Anaesthetic efficacy of eugenol on various size classes of angelfish (Pterophyllum scalare Schultze, 1823)
Abstract
Anaesthetic efficacy of eugenol was investigated on Pterophyllum scalare. A total of 130 fish with average weights of 1.0 ± 0.5, 5.0 ± 1.0 and 10.0 ± 1.0 g were subjected to 1.25, 2.5, 4.0, 5.5 and 7.0 mg/L eugenol, and behavioural responses were observed. Induction and recovery times were significantly affected by the interactive effect of eugenol concentration and fish weight (p < .05). Generally, 49.9–128 s after exposure to 1.25–7 mg/L eugenol, fish reached stage 3. Fish entered stage 4 over 55–135 s post exposure to such concentrations. Recovery time was 393.5–597.7 s in all sizes. Any increase in eugenol concentration led to a significant decrease in the induction time with a subsequent increment of the recovery time. Concentrations of eugenol and fish size along with their interactive effects have significantly contributed to the regression models, with concentration recording the highest beta values for stages 1, 2, 3 and 4 (−0.903, −0.898, −0.976 and −0.864 respectively) and the product of size and anaesthetic concentration for full recovery in smaller fish (0.647) and eugenol concentration in larger ones (0.967). Recovery time was fitted to induction time to stage 4 via quadratic and linear regression models in smaller and larger fish respectively. Results revealed the minimal eugenol concentration to induce anaesthesia in various size classes of angelfish in less than 3 min was 1.25 mg/L. Our results showed eugenol as an effective and safe anaesthetic; however, it is not advisable for live fish transportation.
1 Introduction
Fish may experience stressful condition during fisheries management and aquaculture activities including counting, pathological examinations, hormonal implants or injections, vaccinations, stripping, transfer and hauling and release (Carmichael & Tomasso 1988; Brown 1993; Tarkhani, Imani, Jamali & Sarvi Moghanlou 2016). Such conditions would result in compromised stamina and cause mortality, growth reduction and diseases outbreaks in fish population. In addition, fish response to stressful circumstances via recruiting more energy reserves which later on would affect individuals’ fitness and homoeostasis (Park, Hur, Im, Seol, Lee & Park 2008).
To survive such conditions, application of species-specific concentration and exposure time of an anaesthetic is highly recommended (Summerfelt & Smith 1990). In this regard, the efficacy of several anaesthetic agents including metomidate, 2-phenoxyethanol, quinaldine, tricaine methanesulphonate (MS-222), benzocaine, clove oil and Aqui-STM have previously been examined in different fish species (Pirhonen & Schreck 2003; Iversen, Finstad, McKinley & Eliassen 2003; Coyle, Durborow & Tidwell 2004; Tarkhani et al. 2016). In searching for an appropriate anaesthetic for aquaculture industry, various relevant factors such as low expense, high efficiency, lack of/short withdrawal period, lack of side effects on fish appetite, blood biochemistry and health, wide toxicity threshold both to target animal and labours must be considered (Heo and Shin 2010; Hoseini, Rajabiesterabadi & Tarkhani 2013). Although, MS-222 is the only anaesthetic approved by the Food and Drug Administration of the United States of America (USFDA), its application is mainly limited due to higher cost and lower efficiency of the chemical in controlling plasma cortisol level (Coyle, Dasgupta, Tidwell, Beavers, Bright & Yasharian 2005). Moreover, a 21-day withdrawal period is recommended provided that the fish is intended for human consumption (Ross & Ross 2008). Particularly, such constraints encourage using less persistent and natural anaesthetics such as clove oil (Tarkhani et al. 2016).
Eugenol, 2-methoxy-4-(2-propenyl) phenol, as the major component of clove oil (70%–90% by weight), is generally regarded as safe by FDA (Ross & Ross 2008). Lower price and its safety to both human and environment have encouraged clove oil application as an attractive anaesthetic for fish (Mylonas, Cardinaletti, Sigelaki & Polzonetti-Magni 2005).
Its anaesthetic efficiency has been reported for distinct ornamental fish species including Amphilophus labiatus × Amphilophus trimaculatus (Tarkhani et al. 2016), Pangasius hypophthalmus (Hoseini et al. 2013), Pterophyllum scalare (Mitjana, Bonastre, Insua, Falceto, Esteban, Josa & Espinosa 2014), Pomacentrus amboinensis (Munday & Wilson 1997), Danio rerio (Grush, Noakes & Moccia 2004). With no doubt, one can consider eugenol and isoeugenol as future anaesthetics of choice in the aquaculture industry due to their efficacy, low price, no withdrawal period and lack of side effects on fish appetite (Cupp, Hartleb, Fredricks & Gaikowski 2016). It has been shown that Aqui-S vet. (iso-eugenol) was able to considerably alleviate the primary and secondary stress responses in European eel, Anguilla anguilla L. and improve animal welfare and survival during and after common aquaculture practices (Iversen, Økland, Thorstad & Finstad 2013). The anaesthetic efficacy of eugenol was the subject of several studies on various fish species such as common carp, Cyprinus carpio (Hikasa, Takase, Ogasawara and Ogasawara 1986), rabbitfish (Siganus lineatus) (Soto & Burhanuddin 1995), fathead minnow (Pimephales promelas Rafinesque, 1820) (Palić, Herolt, Andreasen, Menzel & Roth 2006), Common snook (Centropomus undecimalis Bloch, 1792) (Bernardes Júnior, Nakagome, Mello, Garcia & Amaral Júnior 2013) and flower horn (Tarkhani et al. 2016).
The literature suggests that the safely effective concentration and exposure time of eugenol substantially vary depending on fish species and size (Hoseini et al. 2013; Cupp et al. 2016). To determine such prerequisites, various stages of anaesthesia were already described in different fish species according to the behavioural responses of an animal including response to stimuli, opercular rate and fish equilibrium (McFarland 1959; Hikasa et al. 1986; Hoseini et al. 2013). The ideal level of sedation known as deep sedation is indicated by loss of reactivity to external stimuli, decrease in metabolic rate, but maintenance of equilibrium (McFarland 1959) and equals to stage 2 of Anaesthesia as described by Summerfelt and Smith (1990).
At the moment, the ornamental fish market is highly competitive and also demanding with considerably large market values to the extent that they are referred to as living jewels. Their tranquillity, small and colourful body along with astonishingly eclectic shape and behaviour made them popular aquatic pets (Mandal, Mukherjee & Banerjee 2010; Johny & Inasun 2016). Being native to Amazon region of South America including Peru, Colombia and Brazil, the angel fish, P. scalare is considered the most enviable ornamental fish mainly owing to its attractiveness, reproductive capacity, reasonable housing and nutritional requirements and adaptability to captivity (Garcia-Ulloa & Gómez-Romero 2005; Karayucel, Ak & Karayucel 2006; Froese and Pauly 2014). The species enjoys an insatiable worldwide demand among commercial ornamental fish species fostering its fast growing aquaculture and trade (Chapman, Fitz-Coy, Thunberg & Adams 1997). In ornamental fish aquaculture, practice handling and also transportation of live fish especially for a long-distance journey as routine activities impose considerable changes in fish physiology and behaviour. Such events may also result in higher rates of mortality and leave the remaining fish susceptible to various opportunistic fungal or bacterial infections in the one hand and ensue economic loss in the other hand. Among various solutions, using anaesthetics to lower stress to fish and decrease physical injury is highly recommended by experts (Johny & Inasu 2016). Therefore, the present study was to assess the anaesthetic efficacy of eugenol and to establish reliable concentrations for different sizes of angelfish. In the discussion, we shall present appropriate concentrations of eugenol suitable for various management purposes based on our results and also compare the results of this study with the results from previous important studies.
2 Materials and Methods
2.1 Fish and experimental conditions
A total number of 130 angelfish, P. scalare, with three different size classes 1.0 ± 0.5, 5.0 ± 1.0 and 10.0 ± 1.0 g were purchased from a local ornamental fish farm and transferred to laboratory, and each group was separately stocked in 100 L tanks at a density of 10 g/L. Fish were fed at 1.5% of their body weight per day with a commercial diet (Biomar, Nersac, France). All tanks were continuously aerated, and the daily water exchange rate was 80%. Water quality parameters were measured every other day and all quality criteria including pH = 7.0–7.5; temperature = 25.1 ± 1.4°C; N-nitrite = 0.05 ± 0.1 mg/L; total hardness = 140 ± 12.7 mg/L; DO = 6.3 ± 0.2 mg/L were within the optimum ranges for freshwater fish culture. Feeding was given up 24 h before the experiment and continued a day after recovery from anaesthesia.
2.2 Anaesthetic preparation and behavioural observations during anaesthetic exposure
Due to its lower water solubility, a stock solution of eugenol was prepared by mixing eugenol (Sigma, St. Louis, MO, USA; 99% purity) and ethanol (Razi, Iran, with 96% purity) with respective volumetric ratio of 1:2. Final working solutions containing 1.25, 2.5, 4.0, 5.5 and 7.0 mg/L eugenol were freshly prepared right before experimentation. Fish were individually subjected to each anaesthetic solutions (n = 7–10). Plastic 2-L containers with continuous aeration were used (Hoseini et al. 2013). Time required to get different stages of anaesthesia was recorded according to fish behaviour (Table 1). Time required getting complete equilibrium was recorded from transferring the fish to recovery container (60-L aquaria containing 40 L aerated fresh water). Finally, fish were transferred to freshwater aquaria to monitor potential mortality over a 24-h period (Tarkhani et al. 2016). Table 1 contained behavioural responses of fish at various anaesthetic stages; however, more details were illustrated elsewhere (Hoseini et al. 2013). Our preliminary observations on angelfish showed that exposure to ethanol did not bring about anaesthesia or any apparent modifications in fish behaviour implying that the concentration of ethanol used had no effects on the fish during the experiment.
| Stage | Behaviour of fish |
|---|---|
| 0 | Normal |
| I | Relaxation and no response to stimuli: fish were calmed and did not respond to tactile touch |
| II | Imbalance swimming: fish loss their equilibrium and show imbalance swimming |
| III | Total loss of equilibrium: fish laid on lateral side, slightly depressed but regular opercular movement |
| IV | Deep anaesthesia: slow and irregular opercular movement |
| V | Death: opercular movement ceased |
| Recovery stage | Fish regained its equilibrium |
2.3 Statistical analyses
Levene's test and Kolmogorov–Smirnove were used to evaluate the homogeneity of variance of the dependent variables and normality of data set respectively. Two-way ANOVA was used to illustrate whether or not there were significant differences among different experimental groups. The general quadratic equation, Z = b0 + b1X + b2Y + b3X2 + b4XY + b5Y2 + ɛ, applied to test for relationships using polynomial regression; where Z is the response (time to reach each anaesthetic stage), X and Y are the independent variables (e.g. fish size and eugenol concentration). With the use of a p < .001 criterion for Mahalanobis distance, no outliers were found (Steel, Torrie & Dickey 1997; Shanock, Baran, Gentry, Pattison & Heggestad 2010). Model validation analysis was also conducted via cross validation which requires that the regression model for the training sample replicates the pattern of the full data set (Osborne 2000). All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, NY, USA) at the significance level of p < .05. Results were reported as Mean ± SE.
3 Results
After 24 hr, no mortalities were observed in all experimental groups, and the fish were feeding well within 1 day after treatment. Two-way anova revealed a statistically significant interaction between eugenol concentration and average fish body weight with regard to anaesthetic efficacy of eugenol on angelfish (Table 2). The fish sequentially entered different anaesthetic stages. In all stages, any increase in eugenol concentration led to a significant decrease in the induction time with a subsequent increment of the recovery time (Table 3). Any increases in fish weight simultaneously resulted in a significant increment in the induction and recovery time. All size classes showed all anaesthetic stages at 1.25 mg/L eugenol. Results showed that 7.50 mg/L eugenol effectively induced stages 3 and 4 very rapidly in comparison to other concentrations. According to the results, those fish exposed to higher concentrations of the anaesthetic agent required longer time to fully recover and regain their equilibrium (Table 3).
| Source | Dependent Variable | Type III Sum of Squares | d.f. | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|
| Size | Stage 1 | 7044.688 | 2 | 3522.344 | 213.752 | 0.000 |
| Stage 2 | 9432.090 | 2 | 4716.045 | 372.687 | 0.000 | |
| Stage3 | 10970.879 | 2 | 5485.440 | 458.475 | 0.000 | |
| Stage4 | 14054.297 | 2 | 7027.148 | 643.330 | 0.000 | |
| Recovery | 606523.318 | 2 | 303261.659 | 12865.833 | 0.000 | |
| Concentration | Stage 1 | 45552.808 | 4 | 11388.202 | 691.088 | 0.000 |
| Stage 2 | 54406.657 | 4 | 13601.664 | 1074.875 | 0.000 | |
| Stage3 | 58741.261 | 4 | 14685.315 | 1227.403 | 0.000 | |
| Stage4 | 59902.071 | 4 | 14975.518 | 1370.998 | 0.000 | |
| Recovery | 41037.252 | 4 | 10259.313 | 435.250 | 0.000 | |
| Size × Concentration | Stage 1 | 695.874 | 8 | 86.984 | 5.279 | 0.000 |
| Stage 2 | 1234.435 | 8 | 154.304 | 12.194 | 0.000 | |
| Stage3 | 2829.828 | 8 | 353.729 | 29.565 | 0.000 | |
| Stage4 | 3432.788 | 8 | 429.099 | 39.284 | 0.000 | |
| Recovery | 3670.648 | 8 | 458.831 | 19.466 | 0.000 | |
| Error | Stage 1 | 1895.046 | 115 | 16.479 | ||
| Stage 2 | 1455.231 | 115 | 12.654 | |||
| Stage3 | 1375.922 | 115 | 11.965 | |||
| Stage4 | 1256.154 | 115 | 10.923 | |||
| Recovery | 2710.675 | 115 | 23.571 | |||
| Total | Stage 1 | 853460.000 | 130 | |||
| Stage 2 | 1041505.000 | 130 | ||||
| Stage3 | 1237336.000 | 130 | ||||
| Stage4 | 1443671.000 | 130 | ||||
| Recovery | 28340291.000 | 130 |
| Size (g) | Eugenol concentration (mg/L) | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Recovery time |
|---|---|---|---|---|---|---|
| 1 | 1.25 | 96.500 ± 1.204ef* | 106.000 ± 1.238f | 120.200 ± 0.512ij | 125.300 ± 0.578h | 393.500 ± 0.980a |
| 2.50 | 78.300 ± 2.017cd | 85.400 ± 1.213de | 91.100 ± 0.936gh | 94.700 ± 0.978ef | 394.800 ± 1.306ab | |
| 4.00 | 73.200 ± 1.919c | 79.600 ± 1.916d | 84.800 ± 2.097ef | 92.900 ± 2.030de | 415.400 ± 1.641de | |
| 5.50 | 63.200 ± 1.381b | 68.300 ± 1.174c | 73.300 ± 1.325c | 79.400 ± 1.352c | 408.100 ± 1.952cd | |
| 7.50 | 38.800 ± 1.618a | 42.600 ± 1.529a | 49.000 ± 1.826a | 55.600 ± 1.714a | 434.900 ± 1.792f | |
| 5 | 1.25 | 103.000 ± 1.528f | 119.780 ± 0.547g | 126.440 ± 0.603kl | 135.560 ± 0.669i | 402.000 ± 1.667bc |
| 2.50 | 93.200 ± 0.929e | 101.900 ± 0.948f | 111.800 ± 0.712h | 122.100 ± 0.781h | 417.600 ± 1.875e | |
| 4.00 | 80.700 ± 0.978d | 87.800 ± 0.952e | 95.600 ± 0.980h | 106.600 ± 0.499g | 427.700 ± 1.212f | |
| 5.50 | 65.570 ± 0.612b | 73.430 ± 0.719c | 81.140 ± 0.595de | 88.290 ± 0.565d | 451.570 ± 1.587g | |
| 7.50 | 45.250 ± 1.719a | 52.000 ± 1.813b | 59.250 ± 1.319b | 68.380 ± 0.565b | 465.630 ± 1.487h | |
| 10 | 1.25 | 112.430 ± 0.528g | 121.140 ± 0.738g | 128.000 ± 0.617l | 134.140 ± 0.800i | 547.860 ± 2.613i |
| 2.50 | 98.380 ± 0.844ef | 107.630 ± 0.375f | 116.000 ± 0.655i | 127.500 ± 0.707h | 558.500 ± 1.376j | |
| 4.00 | 95.290 ± 1.085e | 107.430 ± 0.922f | 122.140 ± 0.738jk | 133.140 ± 1.243i | 572.000 ± 2.127k | |
| 5.50 | 74.860 ± 0.911cd | 83.000 ± 1.134de | 90.140 ± 1.262fg | 98.860 ± 1.100f | 582.000 ± 0.690l | |
| 7.50 | 60.860 ± 0.508b | 68.430 ± 0.481c | 76.290 ± 0.680cd | 81.860 ± 0.829c | 597.710 ± 1.658m |
- *Values with different superscripts in each column are significantly different at p < .05.
Results from stepwise multiple regression analyses yielded the following regression equations representing the description of time elapsed to reach stage 1, 2, 3, 4 and full recovery from anaesthesia; Stage 1 = 103.675–8.633 × (concentration) + 2.025 × (size), Stage 2 = 113.788–9.472 × (concentration) + 2.338 × (size), Stage 3 = 126.524–10.866 × (concentration) + 1.674 × (size) + 0.205 × (concentration×size) and Stage 4 = 129.038–9.916 × (concentration) + 2.837 × (size). As distinct sizes of angelfish showed different responses to various concentrations of eugenol, time elapsed to fully recover from anaesthesia for smaller fish (1 and 5 g) and the larger ones (10 g) were separately analysed and reported; full recovery from anaesthesia = 386.364 + 4.387 × (concentration) + 1.321 × (concentration×size) for smaller fish and full recovery from anaesthesia = 538.698 + 7.931 × (concentration) for the larger ones. The total variance explained by the model as a whole was 94.4%, F (2, 127) = 1075.998, p = .000 for stage 1, 94.8%, F (2, 127) = 1163.695, p = .000 for stage 2, 93.5%, F (3, 126) = 606.516, p = .000 for stage 3 and 92.1%, F (2, 127) = 737.774, p = .000 for stage 4. However, total variance explained by the model was 90.4%, F (2, 91) = 427.640, p = .000 and 93.6%, F (1, 34) = 495.965, p = .000 for full recovery from anaesthesia in smaller and larger fish respectively.
Both independent variables and also their interactive effects at least in the case of inducing stage 3 and also full recovery of smaller fish were significantly contributed in the model, with concentration recording the highest β value for stages 1, 2, 3 and 4 (-0.903, -0.898, -0.976 and -0.864 respectively) and the product of size and anaesthetic concentration for full recovery in smaller fish (0.647) and eugenol concentration in larger angel fish (0.967). Recovery time was also well fitted to induction time to stage 4 via quadratic and linear regression models in smaller (32.2%, F (2, 91) = 21.656, p = .001) and larger fish (74.5%, F (1, 34) = 99.160, p = .000) respectively (Fig. 1).
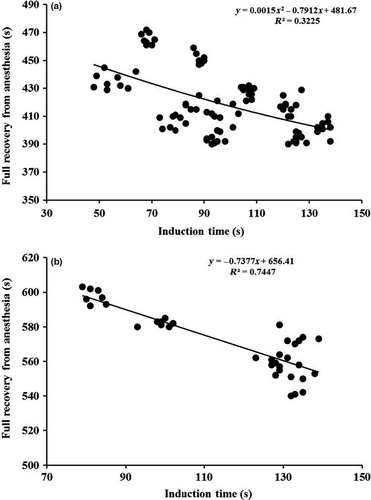
In the analysis of the training samples, the relationships between the predictors and the response variables were statistically significant; 94.09%, F (2, 97) = 767.553, p = .000 for stage 1, 94.67%, F (2, 97) = 845.729, p = .000 for stage 2, 92.93%, F (3, 96) = 418.940, p = .000 for stage 3, 91.78% and F (2, 97) = 534.554, p = .000 for stage 4. The validation procedure for full recovery from anaesthesia also showed that the relationships between the predictors and the response variables were statistically significant; 89.7% F (2, 68) = 298.217, p = .000 and 93.4% F (1, 27) = 382.629, p = .000 for smaller and larger fish respectively. In all stages, the pattern of significance for independent variables in training sample matched the pattern for full data set. The lack of shrinkage in R2 obtained for validation sample in comparison to R2 of training sample indicated that the regression models would be effective in predicting time required to get each anaesthetic stage for other experiments on the species using eugenol as the anaesthetic agent.
4 Discussion
Various anaesthetics have been used in aquaculture industry with their own specific merits and demerits. 2-Phenoxyethanol (2-PE) is selected over tricaine methane sulphonate (MS-222) due to its lower cost and the ease of use (Ortuno et al. 2002); however, its usage has been put under a shadow of doubt by inducing a stress response in fish (Iwama et al. 1989; Thomas & Robertson 1991; Ortuno et al. 2002). Such increased stress response has also been reported for deep anaesthesia induced by MS-222 in fish (Small 2003). Clove oil as a natural commodity is readily available with competitive prices and also is generally considered as safe (GRAS) compound by the U.S. Food and Drug Administration (Summerfelt & Smith 1990). For morphological assessments, taking biopsy samples from tissues and organs and also hand stripping of gametes, where long handling periods outside the water are required, the longer recovery time might be an advantage for clove oil application (Rodri'guez-Gutierrez & Esquivel-Herrera 1995). Also, clove oil may be a more appropriate anaesthetic for use in commercial aquaculture situations, where anaesthetics may be used in large quantities by unskilled labourers and directly released into natural water bodies (Javahery, Nekoubin & HajiMoradlu 2012). Being the major component of clove oil (70%–90% by weight), Small (2003) found that there were not any increase in blood cortisol concentrations of fish anaesthetized with eugenol. However, the anaesthetic is only certified by Japan aquaculture industry. However, narrower safety margin of eugenol compared with other common anaesthetic agents has limited effectiveness of eugenol for surgical manipulations due to its quick effect on respiratory center of fish (Sladky, Swanson, Stoskopf, Loomis & Lewbart 2001; Misawa, Kada & Yoshida 2014).
As application of inappropriate concentrations of chemical may harm or even kill fish, it is important to determine right anaesthetic concentration for each fish species (Hoseini & Jafar Nodeh 2011). Previous researchers found that the ideal times for the induction and recovery from anaesthesia were 3 and 5 min respectively (Hseu, Yeh, Chu & Ting 1998; Marking & Meyer 1985; Gilderhus & Marking 1987). In the present study, all concentrations were efficiently induced anaesthesia within 3 min; however, the time required by fish to regain its equilibrium varied from 393.500 ± 0.980 to 597.710 ± 1.658 s (i.e. 6.5–10 min). It is worth mentioning that according to our preliminary observations on angelfish, time to reach full anaesthesia considerably extended at lower eugenol concentrations. One must know that defining a suitable time for full anaesthesia and recovery may vary depending on fish species and also purpose of inducing anaesthesia (external sampling, fin biopsies, gill biopsies, surgical procedures) require different selection criteria (Stoskopf & Posner 2008).
Our results also showed that 5.5 mg/L eugenol was the best desired dose for induction of anaesthesia in all three size classes of P. scalare. According to table 1, induction and recovery time at 5.5 mg/L eugenol were less than those other concentrations, and at 7.5 mg/L, the recovery time was near critical threshold (> 10 min, Marking & Meyer 1985). Various studied sizes of angelfish (1, 5 and 10 g) exposed to 5.5 mg/L reached the appropriate stage for handling (stage 3) in 73.3, 81.1 and 90.1 s and for surgery and blood sampling (stage 4) in 84.6, 95.5 and 106.2 s respectively. Similar to our results, when Colossoma macropomum (Tambaqui) juveniles were exposed to the ideal concentration of clove oil (65 mg/L), they required 88.8 s to reach the surgical stage, while time was increased to 226.2 s for subadults (Roubach et al. 2005). At ideal concentration of clove oil, Ictalurus punctatus (100 mg/L) required 310.2 s (Waterstrat 1999), and Salmo salar (30-100 mg/L) required 486 s (Iversen et al. 2003) to reach the surgical stage. However, the best clove oil concentrations for the induction of anaesthesia and handling of fish were 50-100 mg/L and 10-30 mg/L respectively (Javahery, Nekoubin & HajiMoradlu 2012). The findings agree well with the existing literature on the efficacy of eugenol on different species as an anaesthetic (Cunha & Rosa 2006; Park et al. 2008; Hoseini et al. 2013, Tarkhani et al. 2016). Similar to our results on angelfish with 1 g body weight, it has also been shown that 4.0 mg/L etomidate was able to anaesthetize 0.3-2 g angelfish in 78 s and fish fully recovered in 240 s (Amend, Goven & Elliot 1982). However, in the present study with eugenol, angelfish required longer time to recover from anaesthesia, which may attributable to the nature of anaesthetic agent and its clearance rate from fish tissue.
In addition, our results showed that the induction time decreased and recovery time increased with any increments in eugenol concentration. In other words, there was a negative correlation between recovery and induction time. In contrast, investigating the effect of eugenol and 2-phenoxyethanol on Dicentrarchus labrax and Sparus aurata, Mylonas et al. (2005) found that longer exposure to anaesthetic agents led to more drug absorption and subsequently lengthened the recovery time. However, Roubach et al. (2005) and Stehly & Gingerich (1999) could not find noticeable relationships between body weight and induction or recovery time. Weyl, Kaiser & Hecht (1996) considered that such contradictory results might be attributable to interspecies differences. Moreover, with regard to the efficacy of anaesthetic agents in aquatics animal health state and stocking density, pH, water temperature, salinity, dissolved oxygen and water mineral content may also come into effect (Josa, Espinosa, Cruz, Gil, Falceto & Lozano 1992; Weyl et al. 1996; Stoskopf & Posner 2008). Nonetheless, it is conceivable that short time exposure to higher concentrations of anaesthetic agents may also lengthen the time required to regain the normal behaviour. Additionally, Weyl et al. (1996) declared that in comparison to exposure time, anaesthetic concentration is more influential on the recovery time, which was reconfirmed by considerably higher β values for eugenol concentration in the present study. With such speculation, it became apparent that faster absorption of anaesthetic agents in smaller fish may lead to quick anaesthesia due to relatively larger gill or body surface to body volume, and subsequently fish may uptake lower quantity of the chemical via gills via gill epithelia. As a result, one might contemplate that smaller fish would recover from anaesthesia more quickly than larger ones, as the rate of anaesthetic clearance from fish body could be a function of concentration gradient and gill surface area to body volume ratio (Javahery et al. 2012; Tarkhani et al. 2016), and fish would recover as soon as the concentration of anaesthetic agent drops to a certain level in their tissues/bodies (Weyl et al. 1996; Javahery et al. 2012). Consistent with our results, previous studies on Gadus morhua (Zahl, Kiessling, Samuelsen & Hansen 2009), D. sargus and Diplodus puntazzo (Tsantilas, Galatos, Athanassopoulou, Prassinos & Kousoulaki 2006) and Ictalurus punctatus (Small 2003) showed that the recovery time was related to body weight or induction time.
Regression analyses revealed that fish with different body size may differently response to anaesthetic agent, to the extent that for smaller fish (1 and 5 g) time required to recover from anaesthesia was mainly affected by the interaction of fish body size and eugenol concentration, while in larger fish (10 g), it was the concentration of eugenol which predominantly affected recovery time. Unfortunately, we did not include larger fish individuals (> 10 g) in our study to fully picture the fish behaviour to eugenol in larger body sizes. Similarly, it has been shown that fish weight and length may also affect the efficacy of anaesthetic and sedative agents (Ross & Ross 2008). Therefore, it is important to examine the dynamics of eugenol absorption, excretion and/or metabolism in angelfish during the induction time and recovery period to thoroughly characterize the extent, quality and magnitude of induction time, anaesthetic concentration and fish size on recovery time and the anaesthetic efficacy of eugenol.
Based on the literature, anaesthesia with eugenol can cause hypoxaemia (75% decrease in arterial oxygen) with subsequent depression to the CNS (Stoskopf & Posner 2008). The situation may lead to medullary collapse and death (i. e., euthanasia) (Kegley, Conlisk & Moses 2010). For instance, mortality due to using high anaesthetic concentrations has been reported by Tsantilas, Galatos, Athanassopoulou, Prassinos & Kousoulaki (2006) and Waterstrat (1999). It is possible that eugenol covers the body and gill surface of fish causing suffocation if used extensively (Sladky et al. 2001). In the present study, no mortality was observed; however, as 7.5 mg/L eugenol may cause angelfish to collapse (with recovery time of near critical threshold of 10 min) and is not suggested to apply to the species. Similarly, no mortality with eugenol was reported on other species such as rainbow trout (Keene et al. 1998), channel catfish (Waterstrat 1999) and kelp grouper (Park et al. 2008).
In conclusion, our results suggested eugenol as an effective and safe anaesthetic agent for different sizes of angelfish. However, none of the studied concentrations of eugenol were advisable for live fish transport as fish would go beyond anaesthesia stage 1 suitable for avoiding physical damage from collision with the container walls and conspecifics (McFarland 1960). It seems that the effective eugenol concentration to induce stage 1 might be less than 1.25 mg/L, which requires further investigation. However, 5.5 mg/L eugenol was the best concentration for angelfish, and further research was quarantined regarding the efficacy of eugenol at different temperatures and determining its lethal concentration for the species.




