Effects of dietary tuna hydrolysate supplementation on feed intake, growth performance, feed utilization and health status of Asian sea bass (Lates calcarifer) fed a low fish meal soybean meal-based diet
[Correction added on 12 May 2022, after first online publication: name order for Khor Waiho has been corrected throughout the article.]
Abstract
This study aimed to examine the effects of dietary tuna hydrolysate supplementation on feed intake, growth, feed utilization and health status of Asian sea bass. Experimental diets included a high fish meal-based diet (HFM diet) containing 45% of fish meal, a low fish meal-based diet (LFM diet) in which 55% of fish meal protein was replaced with soybean meal and the LFM diet coated with 2.5% tuna hydrolysate (LFM + TH diet). Fish were fed diets for 8 weeks. Growth rate, feed intake, feed efficiency, protein efficiency ratio, nitrogen retention, intraperitoneal fat and serum albumin of fish-fed LFM + TH diet were significantly higher than those of fish-fed LFM diet (p < 0.05). No significant differences in carcass chemical compositions, serum cholesterol, triglyceride, aspartate aminotransferase, lysozyme activity, superoxide dismutase and trypsin and lipase activities were found in hepatopancreas and anterior intestine among the dietary treatments. Fish-fed LFM + TH diet displayed a longer length of distal intestine villi than those of fish-fed LFM + TH diet. No histopathological changes in the liver were observed in this study. The results suggest that dietary supplementation of 2.5% tuna hydrolysate is sufficient to enhance the diet palatability, which can increase the replacement levels of fish meal protein with soybean meal up to 55% in a low fish meal soybean meal-based diet without negative impacts on feed intake and growth performance of juvenile Asian sea bass.
1 INTRODUCTION
Fish meal (FM) is commonly used as the main protein source in fish feeds. It is a high-quality protein, has a relatively well-balanced amino acid profile and has high palatability. Fish meal is highly demanded for aquaculture while the total global production of fish meal has declined, resulting in the rising price of fish meal (Olsen & Hasan, 2012; Tacon & Metian, 2008). Particularly, uncertain availability and sustainability of fish meals are critical issues for the aquaculture industry (Gatlin et al., 2007). Whereas the global demand for fish meals continues to grow, the price has historically increased until 2020, with increasing of approximately 350% between 2005 and 2020 up to USD1400 per tones (FAO, 2014, 2021). Many studies have been conducted to identify alternative sources of protein derived from plants such as grains and oilseeds to replace fish meal in fish feeds (Aoki et al., 1996; Carral et al., 2021; He, Li, et al., 2020; He, Yu, et al., 2020; Hosseini Shekarabi et al., 2021; Kumar et al., 2020; Yamamoto et al., 1996). Gatlin et al. (2007) reported that numbers of sustainable plant protein ingredients, namely soy, lupin, rapeseed and pea meals, have potential as alternative sources of protein in diets for farmed fish species. Among the alternative plant protein sources, soybean meal (SBM) has been considered to be a potential candidate as a fish meal replacer in diets for many fish species, namely yellowtail (Seriola quinqueradiata, Nguyen et al., 2015), rainbow trout (Oncorhynchus mykiss, Yamamoto et al., 2012), Atlantic salmon (Salmo salar L., Krogdahl et al., 2015), red sea bream (Pagrus major, Aoki et al., 1997), Asian sea bass (Lates calcarifer, Boonyaratpalin et al., 1998) and largemouth bass (Micropterus salmoides, He, Yu, et al., 2020). Soybean meal is highly potential as an alternative plant protein source due to its sustainable commercial availability, low price, high protein content and exhibit reasonably balanced amino acid profile (Hardy, 2010).
Asian sea bass or Barramundi, Lates calcarifer, is a diadromous species, which returns to estuarine or marine water to breed. Asian sea bass is native to the Indo-Pacific region and its culture is well established in the Indo-Pacific region with significant industries in Australia, Indonesia, Malaysia, Philippines, Taiwan and Thailand (Boonyaratpalin & Williams, 2009). Asian sea bass is a carnivorous fish species, which requires high content of dietary protein in diets. The requirement of dietary protein is recommended in a range from 40% to 50%; nevertheless, the optimal levels of dietary protein in diets for Asian sea bass depends upon the fish size, dietary lipid content and rearing condition (reviewed in Glencross, 2006; Boonyaratpalin & Williams, 2009).
The partial replacement of fish meal protein with soy protein products without any detrimental effects on protein digestibility, growth performance and health of Asian sea bass was revealed by Boonyaratpalin et al. (1998) and Tantikitti et al. (2005). Initially, several types of soybean products, namely solvent-extracted soybean meal, extruded full-fat soybean meal, steamed full-fat soybean meal and soak raw full-fat soybean meal replaced 37% of fish meal protein in fish meal-based diets (a control diet) for Asian sea bass (Boonyaratpalin et al., 1998). In the results of the 10-week feeding study, growth performance, feed utilization and protein digestibility of fish fed a solvent-extracted soybean meal-based diet were significantly higher than those of fish-fed diets containing any other types of soy protein products. Later, Tantikitti et al. (2005) found that only 10% of fish meal protein could be replaced with defatted soybean meal in the diet without any negative impacts on feed intake and growth performance of Asian sea bass. Recently, Ma et al. (2018) found reported that 50% of fish meal protein replaced with soybean meal significantly reduced the growth performance of Asian sea bass, resulting in low feed intake.
Protein hydrolysate processed from by-products derived from several aquatic animals, such as fish, krill and shrimp, is considered potential feed ingredients for fish feed as it is a good source of high-quality protein composed of free amino acids and low molecular weight compounds (Aksnes, Hope, Høstmark, & Albrektsen, 2006; Chotikachinda et al., 2013; Hidaka et al., 2000; Zheng et al., 2012). Previous studies demonstrated that protein hydrolysate has the potential to be as a fish meal replacer, feed attractant and palatability enhancers in diets containing low fish meal-based diets for a number of carnivorous fish species, yellowtail (Seriola quinqueradiata) (Kofuji et al., 2006b), Japanese sea bass, (Lateolabrax japonicus) (Liang et al., 2006), red sea bream (Pagrus major) (Kader et al., 2010; Khosravi, Bui, et al., 2015; Khosravi, Rahimnejad, et al., 2015; Tola et al., 2019a), Asian sea bass (Siddik et al., 2019; Siddik, Howieson, Partridge, et al., 2018), rainbow trout (Oncorhynchus mykiss) (Aksnes, Hope, Jönsson, et al., 2006), Japanese flounder (Paralichthys olivaceus) (Zheng et al., 2012), Atlantic salmon (Salmo salar L.) (Hevrøy et al., 2005), turbot (Scophthalmus maximus L.) (Zheng et al., 2013) and European sea bass (Kotzamanis et al., 2007).
In Asian sea bass, the first study on the inclusion of dietary fish protein hydrolysate as a feed attractant was conducted by Chotikachinda et al. (2013). Fish protein hydrolysate (FPH) was produced from the visceral by-product of skipjack tuna (Katsuwonus pelamis) using the enzymatic hydrolysis technique. This study found that the supplementation of 3–4% fish protein hydrolysate in poultry by-product-based diet significantly enhanced feed palatability and growth performance of Asian sea bass. Besides, the studies of Siddik et al. (2019, 2021) have illustrated that the dietary supplementation of fish tuna hydrolysate possesses plenty of beneficial effects on feed utilization, oxidative status, immune response, disease resistance and modulating the intestinal bacteria of Asian sea bass fed a low fish meal poultry-based diet. The most recent study, Siddik et al. (2021) found the dietary supplementation of fish protein hydrolysate (4.6 g kg−1 diet) could significantly enhance the replacement level of fermented lupin meal to 75% of fish meal protein in the diet for Asian sea bass without the negative impacts on growth, health and immunity.
To our best knowledge, low feed intake in Asian sea bass associated with high levels of fish meal protein replaced with soybean meal is a significant challenge to develop a low fish meal soybean diet for sustainable aquaculture (Ma et al., 2018; Tantikitti et al., 2005). Notably, the poor growth performance of Asian sea bass fed with a soybean meal-based diet was attributed to low caloric intake resulting from depressed feed intake due to poor palatability (Boonyaratpalin et al., 1998; Ma et al., 2018; Tantikitti et al., 2005). Previously published studies showed that replacement levels of fish meal protein with soybean products could be increased by the dietary supplementation of fish protein hydrolysate in carnivorous fish species including red sea beam (Kader et al., 2010; Khosravi, Bui, et al., 2015; Tola et al., 2019b), rainbow trout (Aksnes, Hope, Jönsson, et al., 2006b) and Japanese flounder (Khosravi, Bui, et al., 2015). Based on the previous study findings, this indicates that the supplementation of fish protein hydrolysate as a feed palatability enhancer was an effective approach to develop nutritional values of a low fish meal soybean meal-based diet to maintain feed attractiveness and induce sufficient feed intake of fish.
We hypothesized that if the low feed intake was a reason for the low growth performance of Asian sea bass fed a low fish meal soybean meal-based diet, then the low-caloric intake can be overcome by increasing feed intake induced by the dietary supplementation of fish protein hydrolysate. The present study aimed to examine the effects of the dietary supplementation of tuna hydrolysate on growth performance, feed utilization, digestive enzyme activity and health status, namely serum parameters and histopathological changes in the liver and intestine, of Asian sea bass under the laboratory condition.
2 MATERIALS AND METHODS
2.1 Experimental diets
Tuna hydrolysate liquid (Actipal Fish ML4, AQUATIV Thailand) used in the present study was donated by AQUATIV Thailand. The proximate composition of dietary ingredients is presented in Table 1. Three experimental diets were formulated to contain 45% crude protein and 10% crude lipid. The fish meal-based diet (FM diet) as a control diet was formulated to contain 45% of fish meal. In the soybean meal-based diet (SBM diet), 55.55% of fish meal protein was replaced with 45% of soybean meal. The soybean meal-based diet was supplemented with 2.5% of tuna hydrolysate using a top coating method (SBM + TH diet).
| Chemical composition | Fish meal | Soybean meal | Corn protein concentrate | Tuna hydrolysate | Poultry offal meal | Shrimp head meal |
|---|---|---|---|---|---|---|
| Dry matter | 95.6 | 88.1 | 93.3 | 36.5 | 94.6 | 90.2 |
| Crude protein | 61.1 | 46.9 | 75.7 | 22.3 | 64.7 | 44.3 |
| Crude lipid | 4.4 | 1.3 | 2.9 | 3.4 | 8.9 | 6.4 |
| Ash | 30.2 | 6.5 | 1.1 | 7.0 | 15.6 | 29.6 |
All powdered ingredients (Table 2) were thoroughly mixed with fish oil and boiled water to produce stiff dough. The dough was pelleted using a laboratory pellet mill. The diets were dried at 60°C overnight. In the case of SBM + TH diet, the pellets were harmoniously coated with tuna hydrolysate using a vertical feed mixer. All diets were stored at 4°C until used. The dietary samples were randomly collected for proximate analysis.
| Ingredient (g kg−1 of diet) | Dietary treatment | ||
|---|---|---|---|
| HFM | LFM | LFM + TH | |
| Fish meal | 450 | 200 | 200 |
| Soybean meal | 30 | 400 | 400 |
| Corn protein concentrate | 40 | 40 | 40 |
| Poultry offal meal | 100 | 100 | 100 |
| Shrimp head meal | 10 | 10 | 10 |
| Wheat | 120 | 80 | 80 |
| Rice (polished) | 120 | 63 | 63 |
| Carboxymethyl cellulose | 5 | 5 | 5 |
| Guar gum | 5 | 5 | 5 |
| Choline chloride | 2 | 2 | 2 |
| Vitamin and mineral premixa | 15 | 15 | 15 |
| DL-methionine | 0 | 5 | 5 |
| Dicalcium phosphate | 0 | 15 | 15 |
| Cellulose | 48 | 0 | 0 |
| Fish oil | 55 | 60 | 60 |
| Total | 1000 | 1000 | 1000 |
| Analysed chemical composition (%) | |||
| Dry matter | 96.0 | 95.0 | 96.1 |
| Crude protein | 44.5 | 45.2 | 45.6 |
| Crude lipid | 11.5 | 10.6 | 10.5 |
| Ash | 8.1 | 7.1 | 6.7 |
| Crude fibre | 1.26 | 0.88 | 0.87 |
| Nitrogen-free extractb | 30.6 | 31.2 | 32.4 |
| Gross energy (MJ kg−1)c | 19.4 | 19.2 | 19.3 |
- Abbreviations: HFM, high fish meal diet; LFM, low fish meal diet; LFM + TH, low fish meal diet supplemented with tuna hydrolysate.
- a Vitamin and mineral premix (mg kg−1 of diet): Vitamin A, 18 mg; Vitamin D3, 5 mg; Vitamin E, 150 mg; Vitamin C, 500 mg; Vitamin B1, 16 mg; Vitamin B6, 20 mg; Vitamin B12, 6 mg; Vitamin K3, 18 mg; riboflavin, 40 mg; inositol, 320 mg; calcium-D-pantothenate, 60 mg; niacinamide, 80 mg; folic acid, 5 mg; biotin, 2 mg; ethoxyquin, 100 mg; Na, 30 mg; K, 50 mg; Mg, 100 mg; Cu, 4 mg; Fe, 25 mg; Zn, 35 mg; Mn, 12 mg; I, 1.6 mg; Se, 0.2 mg; Co, 0.8 mg.
- b Nitrogen-free extract (NFE) = % of dry matter – (% of crude protein + % of crude lipid + % of crude ash + % of crude fibre).
- c Gross energy (MJ kg−1) = crude protein (kg kg−1) × 23.6 (MJ kg−1) + crude lipid (kg kg−1) × 39.0 (MJ kg−1) + total carbohydrate (kg kg−1) × 17.0 (MJ kg−1).
2.2 Experimental fish and feeding protocol
The feeding experiment was carried out at the Department of Animal and Aquatic Sciences (Faculty of Agriculture, Chiang Mai University, Thailand). Juvenile Asian sea bass (body weight was approximate 25–30 g), which were adjusted to freshwater, were obtained from Nimit farm, Petchaburi province, Thailand. Fish were acclimatized in the laboratory conditions for 2 weeks. Fish were fed a commercial diet (PROFEED No. 903, Thai Union Feedmill PCL, Thailand) containing 45% crude protein two times a day. The freshwater in all the tanks used for this experiment was recirculated from an external bio-filter with a water refreshment capacity of 10 L min−1. The system was subjected to a photoperiod of 12 h light:12 h darkness. Water quality parameters including temperature, dissolved oxygen and pH (temperature = 23.0 ± 5.6°C, dissolved oxygen = 6.0 ± 1.5 mg L−1 and pH = 7.8–8.1) were measured throughout the experimental period to maintain optimal levels for Asian sea bass.
Groups of 10 fish with a mean initial body weight of 36.2 ± 3.0 g were randomly assigned to nine 200 L fibre-glass tanks. Five fish were randomly collected for chemical composition analysis. Three experimental diets were fed to fish in each tank until satiation once a day for 8 weeks. The feeding was stopped when the feeding response of fish appeared slow.
2.3 Sample collection
Prior to the feeding experiment, all fish were individually measured for initial body weight after 2 days of starvation. The individual fish was measured for the body weight every 2 weeks. At the end of the experiment, fish were starved for 2 days before individually measuring for final body weight. Fish were anaesthetised with 2-phenoxyethanol solution (200 mg L−1). Four fishes from each tank were individually sampled to withdraw blood serum and then dissected to collect the whole viscera. Two fishes from each tank were collected for chemical composition analysis.
2.4 Determination of lipase and trypsin activities and protein contents in hepatopancreatic and anterior intestinal tissues
The hepatopancreatic and anterior intestinal tissues of Asian sea bass were extracted for the crude digestive enzymes according to Rungruangsak-Torrissen et al. (2006). Briefly, the samples were homogenized in 50 mM Tris–HCl buffer pH 8 containing 200 mM NaCl (1:1 w/v). The homogenate was centrifuged at 4°C at 6708 g for 20 min and the supernatant was collected and kept at −20 °C for the determination of total protein content and the activities of trypsin and lipase.
The trypsin and lipase activities were assayed according to the method described by Rungruangsak-Torrissen et al. (2006) and Winkler & Stuckman (1979). Benzoyl-L-arginine-p-nitroanilide (BAPNA) was used as a specific substrate for trypsin assay. The reaction mixture consisted of 1 ml of BAPNA (1.25 mM) dissolved in Tris–HCl buffer (0.2 mM, pH 8.4) and 0.01 ml of tissue homogenate. The assay was performed at 50°C. The production rate of nitroaniline was measured spectrophotometrically at 410 nm (Double Beam Spectrophotometer Libra S80, Biochrom Ltd., Cambridge, England) for 5 min. The trypsin activity was expressed as μmol p-nitroaniline produced h−1 mg protein−1. For lipase activity assay, p-nitrophenyl palmitate was used as a specific substrate. The reaction mixture composed of 0.2 ml of 0.1 M of p-nitrophenyl palmitate was dissolved in isopropanol, 0.8 ml of 0.2 M phosphate buffer (pH 8) and 0.01 ml of the crude enzyme extract. The sample mixture was incubated at 60 °C for 15 min, and then, 1 M Na2Co3 was added to the sample mixture tube to stop the reaction. The mixture was centrifuged at 15,115 g for 15 min. The production of nitrophenol in the supernatant was measured spectrophotometrically at 410 nm and compared with a p-nitrophenol standard curve. The lipase activity was expressed as μmol p-nitrophenol produced h−1 mg protein−1. The total protein concentration of the crude enzyme samples was determined according to Lowry et al. (1951). Bovine serum albumin (BSA standard) was used as a standard. Briefly, the reaction mixture consisted of 0.05 ml of crude enzyme sample, 0.45 ml of distilled water and 2 ml of 100:1:1 ratio (v/v/v) of 2% sodium carbonate, 1% copper (II) sulfate and 2% potassium sodium tartrate. The reaction mixture was mixed well before being incubated at room temperature in the dark for 30 min. Then, 0.2 ml of Folin's reagent that was diluted with distilled water at a ratio of 1:1 (v/v) was added to the reaction mixture. The absorbance was measured at 750 nm. The protein concentration in the mixture sample was determined by comparing the assay response of a sample to that of the BSA standard.
2.5 Determination of histological alteration in liver and distal intestine
Hepatopancreas and intraperitoneal fat from four fish per tank were quickly weighed for hepatosomatic index (HSI, %) and intraperitoneal fat (IPF, %), and then 3–4 mm of hepatopancreatic tissues were collected and immediately fixed in 10% buffered formalin for histological analysis; the rest of hepatopancreatic tissue was stored at −30°C for digestive enzyme analysis.
Different digestive tract segments were separated (e.g. pyloric and anterior intestine, and distal intestine). The tissue of the distal intestine was immediately fixed in 10% buffered formalin for histological analysis. The pyloric caeca and anterior intestine were pooled and stored at −30°C for digestive enzyme analysis.
After the hepatopancreas and distal intestine of Asian sea bass had been fixed in 10% buffered formalin for 24 h at room temperature, they were transferred to a new solution of 10% buffered formalin until processed by standard histological procedures (Clark, 1978). The blocks of the designed sample were dehydrated through a standard ethanol series to 100%, embedded in paraffin wax and sectioned at 4 μm intervals for staining with Haematoxylin–Eosin (H & E) stain. The sections were examined under upright microscopy (Nikon Ci-L, Japan), digital microscopy camera and the imaging software NIS-Elements.
2.6 Determination of serum biochemical and immunological indices
Blood serum was centrifuged (5000 g, 10 min, 4°C), and supernatants were removed and kept at −20°C for analysis of biochemical parameters and immune response.
Serum biochemical indices such as cholesterol, triglyceride, albumin and aspartate aminotransferase were measured using a Chemistry analyser (Mindray BS-200, Shenzhen, China). Serum lysozyme activity was evaluated using a commercially available Lysozyme Activity Assay Kit (ab211113, Abcam, Japan). Superoxide dismutase activity (SOD) in serum was measured using a commercially available Superoxide Dismutase Activity Assay Kit (ab65354, Abcam, Japan). The measurement procedures of lysozyme activity and SOD were conducted following the instruction of the manufacturer. Briefly, the reaction mixture, consisting of 0.2 ml of a water-solution tetratzolium salt (WST-1), 0.02 ml of enzyme working solution and 0.02 ml of serum sample solution, was incubated at 37°C for 20 min. SOD catalyses the dismutation of the superoxide anion into hydrogen peroxide and molecular oxygen resulting in a decrease in WST-1 reduction. This inhibition activity of SOD was measured by the colorimetric method at OD 450 nm (SpectraMax M5, multi-Mode Microplate Reader, Molecular Devices, California, USA). Briefly, the reaction mixture consisted of 0.04 ml of serum sample and 0.01 ml of the prepared substrate, which contained 0.004 ml of lysozyme substrate and 0.06 ml of assay buffer. The reaction mixture was mixed well before being incubated at 37°C for 60 min in the dark. The reaction was stopped by adding 0.05 ml of stop buffer. The fluorescence was measured at 445 nm using a microplate reader. (SpectraMax M5, multi-Mode Microplate Reader, Molecular Devices).
2.7 Proximate analysis
The chemical compositions such as moisture, ash, lipid and protein contents and gross energy were analysed in fish meal, soybean meal, corn protein concentrate, tuna hydrolysate, poultry by-product, shrimp head meal, experimental diets, initial carcass and final carcass, according to the Association of Official Analytical Chemists method (AOAC, 1995). The sample was dried at 60°C for 14 h using the hot air oven and then placed in an electric muffle furnace of 550°C for 5 h. The sample was weighed after cooling in a desiccator for 30 min. Dry matter and ash were calculated. For crude protein, Kjeldahl digestion and distillation units were used for crude protein analysis and the percentage of total nitrogen was determined based on a dry matter basis (%N × 6.25). Crude lipid was extracted from samples using petroleum ether by the Soxhlet apparatus.
2.8 Growth parameter and feed utilization
Body weight gain (%) = 100 × (final body weight–initial body weight/initial body weight).
Average daily gain (ADG, g day−1) = (average final body weight − average initial body weight)/number of feeding days.
Daily feed intake (%) = 100 × total dry feed intake/average body weight at initial and final/feeding days.
Feed conversion ratio (FCR) = dry feed intake/wet body weight gain.
Protein efficiency ratio (PER, %) = body weight gain/protein intake.
Nitrogen retention (%) = 100 × nitrogen gain/nitrogen intake.
Lipid retention (%) = 100 × lipid gain/lipid intake.
Intraperitoneal fat (IPF, %) = 100 × Intraperitoneal fat weight/body weight.
Hepatosomatic index (HSI, %) = 100 × liver weight/body weight.
Survival rate (%) = 100 × (initial fish number–final fish number/initial fish number).
2.9 Statistical analysis
A one-way ANOVA was used to test the mean differences in body weight gain, ADG, daily feed intake, FCR, PER, IPF, HSI, nitrogen retention, lipid retention and survival rate between dietary treatments at p < 0.05. A Tukey's multiple test was used afterwards to determine which mean differs from the rest. The test was performed using graphpad prism version 7.00 for Mac Os X, GraphPad Software.
3 RESULTS
3.1 Growth performance and feed utilization
In the present study, 2.5% tuna hydrolysate was chosen due to our preliminary trial. Asian sea bass was fed a soybean meal-based diet supplemented with graded levels of 0%, 2.5% and 5.0% of fish tuna hydrolysate for 2 weeks. The result showed that the feed intake of Asian sea bass fed a soybean meal-based diet supplemented with 2.5% tuna hydrolysate was significantly higher than that of fish fed with the control diet without tuna hydrolysate. However, there was no significant difference in feed intake between fish fed with 2.5% and 5.0% of tuna hydrolysate (data not shown). The parameters of growth performance and feed utilization of Asian sea bass fed the experimental diets for 8 weeks are shown in Table 3. The final body weight, body weight gain, average daily gain and daily feed intake of fish-fed LFM diet were significantly lower than those of fish-fed HFM diet and LFM + TH diet (p < 0.05); however, no significant differences in the final body weight, body weight gain, average daily gain and daily feed intake were detected between fish-fed HFM diet and LFM + TH diet (p > 0.05).
| Parameters | Dietary treatment | ||
|---|---|---|---|
| HFM | LFM | LFM + TH | |
| Initial body weight | 34.9 ± 4.9 | 39.0 ± 1.4 | 36.8 ± 2.3 |
| Final body weight | 97.3 ± 7.9a | 75.8 ± 4.8b | 90.8 ± 7.1a |
| Body weight gain (%) | 180.8 ± 19.9a | 104.6 ± 5.9b | 146.2 ± 4.4a |
| Average daily gain (g day−1) | 1.12 ± 0.06a | 0.69 ± 0.06b | 0.96 ± 0.09a |
| Daily feed intake (% body weight) | 2.90 ± 0.25a | 2.55 ± 0.10b | 3.00 ± 0.38a |
| Protein efficiency ratio (%) | 1.61 ± 0.08a | 1.06 ± 0.10c | 1.32 ± 0.12b |
| Nitrogen retention (%) | 33.7 ± 2.52a | 25.7 ± 0.58b | 29.0 ± 1.00b |
| Lipid retention (%) | 46.3 ± 2.64a | 19.0 ± 12.8b | 35.2 ± 8.67ab |
| Hepatosomatic index (%) | 3.21 ± 0.85a | 0.44 ± 0.27b | 2.02 ± 0.71ab |
| Survival rate (%) | 96.7 ± 5.77 | 93.3 ± 11.5 | 93.3 ± 5.77 |
- Note: Values are means and standard deviation of three replicate tanks. Values with different superscript letter with different superscripts are significantly different (p < 0.05).
- Abbreviations: HFM, high fish meal diet; LFM, low fish meal diet; LFM + TH, low fish meal diet supplemented with tuna hydrolysate.
The protein efficiency ratio of fish-fed LFM + TH diet was significantly higher than those fish-fed LFM diet (p < 0.05) and significantly lower than that of fish-fed HFM diet (p < 0.05). The nitrogen retention values of fish fed both LFM diet and LFM + TH diet were significantly lower than the fish fed with HFM diet (p < 0.05). The lowest value of lipid retention was significant in the LFM diet-fed fish (p < 0.05), while the highest value of lipid retention was significant in the HFM diet-fed fish (p < 0.05). No significant differences in lipid retention were observed between neither fish-fed LFM + TH diet and LFM diet (p > 0.05) nor fish fed with LFM + TH diet and HFM diet (p > 0.05). The hepatosomatic index of HFM diet-fed fish was significantly higher than that of LFM diet-fed fish (p < 0.05), but the hepatosomatic index of LFM + TH diet-fed fish was not statistically different from neither that of HFM diet-fed fish nor LFM diet-fed fish (p > 0.05). There was no significant difference in the survival rate among the treatments (p > 0.05).
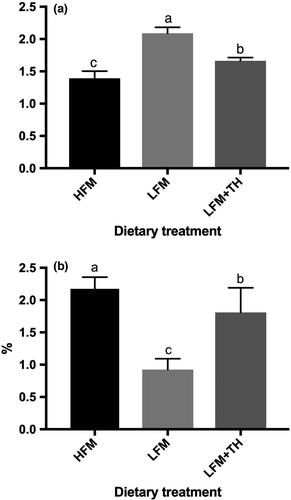
A significantly high value of feed conversion ratio (FCR) was found in fish-fed LFM diet (p < 0.05), whereas the FCR of fish-fed LFM + TH diet was statistically lower than that of fish-fed LFM diet (p < 0.05) and higher than that of fish-fed HFM diet (p < 0.05) (Figure 1a). Intraperitoneal fat (IPF) of fish-fed LFM + TH diet was not significantly different from that of fish-fed HFM diet (p > 0.05) that was significantly higher than that of fish-fed LFM diet (p < 0.05) (Figure 1b).
3.2 Chemical composition
Chemical composition of whole fish of Asian sea bass fed the experimental diets for 8 weeks is presented in Table 4. The crude protein in the whole body of fish-fed LFM diet and that of fish-fed LFM + TH diet tended to be higher than that of fish-fed HFM diet. On the other hand, the crude lipid of fish-fed HFM diet seemed to be higher than those of fish-fed LFM diet and fish-fed LFM + TH diet. The highest value of nitrogen-free extract found in fish-fed LFM diet. Nevertheless, no significance was detected in the aforementioned parameters among the dietary treatments (p > 0.05).
| Parameters | Dietary treatment | ||
|---|---|---|---|
| HFM | LFM | LFM + TH | |
| Proximate composition (%) | |||
| Dry matter | 32.5 ± 0.3 | 32.8 ± 0.5 | 32.7 ± 1.0 |
| Crude proteina | 59.0 ± 1.2 | 61.9 ± 1.9 | 60.0 ± 1.7 |
| Crude lipida | 20.6 ± 0.2 | 14.7 ± 4.3 | 17.8 ± 2.0 |
| Asha | 16.1 ± 0.9 | 18.2 ± 1.8 | 16.8 ± 0.4 |
| Nitrogen-free extractb | 3.3 ± 0.3 | 5.1 ± 1.3 | 4.3 ± 0.2 |
- Note: Proximate composition of initial carcass (dry basis): dry matter: 29.3%; crude protein: 55.1%, crude lipid: 18.5%; ash: 20%; and nitrogen-free extract: 4.04%. Values are means and standard deviation of three replicate tanks. Values with different superscript letter with different superscripts are significantly different (p < 0.05).
- Abbreviations: HFM, high fish meal diet; LFM, low fish meal diet; LFM + TH, low fish meal diet supplemented with tuna hydrolysate.
- a The proximate analysis is in the dry form for crude protein, crude lipid and ash.
- b Nitrogen-free extract (NFE) = % of dry matter − (% of crude protein + % of crude lipid + % of crude ash).
3.3 Serum biochemical and immune parameters
Serum biochemical parameters such as cholesterol, triglyceride and aspartate aminotransferase were not significantly different among the dietary treatments (p > 0.05) (Table 5). Serum albumin of the fish-fed LFM diet was significantly lower than that of the fish-fed HFM diet and the fish-fed LFM + TH diet (p < 0.05).
| Parameters | Dietary treatment | ||
|---|---|---|---|
| HFM | LFM | LFM + TH | |
| Serum biochemical parameters | |||
| Cholesterol (mg dl−1) | 192.0 ± 0.53 | 172.9 ± 10.5 | 169.4 ± 14.3 |
| Triglyceride (mg dl−1) | 212.4 ± 72.7 | 172.9 ± 23.4 | 287.4 ± 44.4 |
| Albumin (g dl−1) | 1.81 ± 0.05a | 1.58 ± 0.08b | 1.87 ± 0.06a |
| Aspartate aminotransferase (U L−1) | 87.2 ± 49.9 | 59.9 ± 26.3 | 95.9 ± 23.1 |
| Immune parameter | |||
| Serum lysozyme activity (mg ml−1) | 131.6 ± 25.2 | 126.5 ± 19.3 | 112.0 ± 12.8 |
| Superoxide dismutase (% inhibition) | 281.0 ± 122.0 | 181.0 ± 128.0 | 110.0 ± 72.9 |
- Note: Values are means and standard deviation of three replicate tanks. Values with different superscript letter with different superscripts are significantly different (p < 0.05).
- Abbreviations: HFM, high fish meal diet; LFM, low fish meal diet; LFM + TH, low fish meal diet supplemented with tuna hydrolysate.
No significant differences in serum lysozyme activity and superoxide dismutase were found among the dietary treatments (p > 0.05).
3.4 Trypsin and lipase activities in hepatopancreatic and anterior intestine tissues
The trypsin and lipase activities in hepatopancreatic and anterior intestine tissues of Asian sea bass were not significantly different among the treatments (p > 0.05; Table 6).
| Digestive enzyme activity (h−1 mg protein−1) | Dietary treatment | ||
|---|---|---|---|
| HFM | LFM | LFM + TH | |
| Hepatopancreatic tissue | |||
| Lipase activity | 304 ± 15 | 306 ± 15 | 329 ± 75 |
| Trypsin activity | 770 ± 601 | 2218 ± 413 | 1982 ± 1510 |
| Anterior intestine tissue | |||
| Lipase activity | 221 ± 15 | 236 ± 41 | 213 ± 7 |
| Trypsin activity | 2607 ± 912 | 2174 ± 1046 | 2013 ± 569 |
- Note: Values are means and standard deviation of three replicate tanks.
- Abbreviations: HFM, high fish meal diet; LFM, low fish meal diet; LFM + TH, low fish meal diet supplemented with tuna hydrolysate.
3.5 Morphology of liver and distal intestine
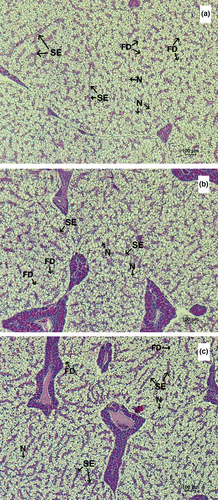
The morphology of the liver and distal intestine of Asian sea bass fed the experimental diets for 8 weeks is exhibited in Figures 2 and 3. Fish from all treatment groups showed the normal structure of the liver parenchyma showing the cords of the hepatocytes separated by sinusoids containing the erythrocytes. The hepatocytes were large with typically center nuclei showing prominent nucleoli. However, the sinusoids of fish fed with the HFM diet (Figure 2a) seemed to contain a smaller number of the erythrocytes than those fed with the LFM diet (Figure 2b) and LFM + TH diet (Figure 2c).
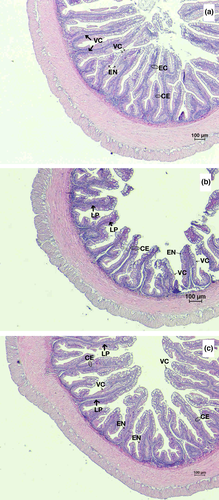
Fish fed with the HFM diet displayed the normal structure of the distal intestine composed of the elongated villi extending in the organ lumen. The small absorptive vacuoles are distributed in the columnar epithelium or the enterocytes. The villous core was filled with connected tissue of lamina propria containing blood and lymph capillaries (Figure 3a). Fish-fed LFM diet had a shorter length of the distal intestine villi (Figure 3b), meanwhile the distal intestine morphology of fish-fed LFM + TH diet showed a similar structure to that of fish-fed HFM diet (Figure 3c).
4 DISCUSSION
4.1 Growth performance and feed utilization
Fish meal substitution with soybean products in formulated feeds has been intensively researched in carnivorous fish species such as yellowtail, rainbow trout, Atlantic salmon, cobia, red sea bream, Asian sea bass and largemouth bass to achieve the sustainable aquaculture (Aoki et al., 1997; Boonyaratpalin et al., 1998; Krogdahl et al., 2015; Liang et al., 2017; Nguyen et al., 2015; Refstie et al., 2001; Salze et al., 2010; Vielma et al., 2002; Yamamoto et al., 2012). In Asian sea bass, few studies have been conducted on the replacement of fish meal with soybean meal in feeds for different sizes of fish. Tantikitti et al. (2005) found that 10% of fish meal protein could be replaced with 76 g SBM kg diet−1 in the formulated diet for 0.9 g juvenile Asian sea bass without reducing the growth rate. Further, the finding of Boonyaratpalin et al. (1998) revealed that 37.5% of fish meal protein could be replaced with 210 g solvent-extracted SBM kg diet−1 in the formulated diet for 1.3 g juvenile Asian sea bass without significantly impacting growth performance in a negative way. Ma et al. (2018) attempted to increase the replacement level of fish meal protein with commercial soybean meal conducted in bigger Asian sea bass (initial body weight = 25–27 g), the substitution of 50% fish meal protein with 300 g SBM kg diet−1 in the formulated diet significantly suppressed growth rate of fish. Those results were consistent with the present study that the replacement levels at 55.5% of fish meal protein with 450 g SBM kg diet−1 in the LFM diet significantly decreased the growth rate of juvenile Asian sea bass (initial body weight = 36 g) in the 8-week feeding trial. It is clearly noted that over 37.5% of fish meal protein substituted with soybean meal and/or high inclusion levels of 300 g SBM kg−1 diet can impair growth performance resulting from poor feed intake of juvenile Asian sea bass (Boonyaratpalin et al., 1998; Ma et al., 2018; Tantikitti et al., 2005).
Low feed intake was commonly recognized as a characteristic of fish fed with soybean-based diets, publishable reported in numeral carnivore fishes including rainbow trout, chinook salmon (Bureau et al., 1998), Asian sea bass (Ma et al., 2018; Tantikitti et al., 2005), spotted rose snapper (Lutjanus guttatus) (Silva-Carrillo et al., 2012), silvery-black porgy (Sparidentex hasta) (Yaghoubi et al., 2016) and red sea bream (Tola et al., 2019a, 2019b). Decreased feeding rates of Asian sea bass fed with the LFM diet were possibly attributed to antinutritional factors in soybean meal. It is well-known that soybean meal contains antinutritional factors, such as saponin, lectin, isoflavone and tannins (Francis et al., 2001; Krogdahl & Bakke, 2015). Though how these antinutritional factors suppress feed intake in soybean meal-based diet-fed fish is still obscure. Bureau et al. (1998) suggested that soyasaponins in soybean meal responded to suppressed-feed intakes of both chinook salmon and rainbow trout fed with a soybean and soy protein concentrate-based diet containing a purified alcohol extract from soybean meal. Another possible reason for low feed intake observed in Asian sea bass fed with an LFM diet could be associated with a lack of attractiveness in the plant protein-rich diets, which contain imbalanced amino acids (Kader et al., 2010; Rhodes et al., 2017). The small soluble molecules, namely amino acids (e.g. taurine, glycine, arginine, glutamic acid, alanine, etc.) betaine, nucleotides and organic acids, which are considered as the feeding stimulants for fishes, are commonly abundant in fish meal (Aksnes, Hope, Høstmark, & Albrektsen, 2006a; Chotikachinda et al., 2013; Harada, 1987; Kader et al., 2010).
The improvement of the feed palatability of soybean meal-based diets by supplementing the tuna hydrolysate has been conducted to successfully overcome certain major limitations with regard to high inclusion levels of soybean meal in fish feeds (Hevrøy et al., 2005; Khosravi, Bui, et al., 2015; Refstie et al., 2004; Tola et al., 2019b). As tuna hydrolysate consists of high concentrations of both free amino acids and total amino acids as flavour and/or taste enhancers including glutamic acid, aspartic and glycine, which have the feeding stimulant property for fishes (Chotikachinda et al., 2013; Tola et al., 2019b). Chotikachinda et al. (2013) succeeded to process tuna hydrolysate made from tuna fish visceral byproduct using enzymatic-alcalase hydrolysis. The authors suggested that dietary supplementation of 3% tuna hydrolysate was sufficient to enhance the palatability of poultry by-product-based diets for juvenile Asian sea bass (initial body weight = 7–8 g). Meanwhile, the present study showed that as low as 2.5% tuna hydrolysate supplementation was adequate to improve the growth rates of Asian sea bass fed with a low fish meal soybean meal-based diet through enhancing feed intake. This lower concentration of tuna hydrolysate supplementation recommended could be due to the different sizes of Asian sea bass used in the present study (initial body weight = 36 g) and in the study of Chotikachinda et al. (2013) (initial body weight = 7–8 g).
The plant protein-rich diets commonly suppress the feed intake of fish resulting in poor growth performance relating to feed deprivation or caloric deficit that adversely affects the biological performance and growth promotion of fish (Ma et al., 2018; Tola et al., 2019a). In the present study, the improved growth rate of Asian sea bass fed LFM + TH diet was directly due to increased feed intake. In comparison to fish fed with the LFM diet, higher values of feed intake appeared in fish fed with the LFM + TH diet leading to the ingestion of sufficient energy and nutrients to meet the requirement for the normal growth of fish (Khosravi, Bui, et al., 2015). The result in the present study agreed with the studies of Kader et al. (2010) and Khosravi, Rahimnejad, et al. (2015), reporting that supplementation of feeding stimulants including tuna hydrolysate, krill meal, fish soluble and squid meal in a low fish meal soy protein concentrate-based diets was able to cover the depleted performance of red sea bream. According to the feeding behaviour observation in Asian sea bass fed the LFM + TH diet, fish grabbed and ingested pellets faster than fish fed with the LFM diet. This feeding behaviour could be explained by the liquid form of tuna hydrolysate used for coating palatability and attractiveness to Asian sea bass. Tuna hydrolysate contains a number of short-chain peptides and free amino acids, which are water-soluble components, and released in the water (Refstie et al., 2004).
In the present study, protein efficiency ratio, lipid retention and nitrogen retention of Asian sea bass fed the LFM + TH diet were significantly greater than that of fish fed with the LFM diet, whereas the feed conversion ratio of Asian sea bass fed LFM diet was significantly higher than that of fish-fed LFM + TH diet. Notably, greater values of nutrient retention and feed efficiency were paralleled with the growth values (e.g. average daily growth and body weight gain) observed in Asian sea bass fed LFM + TH diet, indicating that the growth rate of Asian sea bass fed LFM + TH diet in the present study was attributed to improved feed utilization induced by the dietary supplementation of tuna hydrolysate. The result in the present studies was consistent with previous studies on red sea bream (Kader et al., 2010; Khosravi, Bui, et al., 2015), Atlantic cod (Aksnes, Hope, Høstmark, & Albrektsen, 2006), rainbow trout (Aksnes, Hope, Jönsson, et al., 2006) and yellowtail (Kofuji et al., 2006b; Satoh, 2003; Takii, 1994). These studies revealed that the dietary supplementation of fish protein hydrolysate can ameliorate feed utilization of fish fed the plant protein-rich diets. Khosravi, Bui, et al. (2015) reported that the growth and feed efficiency of red sea bream fed a low fish meal soy protein concentrate-based diets were significantly enhanced by the dietary supplementation of tuna hydrolysate. Similarly, the other studies revealed that the dietary supplementation of fish protein hydrolysate can ameliorate feed utilization of Atlantic cod (Aksnes, Hope, Høstmark, & Albrektsen, 2006), rainbow trout (Aksnes, Hope, Jönsson, et al., 2006) and yellowtail (Kofuji et al., 2006b; Satoh, 2003; Takii et al., 1994), that fed the plant protein-rich diets.
Though it was not clear how the dietary supplementation of tuna hydrolysate improved the feed utilization of Asian sea bass fed with the LFM diet in the present study. It has been reported (Aksnes, Hope, Høstmark, & Albrektsen, 2006; Chalamaiah et al., 2012; Harnedy & FitzGerald, 2012) that certain bioactive peptides derived from the enzymatic hydrolysis processes of fish by-products have a potential for promoting the biological performance of fish. Therefore, some bioactive peptides in tuna hydrolysate could be associated with promoting the growth of Asian sea bass fed with the LFM + TH diet in the present study. Besides, Kofuji et al. (2006a) demonstrated that the dietary supplementations of both synthetic and natural feeding stimulants significantly increased the activities of digestive enzymes, including pepsin, trypsin and chymotrypsin, in the digestive contents of yellowtail fed with the low-protein high-lipid diet during the winter season. These elevated activities of the digestive enzymes in digestive contents were paralleled with increasing values of apparent protein digestibility and growth performance of yellowtail fed with the diet supplemented with feeding stimulants, these results suggest that the dietary supplementations of feeding stimulants promoted the growth via improving protein digestion (Kofuji et al., 2006a). Similar phenomenon was observed in both young and adult yellowtail, revealed by Takii (1994) and Satoh (2003), respectively.
Although feed intake of Asian sea bass that was fed the LFM + TH diet became comparable to those fed the HFM diet, growth rate, feed conversion ratio, protein efficiency ratio, nitrogen retention and lipid retention of fish fed with LFM + TH diet were still inferior to those of fish-fed HFM diet in the present study. This indicates that low growth observed in fish fed with LFM + TH diet was attributed to degraded feed utilization. It has been reported that high levels of fish meal replacement with soybean meal in fish feeds cause adverse impacts on growth performance, feed utilization, digestion of lipid and protein, immune response and health condition of fishes (Krogdahl et al., 2010; Nguyen et al., 2015; Tola et al., 2019a; Van den Ingh et al., 1991; Yamamoto et al., 2008; Zhang et al., 2018). The presence of certain antinutritional factors, such as protease inhibitors, phytase, saponins, lectins and oligosaccharides, in soybean meal was responsible for the poor growth, feed utilization and health of fishes (Francis et al., 2001; Krogdahl et al., 2015). As the results of poor protein efficiency ratio, nitrogen retention and lipid retention of Asian sea bass (LFM + TH fed), compared to fish fed with the HFM diet, the specific reasons for low growth could be due to poor protein and lipid utilizations. In comparison to fish-fed HFM diet in the present study, low nitrogen and lipid utilization of Asian sea bass fed a soybean meal-based diet could not be recovered by the dietary supplementation of 2.5% tuna hydrolysate.
4.2 Chemical composition
Feeding the dietary supplementation of tuna hydrolysate did not significantly affect crude protein, lipid, moisture and ash of Asian sea bass fed the soybean meal-based diets in the present study. Our results were consistent with previous studies (Tola et al., 2019b), crude protein, lipid, moisture and ash in carcasses of red sea bream were not significantly increased by feeding the dietary supplementation of 1.8% tuna hydrolysate. Similarly, Khosravi, Bui, et al. (2015) did not find any significant difference in chemical composition in the carcasses of red sea bream or olive flounder fed with the diets supplemented with 2% of tuna hydrolysate.
The liver is an energy storage for animals. Collins and Anderson (1995) reported that the relative size of the liver of golden perch (Macquaria ambigua) was significantly decreased 30 days after the onset of the feeding restriction period. HSI and IPF are commonly used as an indicator for assessing the health and nutritional status of fish. The low levels of HSI and IPF indicate a negative energy balance in fish (Picha et al., 2006). According to the previous studies, the dietary supplementation of tuna hydrolysate did not statistically increase the hepatosomatic index and visceral fat somatic index of red sea bream fed with the soy protein concentrate-based diet (Tola et al., 2019b) and Asian sea bass fed with the fish meal-based diet (Siddik, Howieson, Ilham, & Fotedar, 2018). However, elevated values of HSI and IPF found in Asian sea bass fed the LFM + TH diet in the present study could be due to sufficient caloric intake resulting from increased feed intake.
4.3 Serum biochemical and immune parameters
Biochemical indices are usually measured to evaluate the nutritional status and health condition of fish (Adams et al., 1993). The serum albumin is commonly used as a marker of the nutrition status, the low value of serum albumin indicates malnutrition or starvation resulting from a mismatch of the nutrient requirement (Keller, 2019). In the present study, serum albumin values of Asian sea bass fed with the HFM diet and LFM + TH diet were significantly higher than that of fish fed with the LFM diet. This result suggested that the dietary supplementation of tuna hydrolysate could improve serum albumin of fish fed a soybean meal-based diet. The values of serum cholesterol and triglyceride were reported in fish-fed soybean meal-based diets due to poor lipid utilization (Nguyen et al., 2015). Nevertheless, total cholesterol, triglyceride and aspartate aminotransferase in the serum of Asian sea bass were not significantly affected by the dietary supplementation of tuna hydrolysate in the present study. These results were in accordance with Khosravi, Bui, et al. (2015) that total cholesterol and triglyceride of red sea bream and Japanese flounder were not significantly affected by the dietary supplementation of tuna hydrolysate. Likewise, Siddik, Howieson, Ilham, and Fotedar (2018) revealed aspartate aminotransferase in serum of Asian sea bass fed poultry by-product-based diets were not significantly affected by the dietary supplementation of tuna hydrolysate. In contrast, Siddik et al. (2021) found that aspartate aminotransferase levels in Asian sea bass fed the diet containing the high levels of lupin meal replaced 75% of fish meal protein without the dietary supplementation of tuna hydrolysate was significantly higher than that of fish fed with the dietary supplementation of tuna hydrolysate. As an increasing value of aspartate aminotransferase in serum points to liver dysfunction in fish, the supplementation of tuna hydrolysate and the high inclusion of soybean meal in the diet do not have negative impacts on the health of Asian sea bass in the present study.
It has been demonstrated that fish protein hydrolysate consists of low molecular weight bioactive peptides, which can stimulate the innate immune response of European seabass, Japanese seabass, Atlantic cod (Gadus morhua), red seabream and Asian sea bass (Gildberg & Mikkelsen, 1998; Khosravi, Bui, et al., 2015a; Kotzamanis et al., 2007; Liang et al., 2006; Siddik, Howieson, Ilham, & Fotedar, 2018a; Siddik, Howieson, Partridge, et al., 2018b). Khosravi, Bui, et al. (2015a) reported that red sea bream fed soy protein concentrate-based diet supplemented with tilapia hydrolysate at 2.88% had significantly greater values of superoxide dismutase (SOD) and lysozyme activity than those fed the diet without tilapia hydrolysate supplementation. Enhanced lysozyme activity associated with dietary fish protein hydrolysate has been reported in Japanese seabass fed with the fish meal-based diet that is supplemented with 15% of fish protein hydrolysate (Liang et al., 2006) and Asian sea bass fed poultry byproduct-based diet or fish meal-based diet supplemented with 5% tuna hydrolysate (Siddik et al., 2019). In contrast, increases in activities of SOD and lysozyme activity of Asian sea bass were not significantly noticed in the present study, which was consistent with the study of Zheng et al. (2013). The author reported that the SOD of Turbot (Scophthalmus maximus) fed with soybean meal-based diets was not significantly affected by the dietary supplementation of 3.7% fish protein hydrolysate. Different results between the present and previous studies (Khosravi, Bui, et al., 2015; Liang et al., 2006; Siddik et al., 2019) could be due to the different supplementation levels of a fish protein hydrolysate, rearing conditions and fish species.
4.4 Trypsin and lipase activities in hepatopancreatic and anterior intestine tissues
It has been demonstrated that high substitution levels of fish meal with soybean meal in diets decrease the activities of hepatopancreatic digestive enzymes namely protease, trypsin and lipase. This was reported in several fish species such as red sea bream (Matsunari, et al., 2018; Murashita et al., 2015), Atlantic cod (Lemieux et al., 1999), Atlantic salmon (Krogdahl et al., 2003), hybrid tilapia (Oreochromis niloticus × O. mossambicus) (Lin & Luo, 2011) and Japanese seabass (Zhang et al., 2018). Trypsin inhibitors and saponins in soybean meal might be responsible for the reduction of digestive enzyme activities in fish fed with the soybean meal-based diets (Hedrera et al., 2013; Huisman & Tolman, 1992; Krogdahl et al., 2003). However, feeding of LFM diets containing 40% soybean meal did not affect the trypsin or lipase activities in hepatopancreatic and anterior intestine tissues of Asian sea bass in the present study.
4.5 Morphology of liver and distal intestine
No evidence of histopathological changes in Asian sea bass livers related to the feeding of a soybean meal-based diet has been previously reported (Boonyaratpalin et al., 1998; Ma et al., 2018; Tantikitti et al., 2005). In the present study, though the liver of Asian sea bass fed with the LFM and LFM + TH diets displayed slightly increased numbers of erythrocytes in sinusoids compared to that of fish fed with the HFM diet, this morphological change might not be associated with the feeding of soybean meal-based diets.
The soybean meal-induced enteritis and severity of morphological alterations associated with diets containing high inclusion levels of soybean meal have been observed in the distal intestines of Atlantic salmon, rainbow trout and Japanese seabass. These morphological alterations include shortened mucosal folds, decreased number of absorptive vacuoles, swollen lamina propria and mucosa, decreased height of microvilli and deceased villus and muscular thickness (Krogdahl et al., 2010; Van den Ingh et al., 1991; Yamamoto et al., 2008; Zhang et al., 2018). Furthermore, Zhang et al. (2018) revealed that feeding soybean meal-induced proinflammatory genes in the intestine of Japanese seabass. Our finding was consistent with the study of Zhang et al. (2018) that Asian sea bass fed with the LFM diet showed shortened villus height in the distal intestine. These destructed intestine microvilli could lead to low absorption of nutrients resulting in poor growth performance of Asian sea bass fed with the LFM diet.
There were positive results of villus height in the distal intestines in Asian sea bass fed with the LFM + TH diet. Siddik, Howieson, Ilham, and Fotedar (2018a) and Siddik et al. (2019) demonstrated that the dietary supplementation of tuna hydrolysate in fish meal and poultry by-product-based diets significantly increased the microvilli height in the distal intestines of Asian sea bass. The improved morphology of distal intestines in Asian sea bass fed with the LFM + TH diet could be attributed to the dietary supplementation of tuna hydrolysate.
5 CONCLUSION
The dietary supplementation of 2.5% tuna hydrolysate could increase inclusion levels of soybean meal up to 40%, which is able to replace fish meal protein for 55% in a low fish meal soybean meal-based diet for Asian sea bass without any negative impacts on feed intake and growth.
Supplementation of the palatability enhancers in diets is an effective approach to develop diets containing high inclusion of soybean meal to maintain feed attractiveness and induce adequate feed intake of fish. This indicates that tuna hydrolysate is a suitable candidate as a palatability enhancer in a low fish meal soybean meal-based diet for Asian sea bass.
AUTHOR CONTRIBUTIONS
Tola S. and Yuangsoi B. were involved in conceptualization; Tola S., Yuangsoi B., and Seel-audom M. designed the experiments; Tola S., Sommit N., Khamtavee P., and Boonmee T. were involved in data analysis; Tola S. wrote the original draft; Tola S., Yuangsoi B., Waiho K., Seel-audom M., and Munpholsri N. reviewed and edited the manuscript. All authors approved the final version of the manuscript for submission.
ACKNOWLEDGEMENTS
Sincere thanks to Dr. Vincent Fournier, Dr. Mikael Herault and Dr. Fabio Soller for their guidance regarding the feed formulation and experimental design. This research was partially supported by Chiang Mai University.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVAL
All experimental procedures were approved by the National Research Council of Thailand (approval no. U1-08621-2562). The study was conducted in compliance with the guideline of the Animal Use Protocol approved by the National Research Council of Thailand, and all methods were in accordance with relevant guidelines and regulations.
Open Research
DATA AVAILABILITY STATEMENT
Data Availability




