na+, K+-ATPase, gills (from one side of each fish) or kidneys of 5 fish in each tank were pooled as one biological sample (N = 1) respectively and frozen in liquid nitrogen immediately and stored at −80°C until use. For total RNA extraction, the brains, gills or kidneys of 3 fish in each tank were obtained as one biological sample (N = 1) respectively and homogenized and stored at −80°C until use.
2.4 Concentration of the RBC
One microliter of the blood sample mentioned above was diluted with 199 μl of Hank's balanced salt solution (HBSS) and then mixed and loaded into a hemoglobinometer (0.1 mm depth). After 3 min of settling, the RBC number was counted under a Nikon microscope (20 × magnification, Eclipse 90i, Nikon), and the RBC concentration (cells/ml) was calculated as the total number of 5 squares (in the corners and the middle) × 25/5 × 104 × diluted multiples. Blood samples from each fish were measured twice to average the concentration of RBCs. The mean RBC concentration of 3 fish measured in each tank was set as one biological sample (N = 1) and 3 biological samples in each group (N = 3).
2.5 Concentration of EE2 in the brain and gill
Gills (from one side of each fish) and brains of 3 fish in each tank were pooled as one biological sample (N = 1). The tissue was thawed at 4°C, and the surface blood stains were washed with HBSS and then blotted dry on filter paper. Precooled 0.86% saline solution was then added to each sample at a ratio of 9:1 (ml/g). The brains were homogenized and centrifuged thereafter at 3000 × g and 4°C for 20 min, the supernatant was removed, and the concentrations of EE2 were immediately measured according to the ELISA Kit (MEIMIAN, China) instructions. The inter- and intra-assay coefficients of variation were 3.4–7.9% and 5.8–8.7% respectively.
2.6 Histology
A histological study was conducted according to Yin et al. (2017). The gill and kidney were fixed in Bouin's solution overnight, followed by dehydration. The tissues were then embedded in paraffin at 60°C, sectioned at 5 μm thickness and stained with haematoxylin and eosin. The slides were then visualized under a Nikon microscope (Eclipse 90i, Nikon).
2.7 Plasma level of cortisol
The determination of cortisol levels in plasma was carried out according to the method described by Xiao et al. (2018). Blood from 3 fish (4 μl/fish) was obtained from the fish using a capillary tube (pre-treated with 10 μg/ml heparin) into a 0.5 ml anti-coagulated centrifuge tube as one sample (N = 1). Blood samples were centrifuged at 5000 × g for 20 min at 4°C, and the supernatant of 3 samples (9 fish, N = 3) was taken from each group for detection. The plasma level of cortisol was measured according to the instructions of the cortisol ELISA Kit (Cayman, USA). The inter- and intra-assay coefficients of variation were 3.7–8.2% and 5.2–7.1% respectively.
2.8 Na+, K+-ATPase activity measurement
The gills and kidneys of 5 fish in each tank were pooled as one biological sample (N = 1). Tissue samples were removed from liquid nitrogen and thawed at 4°C, 50 mg of tissue was removed, and 0.45 ml ice-cold isotonic normal saline was added for homogenization. Aliquots of each homogenate were then stored at −80°C until use. The obtained sample was centrifuged at 3000 × g for 10 min at 4°C to obtain the supernatant and then measured according to the instructions of the Na+, K + -ATPase ELISA Kit (Sino Best Biological Technology Co LTD). The inter- and intra-assay coefficients of variation were 3.2–8.6% and 5.4–7.7% respectively.
2.9 qRT–PCR
Total RNA was extracted from the brain, gill and kidney using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. The brain, gill or kidney of 3 fish in each tank was obtained as one biological sample (N = 1), and 1.0 ml TRIzol (Invitrogen) was added respectively and homogenized at 4°C. The purities and qualities of total RNA were measured with a Nano Drop ND-2000 spectrophotometer (Thermo Electron Corporation) and its quality was verified by 1% agarose RNA gel electrophoresis.
Genomic DNA was removed and cDNA was synthesized using 1 μg total RNA following the instructions of the HiFiScript gDNA Removal cDNA Synthesis Kit (TaKaRa). qRT–PCR was conducted using a Bio-Rad CFX96™ Real-time PCR Detection System (Bio-Rad) and UltraSYBR Mixture (ComWin Biotech) fluorescent labeling (Yin et al., 2017). The primers for the genes in this study (aqp1a1, 3a, atp1a1a.1, −4, crh, crhr, acth and avp) and the internal reference gene were designed with the Primer Premier 6.0 package and their specificities were confirmed by subcloning and sequencing. Primers and their sequences are listed in Table 1. Before analysis, according to the method provided by Lu et al. (2021), a qRT–PCR trial of the expression of the housekeeping gene ef1α in each group was carried out to confirm that the mRNA levels of ef1α were stable under the experimental conditions. The SYBR mix qRT–PCR reactions contained 0.3 μM forward and reverse primers, 1× SybrGreen master mix (TaKaRa), and 2 μl diluted cDNA template (1:9 dilution) in a final volume of 20 μl. The qRT–PCR parameters consisted of an initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s and 72°C for 10 s. All samples were run in triplicate in 96-well PCR plates (Axygen Biosciences). The mean value of all the fish in each tank was set as one biological sample (N = 1) and three samples (N = 3) were used in each group. To ensure that the cDNA stock solutions were not contaminated, dissociation curves and a nontemplate control were performed. The amplification efficiencies of the genes ranged from 98.2% to 103.5%. The relative expression levels of the target genes were evaluated using the 2−ΔΔCT method (Porseryd et al., 2017; Livak & Schmittgen, 2001) with ef1α as an internal control.
| Gene name | Primer sequences (5′–3′) | Accession No. | Size (bp) | References |
|---|---|---|---|---|
| avp | F: TCTCGTCTGCCTGCTACATCC | NM_178293 | 124 | In this sthdy |
| R: CAGATACTGGGGCCAAAACA | ||||
| crh | F: TCAGATCGGTGGTGTCGGTA | JN859047 | 166 | In this sthdy |
| R: CTTGCGGTTGTGGGTTACG | ||||
| acth | F: AGATGCTCGGGTTTGATAGACTG | NM_001083051 | 159 | In this sthdy |
| R: CAGACTCTGCTCCTCTACCTGTTCT | ||||
| crhr | F: CGTCCCTGTTTCAGTTCTTCCT | NM_001113644 | 147 | In this sthdy |
| R: CGATTTCATCCGTGGTGGC | ||||
| aqp1α1 | F: CGAACATTCGGACCAGCAA | NM_207059 | 109 | In this sthdy |
| R: GCAGAAAGTCATAGATTAGCGCA | ||||
| aqp3a | F: CCTTCTTGGGTGCTGCTATTATC | NM_213468 | 236 | In this sthdy |
| R: TTGAGGGATCGGGTTGTTGT | ||||
| atp1a1a.1 | F: AGTTGGTTGGAGGCTGTTATCTT | NM_131686 | 131 | In this sthdy |
| R: TTGCCATTCGTTTGGCG | ||||
| atp1a1a.4 | F: TGGGCGTGTCCTTCTTTATTCT | NM_131642 | 150 | In this sthdy |
| R: CCATTCGTTTGGCAGTGAGAGT | ||||
| ef1α | F: GATCACTGGTACTTCTCAGGCTGA | NM_131263 | 121 | In this sthdy |
| R: GGTGAAAGCCAGGAGGGC |
2.10 Statistical analysis
SPSS 20.0 was used to conduct Levene's test and Shapiro–Wilk's test on the original data. After the homogeneity and normality of variance were tested, the difference analysis was finally conducted by one-way ANOVA with Tukey's range test. Data are expressed as the mean ± standard error of mean (SEM), and the difference was considered significant when the ‘p value’ was greater than 0.05. GraphPad Prism 6.0 was used to generate figures.
3 RESULTS
3.1 Morphological and haematological changes in EE2-treated fish
Compared to the control, no significant morphological change was present in EE2-low fish (Figure 1a). However, body oedema, vessel dilation in the skin and proptosis of the eye were observed in both female and male fish in the EE2-high group (Figure 1a). Compared with the control, a significant increase in the condition factor was only present in the fish of EE2-high (F Female(2,123) = 18.838, P Female = 0.000; F Male(2,123) = 10.8, P Male = 0.005, Figure 1b), and no significant change was observed in the fish of EE2-low (F Female(2,123) = 6.969, P Female = 0.897; F Male(2,123) = 0.01, P Male = 0.561, Figure 1b).
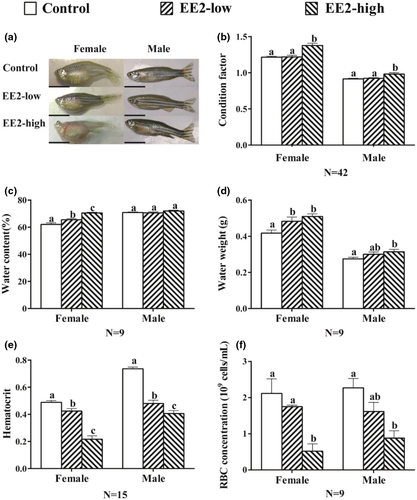
The water content of the female fish showed a significant increase in both treated groups when compared to the control (F[2,24] = 25.497, p = 0.000, Figure 1c). However, the water content remained unchanged in males exposed to each concentration of EE2 (F[2,24] = 1.473, p = 0.249, Figure 1c). The water weight of the fish also increased in both sexes and showed significant changes in the EE2-high group (F Female(2,24) = 6.487, P Female = 0.001; F Male(2,24) = 2.644, P Male = 0.034, Figure 1d). In contrast, the HCT of the fish decreased significantly in both sexes (F Female(2,42) = 55.728, P Female = 0.000; F Male(2,42) = 13.617, P Male = 0.000, Figure 1e). Consistently, both concentrations of EE2 significantly decreased the RBC count in both sexes and decreased significantly in the EE2-high groups (F Female(2,24) = 15.869, P Female = 0.007; F Male(2,24) = 8.536, P Male = 0.018, Figure 1f).
3.2 Histological observations
In the gills of the control fish, normal branchial structures, such as pillar cells, branchial epithelial cells and capillary vessels with RBCs, were present (Figure 2a,d). In the female and male fish of EE2-low, swelling of branchial epithelial cells was observed (Figure 2b,e). In fish of EE2-high, severe swelling and disordered arrangement of branchial epithelial cells, as well as cell adhesion at the basal position of gill lamellae, were observed in both sexes (Figure 2c,f).
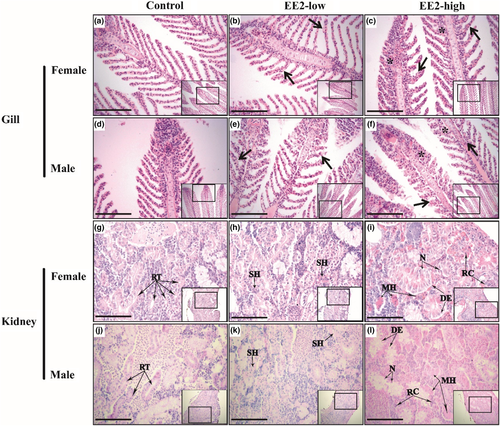
In the kidneys of the control fish, several renal tubules and normal interrenal tissues were present (Figure 2g,j). In the kidney of EE2-low fish, swelling of tubular epithelial cells and small haemorrhages in interrenal tissue were observed (Figure 2h,k). In the kidneys of EE2-high fish, swelling of tubular epithelial cells, multiple haemorrhages, necrotic tubular epithelial cells and degeneration of tubular epithelial cells were further observed (Figure 2i,l).
3.3 Concentrations of EE2 in zebrafish brain and gill
The concentrations of EE2 in the brain and gill of zebrafish in each treatment group are shown in Table 2. These data are expressed as ‘mean ± standard error’. The concentration of EE2 in the zebrafish brain increased in a dose-dependent manner, although there was no significant difference between the EE2-low and EE2-high groups (F female(2.6) = 2.378, P female = 0.198; F male(2.6) = 5.289, P male = 0.083).
| Female (pg/ml) | Male (pg/ml) | |||||
|---|---|---|---|---|---|---|
| Control | EE2-low | EE2-high | Control | EE2-low | EE2-high | |
| Brian | ND | 955.625 ± 7.530a | 1081.156 ± 10.009a | ND | 729.583 ± 10.067a | 895.729 ± 3.610a |
| Gill | ND | 941.042 ± 6.997a | 1209.271 ± 7.773b | ND | 887.396 ± 8.677a | 1073.333 ± 8.067b |
- Note a, b, significantly different. Data are expressed as mean ± standard error of mean (SEM).
- Abbreviation: ND, not detected.
Similarly, the concentration of EE2 in the gill also increased in a dose-dependent manner when compared to the control group. The concentration of EE2 in the gills of the EE2-high group was higher than that of the EE2-low group, and the females had significant differences (F female(2.6) = 23.800, P female = 0.008; F male(2.6) = 6.982, P male = 0.057).
3.4 Expression of aqps in the gill
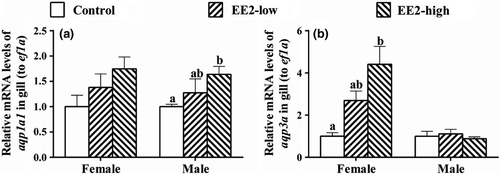
The mRNA levels of aqp1a1 in the gills of females showed a slight but not significant increase in both exposed groups (F[2,6] = 2.355, p = 0.176, Figure 3a); the expression of this gene also showed an increase in male gills in both treated groups, and showed a significant change in the EE2-high group (F[2,6] = 1.757, p = 0.018, Figure 3a). The expression of aqp3a in the gill of female fish increased after exposure and showed a significant increase in the EE2-high group (F[2,6] = 9.228, p = 0.015, Figure 3b), but remained unchanged in the gill of males (F[2,6] = 0.372, p = 0.704, Figure 3b).
3.5 The activity of Na+, K+-ATPase in gills and the expression of atp1a1a.1 in gills and atp1a1a.4 in the kidneys
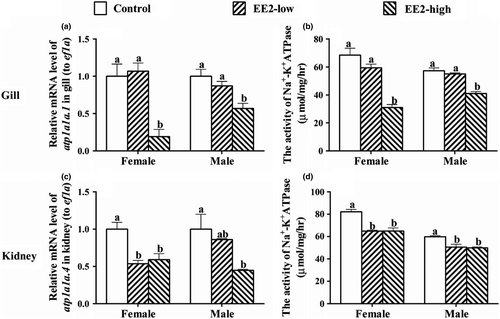
In both sexes, EE2-high significantly down-regulated the mRNA level of atp1a1a.1 (F Female(2,6) = 14.715, P Female = 0.005; F Male(2,6) = 8.62, P Male = 0.017, Figure 4a) and suppressed the activities of Na+, K+-ATPase in the gills (F Female(2,6) = 32.009, P Female = 0.001; F Male(2,6) = 35.802, P Male = 0.000, Figure 4b). Both concentrations of EE2 also down-regulated the expression of atp1a1a.4 in kidneys of females (F(2,6) = 11.684, p = 0.009), while the mRNA level of this gene was significantly suppressed in males in EE2-high (F(2,6) = 6.157, p = 0.035, Figure 4c). In addition, both concentrations of EE2 suppressed the activities of Na+, K+-ATPase in fish of both sexes (F Femaleh(2,6) = 23.583, P Female = 0.001; F Male(2,6) = 11.915, P Male = 0.008, Figure 4d).
3.6 Expression of osmoregulatory genes in the brain and plasma levels of cortisol
After exposure, both concentrations of EE2 significantly suppressed the mRNA levels of crh, crhr, acth and avp in the brains of female fish (F crh(2,6) = 16.658, P crh = 0.004; F crhr(2,6) = 6.375, P crhr = 0.033; F acth(2,6) = 64.342, P acth = 0.000; F avp(2,6) = 38.786, P avp = 0.000, Figure 5a). Both concentrations of EE2 also significantly inhitibted the expression of crhr, acth and avp in the brains of male fish (F crhr(2,6) = 24.785, P crhr = 0.001; F acth(2,6) = 8.147, P acth = 0.019; F avp(2,6) = 38.928, P avp = 0.000, Figure 5b), while the mRNA level of crh remained unchanged (F(2,6) = 1.09, p = 0.395, Figure 5b).
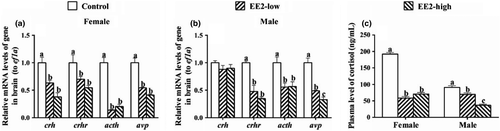
Compared with the control, the plasma levels of cortisol in females declined sharply in both treated groups (F(2,6) = 254.751, p = 0.000, Figure 5c), and the plasma level of cortisol in male fish also showed a dramatic decrease in both treated groups (F(2,6) = 36.552, p = 0.000, Figure 5c).
4 DISCUSSIONS
The present study demonstrated that EE2 can be abundantly enriched in a dose-dependent manner in zebrafish gills and brains. Subacute exposure to EE2 caused severe morphological changes in both sexes of zebrafish (particularly in the EE2-high groups), such as body oedema, trichangiectasis in the skin and proptosis. These data suggested a subacute toxicity of EE2 on zebrafish adults. As a typical xenoestrogen in aquatic environments, EE2 has been well documented to cause reproductive disorders, biased sex ratios and disrupted sexual behaviours and interferes with male–female interactions and non-reproductive behaviour (Nikoleris et al., 2016; Baatrup & Henriksen, 2015; Forette et al., 2015; Volkova et al., 2015; Porseryd et al., 2017). A large number of studies have also used a wide range of concentrations of EE2 in exposure to various fish species, including zebrafish. However, EE2 induced body oedema, trichangiectasis in the skin and proptosis are rarely reported in fish. According to our data, it was highly suggested that the symptoms caused by EE2 in the present study might have occurred in previous studies, whereas they were possibly ignored unintentionally or misdiagnosed as infections.
Intriguingly, treatment with a higher concentration of EE2 significantly increased the condition factors of fish of both sexes. In accordance with this finding, the body weight of the fish in both sexes also increased in a concentration-dependent manner, implying that EE2 might make the fish larger and fatter. However, the water weight of the fish after exposure was dramatically increased in both sexes, indicating that increased water weight might be responsible for the elevation of the condition factor. Moreover, the water content of the fish elevated in both sexes (slightly but not significantly in the male), also agreed with the increased water weight of the fish after exposure. All these data indicated that EE2 exposure might have disrupted the osmoregulation of the fish.
The present study also revealed significantly decreased HCT and RBC counts in both sexes in response to EE2, probably resulting from the dilution of RBC concentration due to excess water intake. In fish, relatively stable blood volume (or blood pressure) is essential for survival (Llorens-Cortes & Moos, 2008). Our findings suggested that short-term exposure to EE2 might have increased the circulating blood volume of zebrafish, possibly resulting in elevated blood pressure, thereby causing morphological changes, such as trichangiectasis in the skin and proptosis.
Generally, the water content of the fish is balanced by water intake and secretion (urination) (Kidder et al., 2006). All the surfaces of FW fish exposed to aquatic environments are capable of absorbing water due to the high osmolarity of the fish (Madsen et al., 2015). Among these surface regions, the gill is the major location of water intake (Madsen et al., 2015). In FW fish, because of the hyperosmolarity of the body, water diffuses freely into ionocytes in the gill through the apical membrane and is then transported into the blood through several AQP proteins (such as AQP1a1 and AQP3a) located on the basolateral membrane of ionocytes (Kwong et al., 2013; Tingaud-Sequeira et al., 2010; Cutler et al., 2007). As one of the most important osmoregulatory organs of fish, the gill is the main determinant of obligatory fluxes of water entering the fish, and theoretically, water permeability should be kept to a minimum (Madsen et al., 2015). In contrast, our study demonstrated that, in the gill, EE2 administration remarkably up-regulated the expression of aqp1a1 in males and aqp3a in females (by 4-fold in EE2-high), suggesting an increase in water intake of zebrafish. It is evident that the expressions of two AQP proteins differs between male and female, which may suggest two different modes of regulation of water homeostasis between sexes under the same conditions. However, the mechanism underlying the differences in aqps expression between sexes remains to be further investigated. This result was consistent with the alterations in morphological and haematological parameters, indicating that EE2 exposure may have damaged water homeostasis in zebrafish by stimulating water intake in the gill.
Aquaporins localizes to the gill epithelium, and its role is mostly related to cell volume regulation (Madsen et al., 2015). In our data, EE2 treatment up-regulated the expression of aqps in zebrafish gills, which also induced histological damage to the gill, such as swelling of epithelial cells, disordered arrangement of gill lamella and cell adhesion between gill lamella. All these histopathological changes indicated a disorder of cell volume control. It is known that cell osmolarity results from the sum of various ion species, proteins and organic compounds inside the cell (Basavappa et al., 1998). Increased osmolarity inside the cell will drive the water flowing into the cell and cause cell swelling (Basavappa et al., 1998).
Na+, K+-ATPase has been well documented to play a critical role in controlling cell volume, which produces a net export of a single positive charge when one ATP molecule is consumed (Nylander-Koski et al., 2005; Yang et al., 2019). Consequently, decreased activity of Na+, K+-ATPase may increase the ion concentration in the cell, thereby resulting in cell swelling (Nylander-Koski et al., 2005). In the present study, the mRNA level of atp1a1a.1 and the activity of Na+, K+-ATPase were both significantly decreased in the gills of the fish treated with higher concentrations of EE2. These data indicated that the sum of positive ions in the ionocytes might have risen, which would lead to higher intracellular osmolarity. High intracellular osmolarity will then drive the water flowing into the cells, thereby inducing cellular damage, such as swelling and necrosis.
Cortisol is the major steroid involved in the regulation of osmotic pressure in fish, and the expression of atp1a1a.1 and the activity of Na+, K+-ATPase are under the control of cortisol in fish (Hu et al., 2019; Guh et al., 2015). The present study showed that plasma cortisol levels were significantly reduced in both female and male zebrafish after exposure to EE2, which is consistent with the low activity and low transcription levels of Na+, K+-ATPase. Lower levels of cortisol inhibit Na+, K+-ATPase activity by reducing binding to the glucocorticoid receptor, thereby importing fewer sodium ions in and exporting fewer potassium ions out, thus increasing the intracellular osmolarity (Guh et al., 2015; Kwong et al., 2016). Recently, the mRNA level of aqp3 in gills was found to be inhibited after the injection of cortisol in eel adapted to FW (Cutler & Cramb, 2002). Breves et al. (2016) also proved in tilapia that cortisol can directly inhibit the mRNA level of aqps in gill and the osmoregulatory balance of fish body (Breves et al., 2016). These findings indicated that cortisol might possess a negative control on aqps expression and a positive control on atp1a1a.1 expression. Moreover, a previous report further demonstrated that E2 treatment suppressed the production of cortisol in the interrenals of juvenile chinook salmon (Oncorhynchus tshawytscha) (McQuillan et al., 2003). Consistent with these evidences, our data revealed a significant decline in the plasma level of cortisol in response to EE2 in both sexes. Accordingly, the mRNA level of aqps was significantly increased, while the mRNA level of atp1a1a.1 and the activity of Na+, K + -ATPase in the gill were suppressed after EE2 exposure in this study. Therefore, it is strongly suggested that the dereased cortisol level in plasma might be one of the underlying causes responsible for elevated water intake and subsequent cell damage in gills.
The production of cortisol in interrenal glands of fish is governed by the HPI axis (Huising et al., 2004; Alsop & Vijayan, 2009). CRH, which is produced from the hypothalamus, activates the synthesis of ACTH through its cognate receptor (CRHR) in the pituitary (Alsop & Vijayan, 2009; Stolte et al., 2008). ACTH is then released and acts on the adrenal gland (interrenal tissues of fish) via the circulatory system, stimulating the expression of steroidogenic enzymes responsible for cortisol synthesis (Alsop & Vijayan, 2009). In the present study, the mRNA levels of crh, crhr and acth were all remarkably suppressed in the female fish of both treated groups. Coincidentally, the expression of the crhr and acth genes in the brains of male fish in both treatment groups was also significantly down-regulated. The down-regulation of these genes indicated the blocked production of cortisol, leading to corresponding changes in the mRNA level of aqps, activity of Na+, K+-ATPase and the subsequent morphological and pathological changes in the gill.
Except for the CRH-ACTH-cortisol pathway, AVP secreted from the pituitary, which acts on the kidney, is also involved in water homeostasis by reabsorption of water in vertebrates (Boone & Deen, 2008). A great number of studies revealed that an elevation of circulating blood volume and blood pressure caused a decrease in AVP secretion, thereby inducing a decline in water reabsorption in the kidney (Zeynalov et al., 2020; Li et al., 2012; Sholokh & Klussmann, 2021). Seawater fish, due to their hypoosmolity, have to drink much sea water and reabsorb the water in the kidney (Kato et al., 2011). However, FW fish secrete hypotonic urine to discharge extra water absorbed through the gill and skin (Nebel et al., 2005). As a water-secreting organ of teleost fish, particularly in FW fish, the kidney needs to be relatively water impermeable to allow strong hypotonic urine excretion (Madsen et al., 2015). Consequently, FW fish remain an extremely low level of reabsorption of water in the kidney. In the present study, increased water content and hypervolemia were in accordance with the decreased mRNA level of avp (suggesting a decreased production of AVP) in the brain. Decreased production of AVP was therefore supposed to inhibit the reabsorption of water and secrete more hypotonic urine to alleviate the hypervolemia and hypertension caused by increased water intake in zebrafish (Warne, 2002; Zeynalov et al., 2020). However, due to the extremely low level of water reabsorption in the kidneys of zebrafish, a decreased level of AVP can hardly decrease water reabsorption. On the other hand, recent studies have shown that AVP plays a critical role in regulating the mRNA level and activity of Na+, K+-ATPase in the kidney (Sakuma et al., 2005; Sherwani & Parwez, 2008). In this study, the mRNA level of atp1a1a.4 and the activity of Na+, K + -ATPase in the kidneys of both sexes of zebrafish were sharply inhibited, indicating an elevation of net positive ions in the cell (particularly tubular epithelial cells). Enhanced osmolity of the cell will drive the water flow into the cell and induce cell swelling. In the present study, various pathological changes, such as multiple haemorrhages, swelling and necrosis of tubular epithelial cells, were present in the kidneys of EE2-treated fish, suggesting histological damage in response to EE2 exposure. All these data demonstrated that short-term exposure to EE2 not only affected the water intake in the gill but also impaired the structure and function of the kidney in zebrafish.
In summary, EE2 was enriched in the zebrafsih brain and gill after short-term EE2 exposure. The enriched EE2 therefore might have inhibited the expression of crh, crhr and acth in the brain, decreased the plasma level of cortisol and elevated the expression of aqps genes in the gill, which led to increased water intake, thereby causing elevated water content and various morphological/haematological damages. In addition, the mRNA level of atp1a1a.1 and Na+, K+-ATPase activity in the gill were suppressed by a lower plasma level of cortisol, giving rise to cellular damage in the gill. Moreover, in response to the hypervolemia and hypertension status of zebrafish, the mRNA level of avp in the brain is inhibited. Decreased avp expression and dereased cortisol levels suppressed the expression of atp1a1a.4 and Na+, K + -ATPase activity in kidney, thereby causing morphological damage in this organ.
5 CONCLUSIONS
The present study demonstrated that short-term exposure of EE2 to zebrafish elevated the water content and water weight, decreased HCT and the RBC count and induced body oedema, trichangiectasis in the skin and proptosis and various cellular damages in the gill and kidney. Further analyses revealed that EE2 decreased cortisol levels in plasma by significantly inhibiting the mRNA levels of genes related to cortisol synthesis in the brain (crh, crhr and acth). Decreased cortisol levels might have up-regulated expression of aqps in the gill, resulting in elevated water intake. Meanwhile, decreased plasma levels of cortisol might also significantly inhibit the mRNA level of atp1a1a.1 and the activity of Na+, K+-ATPase in gill, thereby resulting in various cell damages. In addition, decreased cortisol levels in plasma and mRNA levels of the avp in the brain were supposed to inhibit the mRNA level of atp1a1a.4 and the activity of Na+, K+-ATPase in the kidney. These alterations were then supposed to lead to increased water absorption of cells and subsequent histological damage in the kidney.
ACKNOWLEDGEMENTS
This work was funded by the Chongqing Research Program of Basic Research and Frontier Technology [cstc2020jcyj-msxmX0805, cstc2016jcyjA0133]; the Scientific and Technological Research Program of Chongqing Municipal Education Commission [KJ1600308] and the Open project fund of ‘Key laboratory of Freshwater Fish Reproduction and development (Ministry of Education, China) [FFRD-2015-02]’, China.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
Cheng-Ting Xie, XinTu. Design and execution of project, lab work, data analyses, writing and editing. Mei-Ling Tan. Animal care, help with sampling. Ying-Wen Li D. Assistance of sampling and analysis, project funding and supervision. Yan-Jun Shen D., Qi-Ling Chen D. histology work and editing. Zhi-Hao Liu D. Project conception and design, funding, supervision and editing.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




