Live transport of Atlantic salmon in open and closed systems: Water quality, stress and recovery
Abstract
The goal of the study was to compare open and closed transports of adult Atlantic salmon in terms of water quality and stress (blood chemistry and white muscle biochemistry). The study comprised of two phases: (i) live transport from a commercial farm by vessel (open system) and vehicle (closed system) to flow-through laboratory tanks and (ii) after post-transport recovery, simulated live transports in open and closed systems. The stress reaction the salmon experienced during transfer from fish farm to laboratory tanks was severe. Some delayed mortalities were observed possibly related to extreme acidosis. The fish were left to recover for 65 h before the simulated open and closed transports were carried out. By then, they had not yet fully recovered to baseline levels. The simulated transports did not cause excessive stress reactions relative to the partially recovered fish. However, deteriorating water quality during closed transport eventually affected fish behaviour where fish welfare could be questioned. The main finding was that crowding and transport due to commercial transport overshadowed stress-related results obtained during the laboratory study. The results may also be viewed as effects of repeated fish handling operations, a typical feature of salmon farming.
1 INTRODUCTION
Presently in salmonid aquaculture, there is a trend towards using closed or semi-closed systems for rearing and transporting live fish. Examples of this are recirculation aquaculture systems (RAS), semi-closed on-growing cages in sea and closed transport by well-boats. Closed transports can be desirable since they minimize the risk of spreading diseases when vessels pass, or visit, other aquaculture facilities or wild salmonid populations along their route (Murray et al., 2002). However, deteriorating water quality during closed transport can lead to stress, compromised welfare and, in extreme cases, death. Depending on transport duration, temperature and fish density, and water treatment (in addition to compulsory oxygenation), it can be necessary to maintain adequate water quality. For the personnel involved, frequent monitoring and a more extensive understanding of water chemistry and fish behaviour would be desirable to conduct safe transports under different conditions. Studies of closed transports of salmonids are limited considering water quality, stress, behaviour and welfare. However, Wedemeyer (1996a) gives an overview of different aspects concerning live transport of salmonids. Hatchery-raised smolts are transported to grow-out cages by specialized vehicles in closed tanks, usually in combination with well-boat transport. Upon reaching market size, salmon are transported live by well-boat to processing plants. The physical loading procedure has been shown to be more stressful than the transport itself (Farrell, 2006; Iversen et al., 1998, 2005; Nomura et al., 2009; Specker & Schreck, 1980). Crowding and pumping, during loading and unloading, are the main stressors when fish are transferred between two locations (Erikson et al., 2016; Gatica, Monti, Knowles, Warriss, & Gallo, 2010; Merkin et al., 2010; Roth et al., 2012). Well-boat transport with open valves (flow-through, FT principle) provides good water exchange in the live hold, so the water quality resembles that of the surrounding sea. Under such conditions, the fish remain calm as reflected in white muscle physiology (Erikson et al., 1997). When good water quality conditions prevail in the live hold, Atlantic salmon (Salmo salar) can in fact recover physiologically from loading stress during transport to normal blood chemistry levels (Iversen et al., 2005; Nomura et al., 2009) and bulk oxygen uptake rates (Farrell, 2006; Tang et al., 2009). During such transports, fish welfare is considered good (Farrell, 2006; Tang et al., 2009). Gatica, Monti, Knowles, and Gallo (2010) compared open and closed well-boat transports of adult Atlantic salmon. Both transport systems affected blood chemistry although the closed transport induced more stress as determined by cortisol, lactate, sodium, chloride and osmolality. Nomura et al. (2009) studied blood chemistry and behaviour of Atlantic salmon smolts during transport from freshwater farms to seawater net pens. The fish were transported by vehicle for 30–60 min in closed tanks to dock where the fish were transferred to a well-boat for a 2-h open transport to net pens. Based on blood chemistry levels, only modest stress responses related to loading and truck transport were found. After transfer to well-boat, the fish recovered in the vessel at the dockside. Subsequent transport to net pen did not change the condition of the fish. Video analysis of fish behaviour in the vessel's live hold was consistent with a non-stressful environment, and it was concluded that the transport reflected good fish welfare.
In contrast, a simulated ‘worst-case’ 5-h transport of fasted adult Atlantic salmon in a closed system was carried out at 15°C with fish densities of 227–329 kg m−3 and heavy oxygen supersaturation (200–250%). No water treatment was used except from oxygenation and aeration. Within a few min, fish behaviour changed dramatically. The fish became sluggish, kept their mouth open above the water surface and exhibited a gulping and coughing behaviour. After about 3 h, short sporadic bursts of vigorous activity were observed. After another 1.5 h, most fish tended to lie quietly on the bottom of the tank until the experiment was terminated. The opercular amplitude gradually diminished. Notably, practically, no excessive swimming activity took place during the experiments. Accordingly, the initial pH of the white muscle showed that it was in a rested state. Control fish were exposed to similar conditions, except from that they were exposed to normoxic conditions at 80% dissolved oxygen (DO) saturation. They also exhibited abnormal behaviour although less extreme than in the supersaturated case suggesting other factors than oxygen level only affected the fish (Erikson, 2001). The study shows that deteriorating water quality in closed systems can lead to situations where the welfare of the fish is clearly questionable.
In a commercial case study, market-sized rainbow trout (Onchorhynchus mykiss) were transported to the market by a vehicle for 3.3 h in a closed tank with oxygenation at water temperature about 10°C and a fish density of 169 kg m−3. Due to lack of adequate control of oxygen, the DO level varied between 297% and 331% saturation during transport. Water acidity dropped from pH 7.47 to pH 6.88 after transport. Significant increases in cortisol and lactate were observed just after transport. Immediately after arrival to a live-fish outlet, the fish were transferred to a semi-closed system with aeration and water temperature of about 16°C where they were kept for up to 2 days. The fish did not fully recover from transport stress during this period (Shabani et al., 2016).
Taken together, it seems evident that fish can be affected differently in open and closed systems. We therefore decided to study potential differences in stress reactions under controlled conditions where we hypothesized that transport stress would be more apparent in the physiology of Atlantic salmon after closed transport (without water treatment) than after open (flow-through) transport. Since the experimental fish, obtained from a commercial salmon farm, were transported by vessel (open system), and subsequently by vehicle (closed system) to our laboratory, we decided to study transport and recovery of these fish as an additional feature before the laboratory study was initiated.
2 MATERIALS AND METHODS
2.1 Transport from fish farm to research facility
Atlantic salmon were collected at the Marine Harvest (now MOWI) Persflua fish farm (Lysøysund, Norway) on 4 November 2016. The fish had been offered Marine Harvest MH1200 OCT feed. Veterinary inspection 4 days prior to transport certified the fish as being in good health. Due to the economic implications (reduced on growth) of fasting the whole fish population in the large net pen, our experimental fish were not fasted before transport. Fish were collected from the cage in a sweep-net where they were crowded and subsequently rapidly netted (<10 s) batchwise into the 18.5 m3-hold of the vessel ‘M/S Hepsøfjord’ adjacent to the cage where the seawater temperature was 9.4°C. Twenty-two minutes after the crowding started, all fish had been transferred to the vessel. The round weight and fork length (mean ± SD, n = 105) of these fish were 1641 ± 467 g and 46 ± 5 cm, respectively. Transport was conducted with open valves at a fish density of 9 kg m−3. The duration of the transport to quay was 0.5 h. After 34 min at the quay, salmon were netted batchwise into a vehicle specialized for live-fish (smolt) transport (Kristensens Transport AS). The vehicle, equipped with five transport tanks, routinely pumps a water volume of 2.3 m3 into each tank before loading. The fish were divided equally into three of the tanks. Loading was completed after 68 min. Since clove oil is known to mitigate stress and possibly improve welfare during transport of smolts (Iversen et al., 2009), AQUI-S™ was added to the tanks to give a concentration of 6 ml m−3 to facilitate light sedation. At a fish density of 25 kg m−3 and under constant oxygenation from the vehicle's battery of canisters, the fish were transported for 2.5 h to the laboratory facilities. The seawater pH, measured continuously from just after loading until arrival, dropped from 7.80 to 7.17. During transport, DO in the three tanks ranged between 101% and 123% saturation and water temperature between 7.9 and 10.7°C. Upon arrival, a water sample (500 ml) was taken from each tank for later analysis of total ammonium nitrogen (TAN), total organic carbon (TOC), turbidity and colour. DO, water temperature, acidity and carbon dioxide were also measured in the tanks as 120%–128% saturation, 9.1°C, pH 7.26–7.29 and 5 mg l−1, respectively. Since the fish were not fasted before transport, faeces was to some degree observed on the bottom of the transport tanks. Little foaming was observed, and all fish were swimming calmly. Salmon were then netted and transferred batchwise into a 1000-L tub, containing 0.7 m3 seawater for transfer to a holding tank located indoors. The 4000-L FT holding tank was fed with running sand-filtered seawater (salinity: 34 g kg−1) pumped at a rate of 5 m3 h−1 from 80 m depth. For each batch, transfer time, including loading and unloading, was approximately 10 min. Water was replaced as needed in the transport tub to ensure adequate levels of DO were maintained. Upon completion of transfer, the initial fish density in the holding tank was 48 kg m−3. Throughout the experimental period, the water volume of the holding tank was kept constant at 3.6 m3 (90 cm depth). Based on distinct differences in fish behaviour (swimming vs. ‘resting on bottom’), two groups of fish (n = 13 in total) were sampled between 1 and 2 h after the last fish had been transferred to the holding tank. Each fish was rapidly netted and killed by a sharp cranial blow within 5–10 s. Blood samples were immediately drawn from the caudal vein with heparinized syringes before white muscle pH was measured. During the first hours after arrival, DO varied between 49% and 112% saturation. Eventually, the DO level stabilized as a suitable balance between DO in inlet water and added oxygen through diffusers was established. The pH and water temperature were then 7.63 and 10.2°C respectively. After sampling, the fish were left undisturbed to recover after transport. After 20 h, nine dead fish had been removed from the tank. As a result of the sampling described above and removal of dead fish, the fish density in the holding tank was reduced to 36 kg m−3.
2.2 Experimental design and protocol: simulated transport in open and closed systems
2.2.1 Physiology
After possible recovery from stress related to transport from farm to our laboratory, the first simulated transport experiment was carried out (Open transport). Just before transfer to the transport tank (see below), six individual salmon were netted individually from the holding tank and rapidly killed by a sharp cranial blow within 5–10 s (Control open). Blood samples were drawn from the caudal vasculature for analysis of cortisol, blood pH, glucose, lactate, sodium, potassium, calcium, chloride, partial pressures of oxygen (PO2) and carbon dioxide (PCO2), and haematocrit (Hct). Subsequently, pH in the white muscle was measured. Finally, fish appearance was checked for potential scale loss and haemorrhages (‘red bellies’). The same procedure was carried out before closed transport (Control closed) and after recovery (Recovery-19 h and Recovery 43-h) where 10–12 fish were sampled.
2.2.2 Simulated transport
The duration of each simulated transport was 5 h, corresponding to an intermediate duration of a commercial well-boat transport. For the simulated open transport, a similar adjacent tank (referred to as the transport tank) was used as a common FT system, whereas for the simulated closed transport (same tank), the water supply was stopped and pure oxygen was supplied to the tank through two diffusers (Point Four Systems Inc.). The water level in the tank was adjusted, before fish were added, to a height of 56 cm to mimic a typical commercial fish density. The resulting volume of water was 2.24 m3. Fish density was 54 kg m−3 in the open transport and 42 kg m−3 in the closed transport. Water quality was measured (DO, CO2, pH and temperature) and 0.5-L water samples were taken after 0, 0.5, 2.5 and 5.0 h for later analysis of total ammonium-N (including NH3), total organic carbon (TOC), turbidity and colour. After 5 h transport, ten fish were individually sampled and killed as described above to monitor the stress condition of the fish. The remaining fish in the transport tank were then netted back to the FT holding tank for post-transport recovery.
2.2.3 Experimental timeline
Sixty-five hours after the fish arrived from farm and were transferred to the laboratory holding tank (Day 0), the simulated open transport started. It was anticipated (see Discussion) that this time period was adequate for fish to recover from transport stress. The 5-h open transport was conducted on Day 3. On Day 4, after 14.5 h of recovery, the 5-h closed transport began. In this case, fish were also sampled on Day 5 and Day 6, as well as after 19 and 43 h recovery following the closed transport. An overview of the timeline for the experimental fish groups is shown in Figure 1.
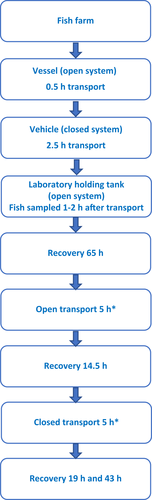
2.3 Analytical methods
2.3.1 Velocity and turbulence measurements
Measurement techniques and post-processing analysis similar to those reported by Plew et al. (2015) and Klebert et al. (2018) were used. The velocities inside the different tanks were recorded using acoustic Doppler velocimeters (Vector, Nortek AS). These instruments have a cylindrical sample volume that is approximately 15 mm high by 15 mm in diameter, located approximately 0.15 m from the instrument probe. Two velocimeters were used at different locations in the tank. During the measurement, the velocities were continuously recorded with high frequency (32 Hz) over several hours. Velocity data from the Nortek Vectors were filtered using a phase space filter (Goring & Nikora, 2002), which removes the velocity spikes that occurred due to Doppler noise, signal aliasing (fish in the sampling volume) or other instrument noise. The mean velocity (time-averaged) values were calculated from the velocity data with the spikes removed. For each velocity components in three spatial directions (U, V and W), the instantaneous fluctuations from the mean values are denoted u’, v’ and w’ respectively. Turbulent kinetic energy (TKE) is evaluated like the following , and the turbulence intensity (TI) is calculated from the TKE as and is used in this study to evaluate the effect of fish on the flow.
2.3.2 Water quality
The dissolved oxygen level was measured using a YSI ProODO meter (YSI Inc.), and the concentration of carbon dioxide was measured by using the OxyGuard® Portable CO2 Analysator (OxyGuard). The instrument measures the level of free dissolved CO2 in the range of 0–50 mg l−1. Water acidity was measured with a shielded glass electrode (WTW SenTix 41, WTW) connected to a portable pH meter (model WTW 315i). For measuring water temperatures, a Testo 110 thermometer (Testo AG) was used. Total ammonium nitrogen (TAN = NH4+ + NH3) was determined according to Norwegian Standard NS 4746, (1975) by measuring the absorbance at 630 nm after a reaction with hypochlorite and phenol at pH 10.8–11.4. The detection limit is 5 μg l−1. The toxic ammonia fraction (NH3) was calculated from data given by Emerson et al. (1975) where the ammonia concentration is computed based on the measured TAN, pH, water temperature and salinity values. The contents of total organic carbon (TOC) in the samples, including precipitate, were analysed by subjecting samples to combustion and IR spectrophotometric analysis of TOC according to NS-EN 1484. The detection limit is 0.5 mg l−1. Turbidity (cloudiness of water) was analysed according to NS-EN ISO 7027. Water samples, transferred to cells, were inserted into a Hach 2100 AN IS Turbidometer (Hach) for measurement of light scattering. The results are presented as NTU (Nephelometric Turbidity Unit). The detection limit is 0.03 NTU. In case of Colour, samples were determined spectrophotometrically according to ISO 7887:2011. The limit of detection is <1 mg Pt l−1. Colour values are reported dimensionless.
2.3.3 Blood chemistry
Cortisol in blood was sampled with heparinized syringes and centrifuged (6000 rpm, 5 min) with a Galaxy Mini Star Silverline C1413-VWR230 centrifuge (Radnor, USA) for extracting blood plasma. The plasma was subsequently stored at −20°C until later analysis. Cortisol was determined by using a radioimmunoassay method as described by Iversen et al. (1998). Whole blood pH was measured immediately after blood withdrawal by a syringe. A similar instrument was used for measurement of water pH. Glucose was measured by dipping the tip of a test strip in whole blood after the blood was sampled with a 5-ml syringe. The strip was then inserted into an Ascensia Contour meter (Bayer HealthCare LLC), and the glucose concentrations were provided in mmol l−1 on the display. Whole blood lactate was assessed with a Lactate Scout+ meter (EKF Diagnostics GmbH) with a measuring range of 0.5–25 mmol l−1. A test strip was inserted into the instrument after it was briefly soaked in blood immediately after the blood was sampled by syringe. While point-of-care devices can be useful as field methods to assess relative differences between fish groups, it should be pointed out that portable lactate and glucose meters generally underestimate the concentrations of glucose and lactate compared with traditional analytical methods (Bakke & Woll, 2014; Stoot et al., 2014; Venn Beecham et al., 2006; Wells & Pankhurst, 1999). Plasma chloride was determined by silver chloride titration using a Radiometer CMT 10 chloride titrator (Radiometer AS). The sodium content of the plasma was analysed by ICP-MS by an Agilent series 7700 instrument according to NS-EN ISO 17294-2 (2007).
2.3.4 Initial pH in white muscle
The initial pH of white epaxial muscle was measured directly in the muscle just after killing. Similar instrument and electrode were used for measuring pH in water.
2.3.5 Respiration and behaviour
Respiration frequency was calculated as a count of the number of opercular beats per minute observed from underwater films (filmed with GoPro 5 cameras). It was determined for five individuals per time point. Recovery after commercial transport was observed 1 and 2 h following commercial transport. Observations during open and closed transport were done at 0, 1, 2, 3, 4 and 5 h after the onset of simulated transports. Potential differences in behaviour among fish in open and closed systems were evaluated by visual observations. Gaping behaviour was defined as a disturbance of water surface by fish with the mouth open. It was measured by counting the number of fish exhibiting this behaviour in a 1-min period and standardized as a proportion by dividing by the number of fish in the tank at that time. The mean of this proportion was calculated for each transport phase, that is during early recovery after commercial transport, as well as during open and closed transports.
2.4 Ethics
The experiments were conducted in accordance with the Norwegian Animal Welfare Act, and the experimental design was approved by SINTEF Ocean personnel authorized by the Norwegian National Animal Research Authority.
2.5 Statistics
Stress data were first tested for normality and homogeneity of variance by using the Shapiro–Wilk and Levene Median tests respectively. If the tests passed, Student t-tests were run for pairwise comparisons between fish sampled from the bottom of the tank and swimming fish (effect of transport from fish farm). Otherwise, Mann–Whitney rank sum tests were used to test significance (p < 0.05). For the laboratory experiment (comparison between open and closed transports including control and recovery groups), either normality or homogenity of variance tests failed in most cases. Hence, the Kruskal–Wallis one-way analysis of variance on ranks method was applied. Where significance was indicated, Dunn's method was used for pairwise comparisons between treatments. Since glucose and sodium data passed both Shapiro–Wilk and Levene Median tests, one-way ANOVAs were applied followed by the Holm–Sidak post hoc test.
3 RESULTS
3.1 Transport from farm to laboratory
3.1.1 Water quality
The water quality in the three closed transport tanks on the vehicle just after arrival, as well as in the FT holding tank during the period when the swimming and ‘bottom’ fish were sampled (see above) is shown in Table 1. The water temperature (9.1°C) was close to that at the farm (9.4°C), similar to the acclimation temperature of the fish. In all tanks, the water was supersaturated with respect to DO (120–128%). In the holding tank, DO varied between 49% and 122% saturation since conditions had not yet stabilized after fish had recently been transferred to the tank. Carbon dioxide levels were only somewhat elevated in all cases (2–5 mg l−1), also verified by the fact that seawater pH was comparatively high in all cases (pH 7.26–7.62). In the closed tanks, TAN had accumulated during transport to 1.12–1.47 mg l−1, whereas in the FT holding tank, the level was lower (0.70 mg l−1). The toxic fraction (NH3) of TAN was, however, low in all cases (2.0–3.0 μg l−1). The TOC levels were clearly elevated after transport (6.2–8.6 mg l−1). Turbidity in the transport tanks was between 2.7 and 4.8 NTU, and 1.8 NTU in the holding tank, whereas the Colour values varied between 8 and 10 in the transport tanks as opposed to 3 in the holding tank.
| Water quality parameter | Closed tank 1 | Closed tank 2 | Closed tank 3 | FT holding tank |
|---|---|---|---|---|
| Temperature (°C) | 9.1 | 9.1 | 9.1 | 10.2 |
| Dissolved oxygen (% saturation) | 128 | 120 | 125 | 49 → 112 |
| Carbon dioxide (mg l−1) | 5 | 5 | 5 | 2 |
| pH | 7.29 | 7.27 | 7.26 | 7.62 |
| TAN (μg l−1) | 1230 | 1120 | 1470 | 704 |
| NH3 (μg l−1) | 2.3 | 2.0 | 2.5 | 3.0 |
| TOC (mg l−1) | 6.2 | 8.4 | 8.6 | 2.3 |
| Turbidity (NTU) | 2.7 | 3.9 | 4.8 | 1.8 |
| Colour (−) | 8 | 9 | 10 | 3 |
- Note: Measured after transport from fish farm to quay by vessel and by vehicle to our laboratory for 2.5 h under constant oxygenation (no water exchange).
3.1.2 Stress
Table 2 shows the stress load of swimming fish and possible moribund fish in the holding tank shortly after arrival from the farm. The cortisol levels (577 and 653 nmol l−1) show that both groups of fish were severely stressed although they were not different (p > 0.05). The mean blood pH of ‘bottom fish’ (pH 7.32) was significantly lower than ‘swimmers’ (pH 7.54) indicating that acidosis was less severe in the latter group. Glucose, sodium and chloride concentrations did not differ between groups (Table 2, p > 0.05). In contrast, the lactate levels of swimming fish (6.0 mmol l−1) were strikingly different from ‘bottom fish’ (17.6 mmol−1). In line with that, the initial muscle pH of the two groups of fish was also significantly different.
| Stress parameter | Fish on tank bottom | Fish swimming in tank |
|---|---|---|
| Cortisol (nmol l−1) | 577 ± 103a | 653 ± 64a |
| Blood pH | 7.32 ± 0.02a | 7.54 ± 0.03b |
| Glucose (mmol l−1) | 2.2 ± 0.4a | 3.5 ± 0.8a |
| Lactate (mmol l−1) | 17.6 ± 3.4a(1) | 6.0 ± 0.7b |
| Sodium (mmol l−1) | 151 ± 3a | 155 ± 1a |
| Chloride (mmol l−1) | 191 ± 6a | 181 ± 4a |
| White muscle pH | 6.83 ± 0.08a | 7.24 ± 0.03 |
- Note: When fish were sampled from the FT holding tank, the water quality was as shown in Table 1.
- Mean values ± SEM (n = 8 for fish on bottom, n = 5 for swimming fish); Different letter, a or b, means the values for the two groups of fish are different (p < 0.05); (1) The measuring range of the Lactate Scout + meter is 0.5–25 mmol l−1. Two fish sampled from the bottom of the tank exceeded the upper detection limit of the instrument. Since these values were not included in the calculation of the mean value, the mean lactate value for the group is therefore underestimated.
3.2 Comparison between simulated open and closed transports and subsequent recovery
The first simulated transport experiment started after the fish had recovered for approximately 65 h (Figure 1) relative to the condition they are in as shown in Table 2.
3.2.1 Hydraulic analysis
Hydraulic analysis was used to monitor environmental conditions in which the fish were submitted during all measurements and also to possibly gather information about their behaviour. Figure 2 shows that the turbulence intensity recorded in the tanks was increasing in the holding tank (Turbulence 2/holding tank) with the stocking density (number of fish). Similar trend occurred in the flow-through transport case (Turbulence 3). The turbulence intensity values in both cases were quite similar. In contrary, in the closed transport case, the intensity (Turbulence 4) was four times higher than the two other cases. Regarding the mean velocities, they decreased in both holding and flow-through tanks with increasing stocking densities and the magnitudes were quite similar. For the closed transport case though, a very low mean flow velocity was registered, where it was basically due to the movement of the fish. The hydraulic conditions affected the swimming pattern of the fish.
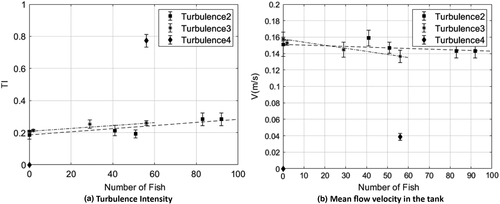
3.2.2 Water quality
Throughout the open system transport, water temperatures varied between 9.3 and 9.7°C, whereas in the closed system, the temperature increased from 9.6 to 10.4°C during the 5-h transports. Changes in water quality during both transports, as well as during closed transport recovery for 19 and 43 h, are shown in Table 3. At DO saturation levels between 92% and 108% in the FT transport and holding tanks (used for recovery), the supply of oxygen was always adequate. In the closed transport tank, DO varied between 90% and 134% saturation. Carbon dioxide was not detected during the open transport (instrument detection limit: 0.0 mg l−1), and during closed transport, CO2 increased gradually, from 7 mg l−1 after 0.5 h up to 24 mg l−1 after 5 h. Acidity in the open system was relatively stable at about pH 8.0 during the entire transport. Due to the gradual accumulation of carbon dioxide during closed transport, acidity dropped to pH 6.65 after 5 h. For both transports, the initial concentrations of TAN were lower than the detection limit of the analytical method (<0.010 mg l−1). After 0.5 h of open transport, TAN had increased to 0.554 mg l−1 followed by a gradual decrease after 2.5 and 5.0 h. During closed transport, TAN increased to 2.95 mg l−1 after 2.5 h and remained at a similar level until the experiment was terminated after 5 h. After subsequent recovery in the FT holding tank for 19 and 43 h, TAN was still somewhat elevated at 0.113 and 0.068 mg l−1. When it comes to the toxic ammonia (NH3) fraction, it is evident that the ammonia levels were very low (≤2.7 μg l−1) at all times in both systems. The level of total organic carbon was fairly stable, between 1.4 and 1.9 mg l−1, during open transport. After closed transport for 2.5 and 5 h, TOC was somewhat higher at 6.2 and 4.9 mg l−1. In line with the elevated levels of TOC during closed transport, turbidity was elevated similarly (3.9 and 3.7 NTU) although not dramatically higher than during open transport (0.4–1.7 NTU). The Colour values were very low in all cases, below or near the detection limit of the method (<1), meaning the water did not become discoloured by, for example, possible blood from haemorrhages (see ‘red-bellied’ fish below).
| Parameter | Open transport (FT) | Closed transport (stagnant) | Recovery (FT) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transport or recovery time (h) | 0 | 0.5 | 2.5 | 5.0 | 0 | 0.5 | 2.5 | 5.0 | 19 | 43 |
| Fish density (kg m-3) | 54 | 42 | 22 | 17 | ||||||
| DO (% saturation) | 92 | NA | 103 | 108 | 118 | 90 | 118 | 134 | 95 | 97 |
| CO2 (mg l-1) | 0.0 | NA | NA | 0.0 | 0.0 | 7 | 13 | 24 | 0.0 | NA |
| pH | 8.00 | NA | NA | 7.88 | 8.07 | 7.19 | 6.95 | 6.65 | 8.03 | 7.96 |
| TAN (μg l-1) | <10 | 554 | 146 | 87 | <10 | 682 | 2950 | 2910 | 113 | 68 |
| NH3 (μg l-1) | <0.1 | NA | NA | 0.6 | <0.1 | 1.1 | 2.7 | 1.4 | 1.2 | 0.6 |
| TOC (mg l-1) | 1.6 | 1.9 | 1.5 | 1.7 | 1.1 | 2.3 | 6.2 | 4.9 | 1.7 | 1.4 |
| Turbidity (NTU) | 0.4 | 1.7 | 0.7 | 0.6 | 0.2 | 1.2 | 3.9 | 3.7 | 1.2 | 0.4 |
| Colour (-) | <1 | <1 | <1 | <1 | <1 | 1 | 2 | 1 | 2 | 2 |
- Note: No water exchange or water treatment other than oxygenation took place during closed transport.
3.2.3 Respiration and behaviour
As shown in Table 4, the mean respiration frequency after commercial transport and during open and closed simulated transports was 161, 137 and 153 beats min−1 respectively. The swimming pattern was very different for fish in the open system, where the water flow was unidirectional with a circular motion, compared with the stagnant water in the closed system (Figure 2). In the open system, the fish distributed themselves in an organized manner against the water current, as commonly observed in fish cultures. In the closed system, however, single fish swam slowly around in the tank in a random fashion. By the end of the simulated closed transport, fish occasionally surfaced and exhibited a coughing behaviour. Gaping behaviour was also observed where fish stayed in a tilted position (tail downwards) at the water surface. Notably, when the fish were transferred to the FT holding tank for recovery, they almost immediately stopped coughing and they no longer stayed close to the surface.
| Transport system | Respiration frequency (opercular beats min−1) | Gaping behaviour (proportion of fish gaping) |
|---|---|---|
| Recovery after commercial transport | 161 ± 20 | 0.03 ± 0.03 |
| Open transport | 137 ± 18 | 0 ± 0 |
| Closed transport | 153 ± 14 | 0.27 ± 0.14 |
- Mean values ± SD, n = 25 & 30 (respiration frequency) and 4 & 5 (gaping behaviour).
3.2.4 Blood chemistry and white muscle biochemistry
Recovery after commercial transport
After arrival from the farm and initial post-transport sampling, the fish were left for 65 h to recover before the open transport experiment was started. Thus, by comparing the stress-related parameters shown in Table 2 with those of the open transport control group (at time 0 h) in Table 5, the extent of recovery can be evaluated. If the values of ‘swimming fish’ (Table 2) are considered, the overall picture was that the open transport control fish had not yet fully recovered to baseline levels. Particularly, cortisol levels were still considerably elevated (454 ± 65 mmol l−1) and not different from the values shortly after arrival (p > 0.05). Furthermore, the levels of blood pH, glucose, sodium and initial pH in the white muscle were also similar to after arrival. On the contrary, substantial recovery was identified in case of lactate and chloride (p < 0.05).
| Stress parameter | Control open | Open transport | Control closed | Closed transport | Recovery-19 h | Recovery-43 h |
|---|---|---|---|---|---|---|
| Cortisol (nmol l−1) | 454 ± 65acY | 479 ± 55a | 277 ± 67bc | 344 ± 65ac | 261 ± 20bc | 316 ± 50bc |
| Blood pHnsd | 7.48 ± 0.06Y | 7.42 ± 0.02 | 7.41 ± 0.03 | 7.40 ± 0.03 | 7.31 ± 0.01 | 7.35 ± 0.02 |
| Glucose (mmol l−1) | 3.5 ± 0.8afhY | 4.5 ± 0.3bdhi | 5.3 ± 0.4befg | 4.1 ± 0.2acgi | 5.8 ± 0.3bc | 6.1 ± 0.5bd |
| Lactate (mmol l−1) | 1.1 ± 0.2bcX | 1.2 ± 0.1bc | 1.2 ± 0.2bc | 0.9 ± 0.1bc | 1.2 ± 0.1bc | 3.2 ± 1.1ac |
| Sodium (mmol l−1) | 157 ± 6aY | 146 ± 3acd | 137 ± 3bce | 178 ± 3d | 144 ± 8ae | NA |
| Chloride (mmol l−1)nsd | 156 ± 2X | 169 ± 3 | 167 ± 9 | 156 ± 4 | 165 ± 4 | 180 ± 9 |
| Potassium (mmol l−1)nsd* | 8.3 ± 1.5 | 5.0 ± 0.3 | 4.4 ± 0.2 | 4.9 ± 0.2 | 5.2 ± 0.5 | 5.9 ± 0.9 |
| Calcium (mmol l−1)nsd* | 1.7 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.3 | 1.8 ± 0.1 | 1.9 ± 0.1 | 2.3 ± 0.3 |
| PO2 corr (kPa)nsd* | 5.04 ± 1.15 | 7.36 ± 1.16 | 5.59 ± 0.97 | 5.14 ± 0.48 | 6.85 ± 0.54 | 6.15 ± 1.18 |
| (mm Hg)nsd* | 37.8 ± 8.6 | 55.2 ± 8.7 | 41.9 ± 7.3 | 38.6 ± 3.6 | 51.4 ± 4.1 | 46.1 ± 8.9 |
| PCO2 corr (kPa)* | 0.45 ± 0.06a | 0.50 ± 0.02a | 0.49 ± 0.04a | 1.77 ± 0.05b | 0.54 ± 0.07a | 0.50 ± 0.07a |
| (mm Hg)* | 3.38 ± 0.45a | 3.75 ± 0.15a | 3.68 ± 0.30a | 13.28 ± 0.38b | 4.05 ± 0.53a | 3.75 ± 0.53a |
| Haematocrit (%)* | 32 ± 4ac | 21 ± 1b | 22 ± 0bc | 25 ± 1ac | 23 ± 1abc | 22 ± 1ab |
| White muscle pH | 7.33 ± 0.04abcY | 7.25 ± 0.02b | 7.40 ± 0.07c | 7.39 ± 0.04c | 7.41 ± 0.03c | 7.45 ± 0.04c |
| Red-bellied fish (#/n)** | 0/6 | 1/10 | 2/6 | 7/10 | 8/10 | 10/12 |
- Note: The number of red-bellied fish at each sampling point was also recorded. Both control groups were sampled from the FT holding tank immediately before the fish were netted to the transport tank. Before sampling of open transport control fish, the fish had recovered for 65 h after the post-transport sampling of fish as shown in Table 3. The recovery period before sampling of control fish for the closed transport was 14.5 h.
- Mean values ± SEM (n = 6, 10 or 12); Different letter, a-i, means the values for the groups of fish are different (p < 0.05);*measured by the epoc™ instrument;**number of red-bellied fish relative to the sampled population at any one time; nsd—not significantly different (p > 0.05); NA—not analysed; ‘X’ means the values are significantly different from swimming fish shortly after arrival from farm, whereas ‘Y’ means the value is not significantly different from swimming fish shown in Table 3.
Open vs. closed transport
Stress-related values of fish subjected to simulated open and closed transport and subsequent recovery are shown in Table 5. The already elevated cortisol values of the control fish did not change significantly during the open and closed transports. Furthermore, no effect of recovery was observed up to 43 h post transport (p > 0.05). Similarly, blood pH did not change during the treatments (p > 0.05). Some significant differences in glucose levels between treatments were observed although in an inconsistent manner. Basically, lactate remained unchanged at low levels (0.9–1.2 mmol−1) during the simulated transports including subsequent recovery for 43 h (1.3–3.2 mmol−1). Open transport did not affect the level of sodium. On the contrary, a significant increase occurred, from 137 mmmol−1 (control closed, 0 h) to 178 mmol−1 at the end of the closed transport. A recovery effect was observed after 19 h (p < 0.05). In contrast, the other ions (chloride, potassium and calcium), as well as the partial pressure of oxygen in the blood, were unaffected by all treatments (p > 0.05). For all fish groups kept in the FT system, the partial pressure of carbon dioxide was relatively stable, between 3.38 and 4.05 mm Hg, where no fish groups were different from each other (p > 0.05). However, after 5 h transport in the closed system, PCO2, increased markedly to 13.28 mm Hg (p < 0.05) before returning 4.05 mm Hg after recovery for 19 h. Regarding haematocrit, some significant differences among treatments were observed within the range of 21–32%. In line with visual observations, showing that no excessive swimming or escape behaviour took place, the lactate levels were low and the values of initial pH in white muscle were comparatively high at pH 7.25–7.45. Some of the sampled fish from each treatment were characterized as ‘red bellied’. Clearly, the number of fish with red bellies (minor haemorrhages) increased over time and it seems that closed transport may have exacerbated this trend. Among the 28 remaining fish after the final sampling (Recovery-43 h), 23 fish were characterized as red-bellied to varying extents, although no severe cases were observed. By the end of the experiment, 5 out of the remaining 28 fish had a moderate loss of scales.
4 DISCUSSION
4.1 Transport from farm to laboratory
4.1.1 Water quality
Overall, it was clear that water quality had deteriorated during transport but probably not to the extent where water quality alone (Table 1) unambiguously could explain altered behaviour and post-transport mortalities for some of the fish. Otherwise, see below for a more detailed discussion on water quality and recommended safe levels for the various water quality parameters. It is likely that TOC predominantly consisted of mucus (glycoproteins) and faeces (observed at the bottom of the transport tank since the fish were not fasted). Colour values in seawater have been determined as <2 (unpublished data). Hence, the Colour values (8–10) in transport tanks must be considered elevated. Possibly, the yellowish tint of the added AQUI-S™ may have affected the Colour values. For comparison with transport water quality (Table 1), another case can be mentioned where fasted freshwater rainbow trout were transported by vehicle at a fish density of 169 kg m−3 for 3.3 h at about 11°C. By the end of the transport, the levels of DO, acidity, TAN, NH3, TOC and Colour were 310% saturation, pH 6.88, 5.0 mg l−1, 8.0 μg l−1, 0.79 mg l−1 and 6 (−) respectively. In this case, no fish exhibited extreme abnormal behaviour and there were basically no delayed mortalities (Shabani et al., 2016).
4.1.2 Stress
The fish, sampled from a large production cage, were not fasted before transport. It is generally recommended that fish should be fasted for at least 48–72 h before transport to reduce metabolic rates, oxygen consumption and ammonia excretion (Wedemeyer, 1996a). Sufficient fasting time for emptying of gut contents before transport would also be beneficial to avoid water fouling during transport. Sufficient fasting time for emptying of gut contents before transport is also beneficial to avoid water fouling during transport. On the contrary, post-exercise recovery rates are not significantly affected by whether fish are fasted or not (Scarabello et al., 1991). The stress level, shortly after transport (Table 2), shows mean cortisol levels 577 and 653 nmol−1. By comparison, the cortisol level of rested salmon is approaching zero (Gamperl et al., 1994; Iwama et al., 2004) while, for example, severe crowding of salmon in a commercial net pen at 12.6°C for 0–2.6 h resulted in cortisol levels of 665–736 nmol l−1 (Erikson et al., 2016). The mean blood pH values after transport were 7.32 and 7.54. When rested salmon, with blood pH 7.85, were exercised to exhaustion, this resulted in extracellular acidosis and a pH-drop to 7.316 (Tufts et al., 1991), similar to the ‘bottom fish’ in the present study. It is difficult to make conclusions regarding the levels of glucose and sodium since their levels were within normal ranges (see below), and we did not know pre-transport levels at the farm. On the contrary, the mean chloride levels (181 and 191 mmol l−1) of both fish groups were extremely high compared with typical levels (approximately 135–165 mmol l−1) from previous studies of salmon in cage cultures (unpublished results). This indicates ionic and osmotic disturbances, something that can be associated with anaerobic exercise (see Cooke et al., 2008). The mean lactate levels of swimming and ‘bottom’ fish were 6.0 and 17.6 mmol−1, respectively, both high compared with 3.1 mmol l−1 after a 3.3-h closed transport of trout (Shabani et al., 2016) as well as with salmon severely crowded in a net pen, where the highest lactate value was 3.4 mmol−1 (Erikson et al., 2016). The same analytical point-of-care method for lactate was used in all cases. In particular, the lactate level of ‘bottom fish’ must be considered dramatically high at >17.6 mmol−1 (the values of two fish which exceeded the upper range of the instrument, 25 mmol l−1, were excluded). At pH 7.24 and 6.83, swimming and ‘bottom’ fish confirmed that the latter group of fish had struggled to near exhaustion, whereas the swimming fish were moderately stressed since it has been shown for rested and exhausted Atlantic salmon that the initial pH are typically within pH 7.5 ± 0.1 and pH 6.7 ± 0.1 respectively (Erikson & Misimi, 2008). By comparison, after the already mentioned live transport of rainbow trout (Shabani et al., 2016) and the crowding of Atlantic salmon in the net pen, the initial pH values were about 6.9 and 7.0–7.1 respectively (Erikson et al., 2016). Regarding the severely disturbed acid–base status including the extremely high lactate levels of the ‘bottom fish’, it could be that both respiratory (water quality issues) and metabolic (exercise to exhaustion) acidosis contributed to the severe condition where the fish might have been moribund. Another indication of this was observed 20 h later where nine other fish were found dead in the holding tank. The appearance of these fish was normal with no prominent occurrences of red bellies, haemorrhages or loss of mucus and scales. These findings (extreme acidosis, exercise to exhaustion and delayed mortalities) may support the hypothesis put forward by Wood et al. (1983) suggesting that post-exercise mortality of rainbow trout may be related to severe intracellular acidosis. In their case, mortalities were observed 4 h post-exhaustive exercise at lactate levels about 12 mmol l−1 (as measured by an established, well-validated laboratory method) and a blood acidity of approximately pH 7.35. AQUI-S™ was added to the closed tanks before the last leg of the journey to our facilities. Although we cannot rule out that it did not provide a stress mitigating effect, the fish nevertheless arrived in a stressed condition. A likely explanation could be that the fish were already severely stressed before AQUI-S™ was introduced into the vehicle's transport tanks, that is, after crowding in net pen, loading to live-fish carrier, and transfer of fish from vessel to vehicle.
4.2 Comparison between simulated open and closed transports and subsequent recovery
4.2.1 Water quality
As a result of repeated sampling of fish, the fish density gradually decreased from 54 kg m−3 in the open system to 17 kg m−3 at final sampling of recovered fish. When fish density per se is taken into consideration, it is unlikely that it would be a significant factor in our study since Thorarensen and Farrell (2011) concluded that there is little, or no consistent effect of density up to about 80 kg m−3 on growth, survival and welfare of Atlantic salmon. Regarding DO, the question is whether the elevated levels of DO up to 134% in the closed system (Table 3) would be sufficiently high to significantly reduce respiration rates, which in turn would cause a build-up of CO2 in the blood and cause acidosis (Hobe et al., 1984). Gas supersaturation can also induce emboli in tissues causing gas bubble disease, which would cause even greater problems if associated with nitrogen (Noga, 2000). When Atlantic salmon juveniles are exposed to DO supersaturated water (110–220%), changes in behaviour with regards to swimming activity, number of turns and panic reactions have been observed, demonstrating signs of pain and discomfort (Espmark et al., 2010). Hyperoxic water can also lead to elevated oxygen levels internally in the fish and might cause oxidative damages (Lygren et al., 2000). Furthermore, exposure of coho salmon smolts (Oncorhynchus kisutch) to hyperoxia (around 340% saturation) for 6 h resulted in osmoregulatory problems (Brauner, 1999). On the contrary, when rainbow trout were reared in FT systems at DO levels 94–180% saturation for 125 days, no differences were observed in growth and feed conversion, nor was mortality affected (Edsall & Smith, 1990). Moreover, no detrimental effects were observed when Atlantic salmon smolts were exposed to 123% DO saturation during a 3-h transport (Hosfeld et al., 2008). Notably, moderate oxygen supersaturation (<140%) did not cause harmful effects on blood chemistry and hepatic gluathione status of rainbow trout (Ritola et al., 1999). Taken together though, it is unclear to what extent DO supersaturation played a role regarding behaviour and stress during the present 5-h closed transport. The highest level of carbon dioxide encountered in this study was 24 mg l−1 after 5 h closed transport (Table 3). The recommended maximum carbon dioxide levels in aquaculture to maintain good fish welfare and to support maximum growth of salmonids varies from 6 to 10 mg l−1 (Fivelstad et al., 1998; Wedemeyer, 1996b) to <20 mg l−1 (Portz et al., 2006; Timmons & Ebeling, 2007). According to Wedemeyer (1996a), the CO2 level in a hauling tank water should be <20–30 mg l−1 to prevent blood CO2 from rising (hypercapnia), which would impair adequate transport of oxygen to tissues (Bohr effect). Rainbow trout change their normal swimming behaviour when CO2 exceeds 35–60 mg l−1 and equilibrium is lost at about 150 mg l−1 (Clingerman et al., 2007). Furthermore, Atlantic salmon are lightly sedated at 70–80 mg l−1 (Erikson, 2011). Therefore, it seems that the duration of the closed transport was not long enough to induce detrimental effects due to elevated levels of carbon dioxide per se. However, the elevated CO2 levels resulted in a drop in acidity where the lowest level was pH 6.65 at the end of the closed transport. This value is still within the recommended levels for fish in aquaculture, namely pH >6 (Randall, 1991) and pH 6.5–8.5 (Timmons & Ebeling, 2007). The increase in TAN after 0.5 h might be explained by an initial stress response (increased metabolic rate) due to the transfer of fish from the holding tank. As the fish subsequently acclimated to the new tank environment, they may have become less stressed (less excretion of ammonia) resulting in diminishing TAN concentrations after 2.5 and 5.0 h. In the closed system, however, TAN gradually accumulated to about 2.9 mg l−1 after 2.5 and 5.0 h. The dominant fraction of TAN, ammonium (NH4+), has generally been considered not harmful (Tabata, 1962). The ammonia fraction was always ≤2.7 μg l−1 in both systems. Although TAN increased significantly in the closed system, ammonia was always kept at bay due to the steadily decreasing pH. With respect to NH3, suggested safe limits for salmonids varies between 12 and 25 μg l−1 (Fivelstad et al., 1995; Timmons & Ebeling, 2007; Wedemeyer, 1996b, 1997). The TOC levels in the present study varied between 1.1 and 6.2 mg l−1. By comparison, Davidson et al. (2009) reported TOC values of 4.64 and 20.52 mg l−1 in RAS with high and low water exchange rates, respectively, where fish survival was high (>99%) in both RAS regimes. It is well-known that adverse water quality and stress can lead to excessive production of mucus (see review by Shepard, 1994). Mucus on gills arches can cause hypoxia, and in such cases, it has been observed that fish try to clear mucus from gills by a coughing behaviour (Ultsch & Gros, 1979). The turbidity levels of both closed (0.2–3.9 NTU) and open (0.4–1.7 NTU) transport were well below the recommended maximum limit of <20 NTU over ambient levels (Wedemeyer, 1997). Moreover, in seawater cage cultures, we have previously measured values around 0.2 NTU (unpublished results).
All in all, the water quality during open transport can be considered good since there were no plausible issues that could have induced severe stress reactions or compromised fish welfare. In case of the closed transport, water quality clearly deteriorated gradually. After 2.5 h, although several water quality parameters were elevated, they were still within acceptable levels according to recommended single-factor values. However, a joint impact of lowered pH and somewhat elevated levels of DO, CO2, TAN, TOC and turbidity might have affected the fish in terms of behaviour, stress and welfare although we are unable to draw categorical conclusions. It should also be kept in mind that the recommended water quality safe limits in aquaculture mostly addresses long-term farming issues such as optimal on growth and health. Fish can probably tolerate less favourable conditions for a relative short period of time such as short, closed transports. Under the environmental conditions prevailing in the present study, it seemed that closed transport could be carried out safely when transport duration is less than approximately 2–3 h. For longer transports, at some point in time, water treatment will eventually become necessary to avoid excessive stress reactions, compromised welfare or mortality.
4.2.2 Respiration and behaviour
The respiration frequencies of fish 2 h after arrival to laboratory as well as during open and closed transports varied between 137 and 161 beats min−1 at 9°C (Table 4). By comparison, the respiration frequency of severely stressed Atlantic salmon at 13°C in a commercial net pen increased to about 80 beats min−1 after crowding for up to 80–100 min from 55–64 beats min−1 in the bulk volume before crowding (Erikson et al., 2016). In rested rainbow trout at 15°C, values of about 53 beats min−1 have been reported (Altimiras & Larsen, 2000). Just after about a 3-h commercial closed transport of adult rainbow trout at hyperoxic conditions at 10°C, the mean respiration rate was 130 beats min−1. Concurrent analysis of blood chemistry showed the fish were severely stressed and the respiration rate remained high at 120 beats min−1 after recovery for 48 h in a semi-closed system (Shabani et al., 2016). Consequently, the high respiration rates (Table 4) show that the fish were severely affected by commercial transport and that they remained high for several days post transport, showing the dominant role the commercial transport had on our fish.
In the closed system, the fish exhibited gaping behaviour and coughing during the last part of the transport. By then, the levels of DO, CO2, TAN, TOC and turbidity were most elevated and the pH was low (Table 3). Related observations include brook trout (Salvelinus fontinales) where fish exposed to low pH often surfaced, a behavioural response indicating that fish are suffering deprivation of oxygen (Packer & Dunson, 1970), whereas gaping and coughing behaviour have been observed during closed transport with heavy oxygen supersaturation (Erikson, 2001). Furthermore, when rainbow trout were exposed to acidic water, an increase in coughing behaviour and ventilation frequency has been observed (Ye & Randall, 1991).
4.2.3 Blood chemistry and white muscle biochemistry
As discussed above, the fish had not yet recovered after the commercial transport before the simulated open transport started. Subsequently, the already elevated values of cortisol basically remained high throughout the duration of the entire experiment (Table 5). Similarly, blood pH did not change significantly and remined about 0.45 pH units below typical values of rested fish (see above), in spite of the drop in water acidity during the closed transport, from pH 8.05 to pH 6.65 after 5 h (Table 3). The levels of glucose varied between 3.5 and 6.1 mmol−1 (Table 5). In rested fish, the concentration of glucose is typically around 3 mmol l−1 and increases slowly after exposure to a stressor. Glucose then remains elevated for a long period of time (Iwama et al., 2004). In our study, the lowest glucose values appeared shortly after the commercial transport (Table 2), whereas the highest levels occurred 19 and 43 h after the simulated closed transport indicating a slow stress response. The lactate levels were low (0.9–3.2 mmol−1, Table 5) throughout the simulated transport experiment. Typical lactate levels in rested salmon are close to, or below, the detection limit of the instrument (0.5 mmol l−1). Lactate recovery from exhaustive exercise in rainbow trout has been shown to take place within 6–12 h depending on blood and white muscle pH (Milligan, 1996; Milligan et al., 2000), or in some cases, within 24–48 h (Franklin et al., 1990). Furthermore, slow recovery of fish can also be explained by elevated levels of cortisol (as in our case) delaying restoration of acid–base status and metabolite levels (Eros & Milligan, 1996; Pagnotta et al., 1994). The low lactate levels (Table 5) confirm our visual observations, that the fish did not respond to open and closed transport with excessive swimming activity. Of the ions (Na+, Cl−, K+ and Ca2+), the only significant change that took place was the marked increase in sodium after the simulated closed transport and the subsequent recovery after 19 h indicating the changes in water quality (Table 3) resulted in an ionic imbalance. Regarding the partial pressures of oxygen and carbon dioxide, the only significant change during the study was the increase in pCO2 from 3.68 to 13.28 mm Hg in case of the closed transport (Table 5). During the transport, water CO2 and pH changed from 0 to 24 mg l−1 and 8.07 to 6.65 respectively (Table 3). On the contrary, DO supersaturation up to 134% did not reduce respiration frequency (Table 4), which could otherwise cause accumulation of CO2 in the blood. In rainbow trout, the venous PCO2 tension ranges from about 3.8 to 11.3 mm Hg (Harter et al., 2014) and according to data from several authors, in rested trout with blood pH of 7.74–8.02, PCO2 ranges from 1.4 to 4.3 torr (as determined by traditional laboratory methods), see Thomas and Poupin (1985). In rainbow trout exercised to exhaustion, arterial pH was reduced from pH 7.88 to pH 7.37. This was accompanied by an increase in arterial PCO2 from 3.03 torr (rested fish) to 7.79 torr. In this case, the fish recovered completely after 2 h (Turner et al., 1983). In another study, a 0.5 pH-unit drop was observed as PCO2 rose from 1.88 mm Hg (rested trout) to 4.50 mm Hg (exhausted trout) as measured by established laboratory methods. The fish fully recovered after 2–4 h (Kieffer et al., 1994). In RAS and FT systems containing Atlantic salmon smolts, mean values of PCO2 have been reported as 11.7–12.7 mmHg, respectively, as determined by the i-STAT system (Kolarevic et al., 2014). Previously reported haematocrit values in salmonids vary considerably making our values (21–32%) difficult to interpret. For example, as based on the traditional centrifugation method, Turner et al. (1983) reported Hct values in rested rainbow trout as about 21%, whereas Kolarevic et al. (2014) measured values around 35% when using the i-STAT system. Furthermore, Morgan and Iwama (1997) reported that normal values in fish are in the range of 30–50%. The initial pH in white muscle (pH 7.25–7.45, Table 5) were approaching the levels of rested salmon (pH 7.5 ± 0.1, see above). This is in line with visual observations and the low lactate levels discussed above. The gradual increase in the number of red-bellied fish (Table 5) might be a delayed effect of the severe stress bout during the commercial transport. Previously, we have occasionally observed more extreme cases ultimately leading to death several days after transport from farms (unpublished results). Such fish were always kept in FT tanks with good water quality and were not subjected to additional handling or experimentation.
Before the simulated transports were carried out, the fish were clearly stressed (control open, Table 5). It is, however, difficult to assess how stress responses would have been if the fish had been in a rested state before the simulated transport experiments were initiated. It could well be that moderate stress effects may have been masked to some extent (overshadowed by stress due to the commercial transport to the laboratory).
4.3 Assessment of epoc™ as a potential field instrument
Blood chemistry by epoc™ was assessed, but not validated, in the present study at 18°C. Two out of the 46 tested cartridges did not work (no results displayed), and in 34 out of 46 cases, no pH values were displayed (data not shown). This result was unusual since pre-tests had shown that pH values are normally displayed without problems. In fact, temperature-corrected pH values (Ashwood et al., 1983) correlates well with pH meter readings of fish blood acidity. Lactate by epoc™ readings (data not shown) showed good correlation with the lactate meter values (R = 0.988, p < 0.001) although the epoc™ values were consistently 0.4–1.4 mmol l−1 lower than meter readings. Glucose readings by epoc™ (data not shown) also correlated well with glucose meter readings (R = 0.838, p < 0.001) although there was a tendency that epoc™ values were slightly higher. All measured lactate and glucose concentrations were within the stated range of the epoc™ instrument. In case of sodium, 32 of the 46 blood samples exceeded the instrument's upper limit (Na+ > 180 mmol l−1, data not shown). Otherwise, the concentrations of potassium, calcium and haematocrit (Table 5) were all within the measuring range of the instrument as well as within the physiological ranges reported for salmonids (see Cooke et al., 2008). The temperature-corrected values of PO2 and PCO2 were also within the measuring range of the instrument. However, the levels, ranged from 37.7 to 55.2 mm Hg, are considerably lower than the levels 87.2–89.4 mm Hg (measured by electrode) reported by Tufts et al. (1991) in rested Atlantic salmon at 18°C. On the contrary, comparable levels for both partial pressures are reported when other analytical methods were employed (see above). In any case, all analytes of interest measured by the epoc™ should be validated against traditional analytical methods over a broader concentration range than presented here. Since the epoc™ does not operate at ambient temperatures lower than room temperature, field usage can at times be rather cumbersome. Due to the technological resemblance with the i-STAT system, it is likely that issues related to analysis of fish blood, validation and limitations of the i-STAT system (Cooke et al., 2008; DiMaggio et al., 2010; Harter et al., 2014; Stoot et al., 2014) are also more or less relevant for the epoc™. If we consider measuring blood pH, glucose and lactate alone, we cannot recommend using the epoc™ as a substitute for the meters used in this study since the epoc™ method is more expensive (price of instrument and costs of cartridges vs. meter strips), less reliable, as well as due to it is dependence of ambient temperature to operate properly. Moreover, common stress indicators for fish, like chloride, and to some extent sodium, are not measured by the instrument.
5 CONCLUSIONS
The stress reaction salmon experienced during crowding and subsequent transfer by vessel and vehicle, from fish farm to laboratory tanks, was severe and resulted in some delayed mortalities. These fish exhibited extreme acidosis. Recovery for 65 h, before the laboratory study of simulated open and closed transports was carried out, showed that the fish still had not fully recovered. Subsequent open transport did not cause excessive stress reactions relative to the state of fish after partial recovery from stress associated with commercial transport to laboratory. On the contrary, deteriorating water quality during closed transport eventually affected fish behaviour where the welfare of the fish could be questioned. Unless transport duration is comparative short (less than about 2 h under the present conditions), water treatment is therefore necessary to avoid compromised welfare. Stress associated with crowding and transport under commercial conditions largely overshadowed potential stress effects of the laboratory study. Overall, the results from this study can be viewed as the effect of repeated handling operations, which is a typical feature in salmon aquaculture.
ACKNOWLEDGEMENTS
The study was funded internally by SINTEF Ocean as part of the Future Containment Systems for Aquaculture project.
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest associated with the present publication.
Open Research
DATA AVAILABILITY STATEMENT
Raw data that support the findings of this study are available from the corresponding author upon reasonable request.




