The observed oogenesis impairment in greater amberjack Seriola dumerili (Risso, 1810) reared in captivity is not related to an insufficient liver transcription or oocyte uptake of vitellogenin
Abstract
The greater amberjack Seriola dumerili is an excellent candidate for the Mediterranean aquaculture, due to its large body size and high growth rate, as well as its high flesh quality and commercial value worldwide. For its successful incorporation in the aquaculture industry, an in-depth understanding of the reproductive function of the species under rearing conditions is necessary, since completion of oogenesis in captivity is currently a bottleneck for the commercial production of the species. Liver and ovary samples from wild and captive-reared greater amberjack females were collected at three different phases of the reproductive cycle: early gametogenesis (EARLY, late April-early May), advanced gametogenesis (ADVANCED, late May-early June) and spawning (SPAWNING, late June-July). The cDNAs of three vitellogenins (VtgA, VtgB and VtgC) were partially sequenced and a qRT-PCR for their expression was used to compare ovarian maturity stage and liver vitellogenin transcript levels between wild and captive-reared individuals. An extensive atresia of late vitellogenic follicles, which prevented any further oocyte development and spawning was observed in captive-reared individuals during the ADVANCED phase. The expression levels of the three vitellogenins, as well as the amount of yolk globules in vitellogenic oocytes, did not differ significantly between captive-reared and wild females, indicating that the observed oogenesis impairment in greater amberjack reared in captivity was not related to an insufficient liver synthesis or a reduced oocyte uptake of vitellogenin.
1 INTRODUCTION
The greater amberjack Seriola dumerili (Risso, 1810) is an excellent candidate for aquaculture production due to its large body size and fast growth rate, the high quality of its flesh (Nakada, 2000; Sicuro & Luzzana, 2016) and the high consumer acceptability worldwide (Lovatelli & Holthus, 2008; Sicuro & Luzzana, 2016). The greater amberjack is a large migratory pelagic fish, with a cosmopolitan distribution in tropical and temperate waters (Bauchot, 1987; Cervigón, 1993; Smith, 1997), and females exhibit a group-synchronous ovarian development with a multiple spawning pattern (Díaz, García & Agulleiro, 1997; Marino, Mandich, Massari, Andaloro & Porrello, 1995).
Among the major bottlenecks for the incorporation of a new species in the aquaculture industry, reproductive dysfunctions are very common and limit the production of viable eggs. Reproductive dysfunctions have been also documented in captive-reared greater amberjack confined in sea cages or tanks (Díaz et al., 1997; Micale, Maricchiolo & Genovese, 1999; Mylonas, Papandroulakis, Smboukis, Papadaki & Divanach, 2004). A scarce development of the ovaries during the reproductive period and lower sex steroid plasma levels were observed in greater amberjack reared in sea cages in the Aegean Sea (Salamina Island, Greece), compared to wild populations (Zupa et al., 2017). This severe impairment of oogenesis resulted in an extensive atresia of late vitellogenic oocytes during the advanced vitellogenesis and spawning phases, and was associated with differences in total polar lipid and essential fatty acid content in the gonads (Zupa et al., 2017).
In teleost fish, as well as in other oviparous animals, the process of vitellogenesis consists of an ordered sequence of events. These include the liver synthesis of different vitellogenins (egg yolk precursor proteins, Vtgs) under oestrogen stimulation (Ng & Idler, 1983; Sawaguchi et al., 2006; Susca, Corriero, Bridges & De Metrio, 2001), their release in the bloodstream as homodimer lipoprotein complexes (Finn, 2007), their uptake by developing oocytes through endocytosis mediated by receptors belonging to the low density lipoprotein receptor (LDLR) family (Mizuta et al., 2013; Stifani, Barber, Nimpf & Schneider, 1990), and their final cleavage into egg yolk proteins that are accumulated in yolk globules or platelets (Wallace & Selman, 1990). Yolk content is an important determinant of egg and larval quality in fish, as it represents the major nutrient for the developing embryo/larva during the first days of endogenous feeding (Bobe & Labbé, 2009; Brooks, Tyler & Sumpter, 1997). Multiple vitellogenins have been described in a number of fish species including the white perch Morone americana (Gmelin, 1789) (Schilling et al., 2014), the Atlantic bluefin tuna Thunnus thynnus (Linnaeus, 1758) (Pousis et al., 2011), the grey mullet Mugil cephalus (Linnaeus, 1758) (Amano et al., 2008), the zebrafish Danio rerio (Hamilton, 1822) (Wang, Tan, Emelyanov, Korzh & Gong, 2005) and the red seabream Pagrus major (Temminck & Schlegel, 1843) (Sawaguchi et al., 2006). During oocyte maturation in marine fish that spawn floating eggs, the yolk protein domains of these three Vtgs undergo varying degrees of proteolysis into lipids and free amino acids (FAAs), causing oocyte hydration (Cerdà, Fabra & Raldúa, 2007; Schilling, Loziuk, Muddiman, Daniels & Reading, 2015).
In wild greater amberjack, total plasma vitellogenin levels have been determined during different phases of the reproductive cycle using heterologous antibodies in an immunoenzymatic assay (Mandich et al., 2004), but no information on the different Vtgs genes is available. The aim of this study was to compare liver Vtgs expression levels and oocyte yolk accumulation in wild and captive-reared breeders during different periods of the reproductive cycle, in an effort to improve our understanding of the oogenesis impairment observed when these fish are reared in captivity (Mylonas et al., 2004; Zupa et al., 2017).
2 MATERIALS AND METHODS
2.1 Sampling
A total of 23 wild and 12 captive-reared greater amberjack females were sampled at three different phases of the reproductive cycle (Zupa et al., 2017) that were determined according to the available literature (Mandich et al., 2004; Sley, Hadj Taeib, Jarboui, Ghorbel & Bouain, 2014): early gametogenesis (EARLY, late April-early May), advanced gametogenesis (ADVANCED, late May-early June) and spawning (SPAWNING, late June-July). Wild fish were sampled soon after their capture on board a professional purse-seine fishing vessel operating around the Pelagie Islands (Sicily, Italy) during the fishing seasons 2014 (n = 11), 2015 (N = 10) and 2016 (N = 2). Captive-reared individuals belonged to a broodstock captured as juveniles (~1 kg body weight) in 2011 in the area of Astakos (Ionian Sea, Greece). In September 2013, the fish (5–7 kg in body weight) were transferred to a sea cage of Argosaronikos Fishfarming S.A. (Salamina Island, Greece), where they were reared for 2 years according to standard farming practices. The fish were fed to apparent satiation every other day, using a commercial extruded broodstock diet (Vitalis Cal; Skretting, SA, Norway), until they were killed for research purposes during the three above mentioned phases of the reproductive season of 2015 (N = 4 per reproductive phase).
Before sampling, fish were confined in a small cage area using a net and were led into a PVC bag (2 × 8 × 2 m), where they were slightly anesthetized with 0.01 ml/L clove oil. Then, they were gently directed into a PVC stretcher, brought on board of a service vessel and anesthetized deeply with 0.03 ml/L clove oil for sex recognition by means of gonad cannulation. Subsequently, fish were euthanized by decapitation and were placed on crushed ice and transferred to the farm facility. For each fish, biometric data (fork length, FL, nearest cm; body mass, BM, nearest kg; ovarian mass, OM, nearest g) were recorded and liver and ovary samples were taken. Liver samples, destined for molecular biology studies, were stored in RNA later® at 4°C and then transferred at −80°C. Ovarian samples, destined for histological analysis and for the assessment of oocyte yolk content, were fixed in Bouin's liquid for 4–6 hr and then stored in 70% ethanol.
2.2 RNA extraction and reverse transcription
Frozen tissue (about 50 mg) was powdered under liquid nitrogen with a porcelain mortar and pestle. RNA extractions from liver tissues, included treatment with the RNase Free Dnase set, to prevent genomic DNA contamination, and were carried out with Qiagen RNaesy® Lipid Tissue Mini Kit as described by the manufacturer. The RNA was resuspended in 50 μl of Rnase free water and stored at −80°C until used. To have an equal amount of total RNA to perform reverse transcription, RNA quantification was necessary. Quality and concentration of the RNA preparation were determined in 1% agarose-Tris-acetate buffered 1% gels stained with ethidium bromide and by spectrophotometric measurements at 260, 280 and 230 nm using NanoDrop® ND-1000 spectrophotometer (ThermoFisher Scientific Inc., MI, Italy). Moreover, to confirm that the isolated RNA was DNA free, a PCR was performed using RNA as template, with β-actin primers. Reverse transcription of 1 μg of total RNA was performed using SuperScript® III Reverse Transcriptase as described by the manufacturer (Invitrogen). Random hexamer primers were used for the first-strand cDNA synthesis. cDNA was kept at −80°C until used in the real-time PCR assay.
2.3 Cloning of greater amberjack vitellogenins cDNA
The partial cDNA of VtgA, VtgB, VtgC, and β-actin were amplified from total cDNA by means of overlapping PCR reactions, using primer pairs for Atlantic bluefin tuna Vtgs and β-actin, according to Pousis et al. (2011). All PCR were performed on a PCR Sprint Thermal Cycler using 50 ng cDNA. PCR-generated DNA fragments were resolved in 1× Tris-acetate buffered 1.2% agarose gels and visualized by ethidium bromide staining. Amplification product was excised from a 1.2% agarose gel, purified using Nucleo Spin extract II (Macherey-Nagel), ligated into the pCR 2.1 TOPO cloning vector (TOPO TA cloning kit; Invitrogen) and transformed into Escherichia coli competent cells (One Shot TOPO 10 chemically competent cells; Invitrogen), all the procedures according to the manufacturers’ instructions. Recombinant plasmids were purified and sent to the Eurofin Genomics Sequence Service (Ebersberg, Germany) for sequencing. The partial cDNA sequences of Vtgs and β-actin were obtained by comparing overlapping fragments.
2.4 Real-time PCR
Relative levels of Vtgs mRNA in the liver were examined by real-time PCR, using 96-well microwell plates and a QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems®) according to the manufacturer's instructions. Total RNA from liver (1 μg) was reverse transcribed as described previously. Once the greater amberjack Vtgs homologous sequences were obtained in this study, Vtgs and β-actin specific primers were designed (Table 1) using the Primer3 software (Rozen & Skaletsky, 2000) and their specificity was checked both in silico (NCBI Primer-BLAST tools) and in vitro by agarose gel electrophoresis. For gene expression analysis, qRT-PCR experiments were carried out in triplicate using 1 μl of diluted cDNA (1:10) as template for each reaction with SYBR Green PCR Master Mix (Bio-Rad). The quantification of the β-actin gene was used as the endogenous control, since its expression was already proved to be stable in the congener Seriola quinqueradiata (Temminck & Schlegel, 1845) (Kawanago, Takemura, Ishizuka & Shioya, 2014). This was also tested in this study and no statistical difference (ANOVA, p > .05) was found either among the three sampling periods, or between wild and captive-reared fish. Amplification parameters were as follows: hot start at 95°C for 15 min; 40 amplification cycles (95°C for 15″, 60°C for 30″, 72°C for 30″). Melting Curve (dissociation curve) analysis of amplification products was performed at the end of each PCR to confirm that a single PCR product was detected. Fluorescence raw data were exported from the QuantStudio Real Time PCR software (Applied Biosystems®) and analysed with the DART-PCR Excel workbook (Peirson, Butler & Foster, 2003). The mean amplification efficiency values for each amplicon (VgA 1.07 ± 0.11; VgB 1.03 ± 0.11; VgC 1.09 ± 0.08; β-actin 0.98 ± 0.04) were used to correct Ct values before applying the ΔCt method. Gene expression levels were calculated as:  (Livak & Schmittgen, 2001).
(Livak & Schmittgen, 2001).
| Target | Primer | Sequence | Tm |
|---|---|---|---|
| VtgA | Forward | GCAGCTTGAGACTGAGATCAG | 64 |
| Reverse | GGTACAGAAACAGGCAGAGCT | 64 | |
| VtgB | Forward | CAGCTGCTGGACCAGTCATC | 64 |
| Reverse | CAGGAACCAAGATATTCTTGAGT | 64 | |
| VtgC | Forward | GAGCCAGAATGTGCGCTGAG | 64 |
| Reverse | GCGTGTGCTCATCGGATGTC | 64 | |
| β-actin | Forward | CCCTGTCCTGCTCACAGAGG | 64 |
| Reverse | CAAGTCCAGACGCAGGATGG | 64 |
2.5 Sequence analysis
Comparison of greater amberjack Vtgs aminoacidic sequences with those of other Perciform species available in GenBank was performed using Clustal W program (Thompson, Higgins & Gibson, 1994) and BLASTP 2.2.24+ (http://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al., 1997, 2005).
2.6 Histological analysis of the ovaries and oocyte yolk accumulation
Fixed ovarian samples were dehydrated in increasing ethanol concentrations, clarified in xylene and embedded in paraffin wax. Five-μm thick sections were cut, deparaffinized and stained with haematoxylin-eosin. For each ovary sample, the most advanced oocyte stage, the percentage of atretic vitellogenic follicles and the presence of post-ovulatory follicles were recorded (Corriero et al., 2003, 2007; Zupa et al., 2017).
To compare oocyte yolk accumulation in wild and captive-reared individuals, oocytes at early and late stage of vitellogenesis, having a large and centrally located nucleus were selected. Oocyte diameter (μm) and surface occupied by yolk granules (μm2) were measured from microphotographs taken with a digital camera (DFC 420; Leica, Cambridge, UK) connected to a light microscope (DIAPLAN; Leitz, Wetzlar, Germany), using an image analysis software (version 3.3.0; Leica Application Suite, Cambridge, UK).
2.7 Statistical analysis
Differences in mean values of (i) Vtgs expression, (ii) oocyte diameter of oocytes at early and late vitellogenesis stage and (iii) oocyte surface occupied by yolk granules, were assessed by Student's t-test between the following pair of groups: wild specimens sampled in consecutive phases of the reproductive cycle; captive-reared specimens sampled in consecutive phases of the reproductive cycle; wild vs captive-reared specimens sampled in the same phase of the reproductive cycle. All the results are presented as means ± SE; the statistical probability significance was established at the P < .05 level.
3 RESULTS
The list of the fish used in this study, including biometric data, most advanced oocyte stage, presence of post-ovulatory follicles and percentage of atretic oocytes is reported in Table 2.
| Fish origin | Sampling date | FL (cm) | BM (kg) | OM (g) | Most advanced oocyte stage | α-atretic vitellogenic follicles (% of total vitellogenic oocytes) |
|---|---|---|---|---|---|---|
| Early gametogenesis (EARLY) | ||||||
| Wild | 01 May (SST = 18.1°C) | 103 | 14 | 100 | Cortical alveoli | – |
| 112 | 19 | 200 | Early vitellogenesis | – | ||
| 116 | 20 | 300 | Early vitellogenesis | – | ||
| 103 | 15 | 200 | Cortical alveoli | – | ||
| 106 | 13 | 100 | Perinucleolar | – | ||
| Captive | 24 April (SST = 17.5°C) | 87 | 10 | 85 | Cortical alveoli | – |
| 97 | 14 | 155 | Early vitellogenesis | – | ||
| 96 | 14 | 125 | Early vitellogenesis | – | ||
| 100 | 14 | 160 | Early vitellogenesis | – | ||
| Advanced gametogenesis (ADVANCED) | ||||||
| Wild | 31 May (SST = 19.3°C) | 114 | 21 | 1600 | Late vitellogenesis; post-ovulatory follicles | 9.5 |
| 117 | 22 | 1650 | Late vitellogenesis; post-ovulatory follicles | 13.3 | ||
| 04 June (SST = 19.6°C) | 101 | 15 | 1100 | Late vitellogenesis; post-ovulatory follicles | 12.5 | |
| 98 | 14 | 1050 | Late vitellogenesis; post-ovulatory follicles | 11.5 | ||
| Captive | 04 June (SST = 20.0°C) | 97 | 13 | 335 | Late vitellogenesis | 67.6 |
| 97 | 13 | 920 | Late vitellogenesis | 39.2 | ||
| 106 | 17 | 305 | Late vitellogenesis | 89.1 | ||
| 101 | 12 | 660 | Late vitellogenesis; | 51.3 | ||
| Spawning (SPAWNING) | ||||||
| Wild | 29 June (SST = 23.8°C) | 101 | 14 | 500 | Late vitellogenesis; post-ovulatory follicles | 0.0 |
| 114 | 19 | 1000 | Late vitellogenesis; post-ovulatory follicles | 0.0 | ||
| 109 | 16 | 700 | Late vitellogenesis; post-ovulatory follicles | 2.2 | ||
| 30 June (SST = 23.4°C) | 99 | 11 | 500 | Late vitellogenesis; post-ovulatory follicles | 2.7 | |
| 100 | 12 | 490 | Late vitellogenesis; post-ovulatory follicles | 0.0 | ||
| 97 | 12 | 450 | Late vitellogenesis; post-ovulatory follicles | 5.3 | ||
| 100 | 12 | 400 | Late vitellogenesis; post-ovulatory follicles | 20 | ||
| 98 | 12 | 500 | Hydrated | 0.0 | ||
| 96 | 12 | 390 | Late vitellogenesis; post-ovulatory follicles | 4.3 | ||
| 102 | 13 | 600 | Late vitellogenesis; post-ovulatory follicles | 4.3 | ||
| 104 | 14 | 950 | Hydrated | 0.0 | ||
| 95 | 12 | 450 | Late vitellogenesis; post-ovulatory follicles | 4.3 | ||
| Captive | 02 July (SST = 25.5°C) | 92 | 8 | 95 | Perinucleolara | |
| 96 | 12 | 130 | Perinucleolara | |||
| 95 | 11 | 135 | Perinucleolara | |||
| 97 | 12 | 140 | Perinucleolara | |||
- FL, fork length; BM, body mass; OM, ovarian mass; SST, Sea Surface Temperature.
- a α and β atresia of vitellogenic follicles.
3.1 Vitellogenin cDNA sequences and relative quantification of vitellogenin expression
Partial cDNA sequences encoding greater amberjack VtgA (GenBank accession no. KX570958), VtgB (GenBank accession no. KX570959), VtgC (GenBank accession no. KX570960) and β-actin (GenBank accession no. KX570957) were obtained. The deduced partial Vtgs aminoacidic sequences showed high similarities with those of all the other Perciform species available in GenBank, with a maximum of 99% between greater amberjack and yellowtail amberjack Seriola lalandi (Valenciennes, 1833) VtgA (GenBank accession number KX289700.1).
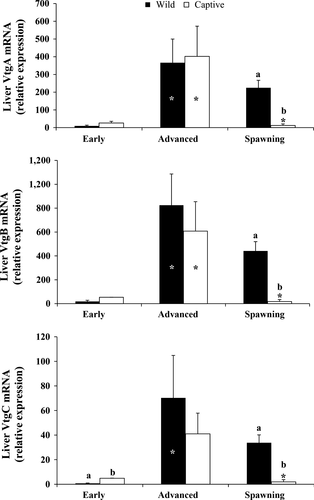
Transcript abundance of Vtgs genes during the three sampling phases was expressed as relative units, normalized to β-actin expression (Figure 1). The trend of Vtgs transcription levels in wild and captive-reared greater amberjack was similar for all the analysed Vtgs, showing an increase from EARLY to ADVANCED and a decrease during the SPAWNING phase, although this expression decrement was dramatic and statistically significant only in captive fish. Relative expression levels of VtgC were significantly lower in wild than in captive-reared greater amberjack during the EARLY phase (P < .01). All three Vgs were more expressed in wild than in captive-reared fish during the SPAWNING phase (P < .05).
3.2 Histological analysis of the ovaries and oocyte yolk accumulation
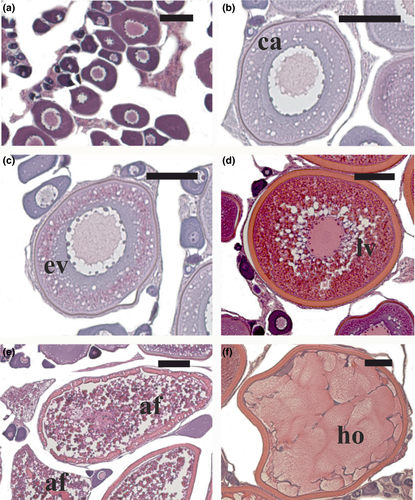
The ovaries of wild and captive-reared specimens sampled during the EARLY reproductive period exhibited primary growth (perinucleolar or cortical alveoli stage; Figure 2a,b) or early vitellogenesis (Figure 2c) as the most advanced oocyte stages. During the ADVANCED reproductive period, oocytes at late vitellogenesis stage (Figure 2d) and post-ovulatory follicles (a sign of recent spawning) were observed in the ovaries of the wild fish. The ovaries of captive-reared fish exhibited late vitellogenic oocytes as the most advanced oocyte stage, and some of these oocytes were atretic, while in two of the females the majority of vitellogenic follicles were atretic (Figure 2e). During the SPAWNING period, all the wild fish were in spawning condition, having ovaries with hydrated oocytes (Figure 2f) or post-ovulatory follicles. All the captive-reared fish sampled in this period were reproductively inactive, since their ovaries contained oocytes at the perinucleolar stage along with atretic vitellogenic follicles.
The comparative analysis of oocyte yolk accumulation did not show any significant difference in the yolk content of oocytes at the same development stage (early and late vitellogenesis) of wild and captive-reared specimens (Table 3).
| Oocyte stage | Fish condition | Oocyte diameter (μm) | Yolk surface (μm2) |
|---|---|---|---|
| Early vitellogenesis | Wild | 362.5 ± 3.5 | 55,584.9 ± 1,513.4 |
| Captive-reared | 356.5 ± 6.9 | 55,760.8 ± 3,238.2 | |
| Late vitellogenesis | Wild | 453.7 ± 3.5 | 84,660.1 ± 1,368.3 |
| Captive-reared | 453.0 ± 9.3 | 90790.6 ± 3,650.1 |
- No statistical significance existed in oocyte diameter and yolk surface between captive-reared and wild specimens (Student's t-test, P > .05).
4 DISCUSSION
In this study, the reported severe impairment of oogenesis, leading to vitellogenic oocyte atresia (Zupa et al., 2017) was examined in relation to the expression of the three genes encoding for Vtgs in captive greater amberjack reared under routine farming conditions. Reproductive dysfunctions are common in a variety of fish when they are reared in captivity (Mylonas, Fostier & Zanuy, 2010). These dysfunctions probably result from a combination of captivity-induced stress (Pankhurst & Van der Kraak, 1997; Sumpter, Pottinger, Rand-Weaver & Campbell, 1994), the lack of environmental conditions suitable for reproduction (Battaglene & Selosse, 1996; Ohta et al., 1997; Yaron, 1995; Zohar, 1989a,b) and nutritional deficiencies (Izquierdo, Fernández-Palacios & Tacon, 2001; Mylonas & Zohar, 2001). The most common type of reproductive dysfunction is the absence of oocyte maturation at the completion of vitellogenesis. Vitellogenesis progresses normally, but once it is completed, oocytes fail to undergo oocyte maturation and ovulation, and undergo atresia (Berlinsky, William, Hodson & Sullivan, 1997; Larsson, Mylonas, Zohar & Crim, 1997; Mylonas, Magnus, Gissis, Klebanov & Zohar, 1997; Tucker, 1994). In addition to the greater amberjack, this reproductive dysfunction has been already documented in other large pelagic fish as well, such as the Atlantic bluefin tuna (Corriero et al., 2007; Micale et al., 1999; Mylonas et al., 2004, 2007; Zupa et al., 2017). Atlantic bluefin tuna caught from the wild and reared in captivity exhibited a reduced capacity to undergo oocyte maturation and ovulation spontaneously (Corriero et al., 2007). Although rearing in captivity did not seem to affect Vtg gene expression (Pousis et al., 2011) as in this study, the number of vitellogenic oocytes was lower compared to wild Atlantic bluefin tuna, and the ovaries of half of the fish reared in captivity showed major α-atresia of vitellogenic follicles (Corriero et al., 2007). Similarly, wild-caught greater amberjack reared in outdoor tanks in an earlier study, failed to undergo oocyte maturation with consequent extensive atresia (Micale et al., 1999). Mylonas et al. (2004) studied the reproductive maturation of two greater amberjack broodstocks reared in 30–40-m3 tanks: in all the females of the first stock (n = 4), oogenesis was arrested at the primary growth or early vitellogenic stage; the only one female of the second stock was able to complete vitellogenesis and was induced to mature and spawn through the administration of gonadotropin releasing-hormone agonist (GnRHa) implants. Therefore, the results of this study corroborate earlier works, showing that even when maintained in cages, captive-reared greater amberjack may exhibit severe reproductive dysfunctions.
In teleost fish, multigene families for Vtg have been reported (LaFleur et al., 1995, 2005; Pousis et al., 2011; Sawaguchi et al., 2005, 2006). In some marine teleosts spawning pelagic eggs, virtually all yolk proteins derived from the VtgA gene may be proteolytically cleaved into FAAs during oocyte maturation, whereas the major products of the VtgB and VtgC genes remain largely intact (Cerdà et al., 2007). Free amino acids act as osmotic effectors of oocyte hydration and serve as diffusible nutrients utilized selectively by early embryos, while the large lipoproteins derived from VtgB and VtgC are utilized later by the larvae, during yolk absorption (Cerdà et al., 2007; Matsubara, Ohkubo, Andoh, Sullivan & Hara, 1999; Matsubara et al., 2003). Thus, the multiple Vtg system provides for proper adjustment of egg buoyancy and delivers the appropriate types of nutrients for each stage of early development.
Stress and cortisol are known to affect negatively vitellogenesis in oviparous species (Lethimonier, Flouriot, Kah & Ducouret, 2002). However, the effect of cortisol-mediated stress on vitellogenesis is controversial and species-specific. Cortisol has been shown to downregulate 17β-estradiol (E2)-induced Vtg expression in Arctic char Salvelinus alpinus (Linnaeus, 1758) (Berg, Westerlund & Olssona 2004) and to reduce plasma Vtg levels in brown trout Salmo trutta (Linnaeus, 1758) (Carragher, Sumpter, Pottinger & Pickering, 1989). However, cortisol exposure potentiated E2 induction of Vtg in catfish Heteropneustes fossilis (Bloch, 1794) (Sundararaj, Goswami & Lamba, 1982) and glucocorticoid treatment has been found to downregulate Vtg in cultured rainbow trout Oncorhynchus mykiss (Walbaum, 1792) hepatocytes (Mori, Matsumoto & Yokota, 1998; Pellisero et al., 1993) and to upregulate Vtg in blue tilapia Oreochromis aureus (Steindachner, 1864) (Ding, Lim & Lam, 1994). Furthermore, cortisol has been shown to reduce Vtg plasma levels without affecting Vtg mRNA levels in Arctic char (Berg, Modig & Olsson 2004). Therefore, cortisol can exert its effects on vitellogenesis both at the transcriptional and at the post-transcriptional level.
In this study, greater amberjack liver VtgA and VtgB gene expression was comparable between wild and captive-reared individuals during the EARLY and ADVANCED phases of the reproductive cycle. On the contrary, liver VtgA and VtgB gene expression differed only during SPAWNING, being higher in wild fish. Plasma levels of E2 (Zupa et al., 2017) and liver Vtgs gene expression (present results) increased from the EARLY to the ADVANCED phase both in wild and captive-reared greater amberjack, but during the ADVANCED phase only E2 plasma levels were significantly lower in captive individuals compared with wild fish. The lower E2 circulating levels reported by Zupa et al. (2017) for captive-reared individuals were not associated with a significant decrease in Vtgs gene expression. At the same time, an abnormal rate of atresia of vitellogenic follicles was observed in captive-reared fish. Later, during SPAWNING, ovaries of captive fish were in a regressed state showing only atretic vitellogenic follicles, contrary to that observed in wild fish, which presented fully vitellogenic ovaries. Therefore, this study suggests that the impaired oogenesis described by Zupa et al. (2017) in captive-reared greater amberjack was not related to lower expression of the Vtgs genes, since these genes did not exhibit lower expression at the time of the first occurrence of atretic vitellogenic oocytes during the ADVANCED period, but only later during the SPAWNING period. Although a negative effect of captivity-induced stress on greater amberjack vitellogenesis at the post-transcriptional level cannot be excluded, the similar pattern of yolk accumulation in captive-reared and wild fish, apparently indicates that the whole vitellogenic process from liver vitellogenin gene expression to oocyte vitellogenin uptake, was not affected negatively by rearing conditions.
Reproductive maturation in fish, as in all vertebrates, is controlled by the gonadotropin hormones (GtHs), follicle-stimulating hormone (FSH) and luteinizing hormone (LH). During vitellogenesis, FSH stimulates testosterone production by thecal cells and its aromatization to E2 by granulosa cells (Kagawa, Young, Adachi & Nagahama, 1982; Nagahama, 1987, 1994), while LH promotes oocyte maturation through the stimulation of 17α-hydroxyprogesterone production by the thecal cell layer and its conversion into 17,20β-dihydroxypren-4-en-3-one by the granulosa cell layer through 20β-hydroxysteroid dehydrogenase (Nagahama & Yamashita, 2008; Nagahama, Yamashita & Tokumoto, 1994; Young, Adachi & Nagahama, 1986). In captive-reared greater amberjack, 17,20β-P plasma levels were significantly lower compared to wild fish during the EARLY and ADVANCED phases (Zupa et al., 2017). This hormone is essential in a number of fish in inducing oocyte maturation, a process that includes the germinal vesicle breakdown and yolk globule coalescence (Mylonas et al., 1997). This role of the hormone has been confirmed also for greater amberjack and its congeners (Mandich et al., 2004; Poortenaar, Hooker & Sharp, 2001; Rahman et al., 2001). The observed oogenesis arrest and high occurrence of apoptosis in this study may be ascribed to low 17,20β-P plasma levels rather than to insufficient E2–induced liver synthesis of Vtgs. Studies in the gilthead seabream Sparus aurata (Linnaeus, 1758) and striped bass Morone saxatilis (Walbaum, 1792) showed that the failure of captive fish to undergo oocyte maturation in captivity was due to the lack of pituitary LH release, and to the consequent low plasma levels of 17,20β-P during the spawning season, even though LH accumulated in the pituitary during oogenesis (Mylonas, Woods, Thomas & Zohar, 1998; Mylonas et al., 1997; Zohar, 1988). Similarly, in cultured Atlantic bluefin tuna that failed to undergo oocyte maturation, ovulation and spawning, a high pituitary LH content was associated with low plasma LH levels, presumably because of insufficient pituitary stimulation by GnRH from the hypothalamus (Rosenfeld et al., 2012). So, in all these species it seems that low plasma 17,20β-P during the spawning season may be responsible, at least in part, for the failure of females to complete vitellogenesis and undergo oocyte maturation.
In teleost fish, as in other oviparous animals, Vtg is specifically incorporated in the developing oocytes through a process of endocytosis, mediated by vitellogenin receptors (VtgRs) that are orthologous to the mammalian very low density lipoprotein receptor (VLDLR; Hiramatsu, Chapman, Lindzey, Haynes & Sullivan, 2004). The mechanisms that regulate VtgR and other VLDLR expression in fish have not yet been clarified (Chakraborty et al., 2011; Dominguez et al., 2012; Pousis et al., 2012). However, a role of the diet, particularly of lipid intake, in the regulation of VtgR expression and Vtg oocyte uptake is expected, based on evidence on Senegalese sole Solea senegalensis (Kaup, 1858) in which liver VLDLR overexpression was associated to a high fat diet (Borges et al., 2013). It is well documented that the nutritional state of a broodstock plays an important role in gonad development and reproductive success (Izquierdo et al., 2001; Tocher, 2010; Verakunpiriya et al., 1996). In captive-reared Atlantic bluefin tuna, the administration of an improved diet based on squid (Loligo and Illex spp.) to increase the content of high quality fatty acids and protein in the diet, was associated with higher VtgR expression levels (Pousis et al., 2012). Although in individual oocytes of captive-reared greater amberjack a normal yolk accumulation was observed, the scarce ovarian development (lower gonado-somatic index) was likely associated to a reduced production of yolked oocytes. Considering that the ovaries of the captive-reared greater amberjack stock showed a different total polar lipid profile and a lower content of essential fatty acids compared with the wild population (Zupa et al., 2017), the possibility that a sub-optimal diet may have altered the physiological expression of VtgRs should be examined.
5 CONCLUSION
In this study, the reported severe impairment of oogenesis, leading to vitellogenic oocyte atresia and reduced gonado-somatic index (Zupa et al., 2017), was examined in relation to the expression of the three genes encoding for Vtgs in captive-reared greater amberjack. Although both plasma E2 and 17,20β-P levels were lower in captive-reared compared to wild fish (Zupa et al., 2017), Vtgs gene expression and oocyte yolk uptake did not seem to be affected by these low steroid levels and the reported impairment of oogenesis in captive-reared greater amberjack could not be ascribed to a dysfunctional vitellogenic process.
ACKNOWLEDGMENTS
This project has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration (KBBE-2013-07 single stage, GA 603121, DIVERSIFY). Thanks are due to Mr Peppe, Giovanni and Vincenzo Billeci, and all the crew of the purse-seine fishing vessel ‘Graziella’ for their hospitality on board and assistance during wild greater amberjack sampling. Special thanks are due to Mr Tasos Raftopoulos of Argosaronikos Fishfarming S.A. (Greece) for the hospitality in his farm, and the maintenance and sampling of captive-reared greater amberjack broodstock.




