Evaluation of culture of the mussels Choromytilus chorus and Aulacomya ater (Molina) in northern coasts of Chile
Abstract
The culture of Aulacomya ater and Choromytilus chorus in northern Chile was evaluated by collecting spat from both species from their natural environments and culturing them in an area isolated from natural populations. The spat collection at the site selected allowed the establishment of four cohorts of A. ater and six cohorts of Ch. chorus after 131 days. These cohorts were transported 250 km south for further growth, resulting in 290 ropes of Ch. chorus and 30 ropes of A. ater with an average of 900 specimens with a 13.5 mm mean size. After 29 months of cultivation, the yield was 623.7 ± 62.2 specimens of A. ater and 575.3 ± 49.3 specimens of Ch. chorus per rope of 3.5 m long. The growth attained during this period indicated values of L∞ = 136 mm and k = 0.58 for Ch. chorus and L∞ = 107 mm and k = 0.66 for A. ater. The Ch. chorus specimens reached greater lengths at month 15 than the A. ater specimens with the same age. For the total length–weight ratio and the soft part length–weight ratio for specimens above 60 mm in length, A. ater presented higher weights at the same lengths than Ch. chorus. We established that Ch. chorus reached its optimum soft part weight after 19 months and at a size of 82 mm, whereas this optimum was reached by A. ater after 18 months and at 68 mm. We concluded that farming both species in northern Chile was viable.
Introduction
Recently, Chile has become the world's second largest producer of farmed mytilidae. Chile reached a production of 244 137 tons of Mytilus edulis, 1995 tons of Aulacomya ater and 339 tons of Choromytilus chorus in 2012. These amounts represent 13.5% of the global aquaculture production (1 828 845 tons) produced that year (FAO, 2014). In Chile, the farming of these species is exclusively sustained by spat collection in natural environments, of which 99% are concentrated in the Lakes Region (40°S). In contrast, no mytilid farms are located in the Humboldt Current upwelling system in northern Chile. Nevertheless, between 1973 and 1981 A. ater was cultured in the Mejillones del Sur Bay (23°S), where it reached a production level higher than 500 tons per year (Avendaño 1984). This species is distributed along the Pacific coastline from El Callao, Peru, to the Strait of Magellan in Chile and throughout the Atlantic coastline from the south of Argentina to southern Brazil (Cancino & Becerra 1978; Navarro & Gutiérrez 1990; Zaixso 2004). The species can also be found along the South African coast (Griffiths 1977) and is the most significant mytilid species farmed at the commercial level in northern Chile.
Choromytilus chorus has been reported from Callao, Peru, to the Strait of Magellan and the Beagle channel in Chile and following the Atlantic coast up to southern Brazil and including the Falkland Islands (Osorio 2002). In Chile, the highest concentrations have been found in the channel area south of Puerto Montt (ca. 42°S) (Hancock 1969; Ramorino 1974) and north of the Coquimbo Region (ca. 30°S) (Bellolio, Toledo & Dupré 1996). Until the end of the last century, there were no records of this species from the northern area of the country (ca 23°S) to Peru. However, Avendaño and Cantillánez (2011) confirmed its recovery in the shallow area of the Big North of Chile at the beginning of 2000, which provoked the displacement of A. ater to the deeper areas and banks.
Mytilid farming in Chile is concentrated between the parallels 40°13′ and 44°3′ south in the Lakes Region, which is where 60% of Chilean aquaculture production is conducted (Sernapesca 2014). This concentration has led to a saturation in adequate sites for the expansion of the growth of this activity. The increase in the farming period of Mytilus chilensis (Hupé) in the past and the sparse supply of its spat from natural environments have enhanced the possibilities for other regions, such as the upwelling areas of northern Chile, to participate in mytilid aquaculture production (Subpesca 2013).
Chile has recently established improved access to Small Scale Aquaculture (Acuicultura de Pequeña Escala – APE) and Areas for Management and Exploitation for Benthic Resources (áreas de manejo y explotación de recursos bentónicos – AMERBs) as one the primary challenges of the country's aquaculture sector and a productive alternative for coastal and artisanal fishery communities. Accordingly, in 2013, the Chilean government proposed a bill creating the Institute for the Development of Artisanal Fisheries and Small Scale Aquaculture (Instituto de Desarrollo de la Pesca Artesanal y de la Acuicultura de Pequeña escala – IDEPA). In this context, this study on experimental farming is focused on the re-adoption of A. ater cultivation and the development of integrated cultivation with Ch. chorus evaluating your feasibility in the Chilean Far North. Our strategy involved the transfer of technology to coastal areas that are currently linked to artisanal fisheries.
Material and methods
Study area
The study was conducted in two localities in the Antofagasta region (Chile): Punta Arenas Cove and Errázuriz Cove (Fig. 1). Punta Arenas Cove (21°38′S; 70°09′W) has a natural bed of A. ater distributed from 15 m down to depths beyond 30 m. Choromytilus chorus inhabits sectors in the shallower areas that range from 4 m to 13 m in depth (Avendaño & Cantillánez 2011). The presence of both species permits the spat to anchor onto the collectors in this area (Avendaño & Cantillánez 2013), which is why this area was selected for collection. Errázuriz Cove (23°24′S; 70°35′W) is located 250 km south of Punta Arenas Cove and does not have beds with these resources; however, its protected bay conditions have permitted the existence of an authorized centre for the farming of the pectinid Argopecten purpuratus (Lamarck) (Avendaño & Cantillánez 2013), which led it to be chosen as a place to cultivate both species.
Oceanographically, both locations are in a subtropical transition area where a mass of sub-Antarctic water (SAW) dominates the upper 200 m of the northern branch of the prevailing Humboldt cold current during normal years. These SAW waters are mixed with a smaller proportion of subtropical waters (STW) with greater salinity and temperature. These waters also periodically mix with cooler waters from deeper areas corresponding to equatorial subsurface waters (ESW) that ascend towards the coast due to upwelling induced by southern and south-western winds (Escribano, Rodríguez & Irribarren 1995).
These phenomena, which include the mixture of ESW and SAW waters during spring, summer and sometimes autumn, bring nutrients to the upper levels and permit the existence of typical phytoplankton communities in the upwelling areas (Avaria & Muñoz 1982; Rodríguez 1987; Rodriguez & Escribano 1996). On the microscale, the area is characterized by alternating current fluxes as a consequence of the predominance of moderate intensity winds from the south and south-west that result in a predominant north-eastern current. This situation is the case in the Punta Arenas Cove, which meets the barrier created by its beach (Avendaño, Bórquez, Follegati, Marín, Porflitt, Ramos, Rodriguez, Tapia & Zuñiga 1988).
Collection, fattening and harvest experiments
Based on the previous significant spawning events reported for A. ater in the month of November at Punta Arenas Cove (Avendaño & Cantillánez 2013), a survey of the planktonic assemblages was conducted upon the installation of the collectors on 4 November 2010, to ascertain the presence and state of their larvae. Once their presence was verified, a second sampling was conducted two weeks later to detect whether there had been an increase or decrease in these larvae in the area. Triplicate samples were obtained along an 18 m deep water column by vertically pulling a 53-μm plankton net. These samples were subsequently transported to the laboratory, where the larvae were identified, counted and measured following the methods of Avendaño, Cantillánez, Le Pennec, Varela and Garcia (2011).
The population size structure of the larvae of both mytilidae during both sampling surveys was later subjected to a discriminant cohort analysis (Avendaño et al. 2011) to identify the number of microcohorts, their mean sizes and their representativeness using the program MIX 3.1a (MacDonald & Pitcher 1979). Size frequency histograms were represented based on a normal distribution (significance level = 0.05).
The installed collectors consisted of a 0.10 m wide by 12 m long anchovy net fringe tied to a long line that was placed in an 18 m water column. These collectors were suspended from a depth of 1 m and distributed every 0.8 m along the line. To maintain their verticality, a sinker was affixed to their lower end (Avendaño 1984). The long line consisted of a 100 m long double line holding 39 cylindrical buoys with a 350 L displacement that was anchored using concrete blocks.
To evaluate the postlarval settling efficiency in the collectors, one collector was extracted randomly after 28 days and transported to the laboratory. Three 100 cm2 sections were obtained from the upper, medium and lower parts. These sections were washed separately over a 180 μm mesh to detach all fixed postlarvae (Cantillánez, Thouzeau & Avendaño 2007). The postlarval concentrate obtained from each section was homogenized in a 10-division plankton splitter. Two subsamples of 1/10 each were fixed in 70% alcohol and analysed under a stereomicroscope with a graduated eyepiece. The postlarvae were identified to the species level, measured and counted (Avendaño, Cantillánez, Thouzeau & Peña 2007). The mean value of attached postlarvae per species standardized per 600 cm2 of collector was obtained with the values recorded in the three analysed sections of the collector (considering that fixation occurred on both sides of each section).
The size structure shown by the spat measured for each mytilid species was analysed to identify microcohorts of fixed postlarvae using the program MIX 3.1.a (MacDonald & Pitcher 1979). The size frequency histograms built with 50 μm intervals were broken down following a normal distribution (significance level = 0.05) to provide the mean size and proportion of each identified group (Avendaño & Cantillánez 2013).
After the collectors remained in place for 131 days in Punta Arenas Cove, the fixed spat were extracted from eight of them. A total of 250 kg of spat were obtained and transported in a covered vehicle to the farming site in Errázuriz Cove. Once there, the spat were deposited in a semi-submerged cage and separated by species. They were sowed on a rope (stringing), hung from a long line with the same characteristics as the line used for the collection and deployed in a 16 m deep water column.
Before stringing the spat, the maximum length of a representative sample of approximately 300 specimens per species was measured to discriminate the number of cohorts that were eventually fixed in the collectors and to record the mean size of the initial cultures. Stringing was manually performed using a sowing tray and the modified ‘French system’ technique currently used in southern Chile. Briefly, a 15 cm wide by 3.5 m long piece of anchovy net was assembled with the spat wrapped inside a tubular cotton hose. This hose degrades naturally and provides time for the spat to attach to the central rope. Additionally, each rope was attached to a sinker at its base to maintain its verticality. The ropes of each species were hung from the culturing surface with 60 cm of separation and maintained in suspension from March 2011 to August 2013. No manipulation of the rope was conducted to avoid detaching the specimens. To establish the size structure and estimate their growth, the total length of a sample of >100 individuals obtained from one of the ropes was recorded at different time points. At the same time, the total weight and the weight of the soft parts was determined from 20 representative specimens from each of these samples.
Size and mortality estimations
The size estimations of both species were conducted by modal monitoring of the length structures of the farmed specimens. The specimens conformed to unimodal structures because they were sown in cultivation ropes. Their growth was modelled using the von Bertalanffy equation Lt = L∞ (1 − e−k(t – t0)). The equation parameters were calculated by minimizing the sum of the squares into the observed length, which was equivalent to the mean monthly length, and the length expected under von Bertalanffy's equation. The square minimization procedure is given by SSM = [Lt − L∞ (1 − e−k(t – t0))]2, where Lt is the mean length of the cohort observed at a certain age (months). The algorithm gives the growth parameters and the sum of the residual squares for each iteration. The iterative process is stopped when the SSM is minimized. Adjustments were performed using the solver routine included in Excel with the nonlinear GRG (Grade Residual Gradient) optimization method. The growth index phi prime (Φ′), defined as: Φ′ = log10 (L∞) + 2 log10 K (Pauly & Munro 1984), was used to measure growth performance. This criterion was chosen because the negative correlation between K and L∞ (Pauly & Munro 1984) invalidates comparisons based on individual parameters.
The length–total weight (PT) and length–soft part weight (PPB) ratios of both species were established via a potential regression following the equation W = aLb, where W is weight (g), L is the length (mm), a is the regression constant and b is the regression growth coefficient.
During the last sampling, the final yield of the cultivation ropes from each species was obtained by weighing and counting the number of specimens present in three ropes. The total natural mortality (M) of the cohorts of both species was estimated from the difference between the initial size (N0 = 900 seeds) and the size present after 29 months (Nt) using the formula Nt = N0*e−Mt, where the parameter M was expressed in monthly terms (Ricker 1975).
Optimal time – length harvest estimation
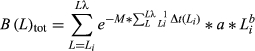
The age–length ratio that maximizes the growth in the total weight and/or the soft part weight of a cohort (critical length) is determined when the derivative of the previous equation with respect to length equals 0 (Ricker 1975).
Temperature in the experimentation areas
Data loggers (Tidbit, v2 Temp model UTBI-001, Onset Computer Corporation, Bourne, MA, USA) were installed at a depth of 3 m to detect cold water upwelling at both the farming site and the collector installation location. The data loggers were programmed to record temperature measurements every hour throughout the respective experimental periods at each location.
Results
Collection, spat harvest and fattening
The results of the plankton surveys conducted in Punta Arenas Cove indicated the presence of substantial larval groupings of both A. ater and Ch. chorus (Table 1). During the first survey, A. ater reached an average amount of 998 ± 175 larvae m−3 composed of two microcohorts whose mean lengths ranged from 330.1 to 207.2 μm. Choromytilus chorus reached an average quantity of 251 ± 136 larvae m−3 and was composed of three microcohorts with average lengths that varied between 356.2 and 137.1 μm (Table 1). During the November 18th survey, the amount of A. ater larvae declined to 226 ± 93 larvae m−3 and the number of Ch. chorus larvae increased to 4531 ± 1719 larvae m−3. Both species maintained the number of cohorts identified during the first survey (Table 1).
| Date | Mean larvae per m3 ± SD | Cohort 1 | Proportion (%) | Cohort 2 | Proportion (%) | Cohort 3 | Proportion (%) |
|---|---|---|---|---|---|---|---|
| Length ± SD (μm) | Length ± SD (μm) | Length ± SD (μm) | |||||
| A. ater | |||||||
| 11-04-2010 | 998 ± 175 | 330.1 ± 6.0 | 8.1 | 207.2 ± 1.4 | 91.9 | – | – |
| 11-18-2010 | 226 ± 93 | 287.7 ± 23.2 | 21.7 | 127.2 ± 19.3 | 78.3 | – | – |
| Ch. chorus | |||||||
| 11-04-2010 | 251 ± 136 | 356.2 ± 27.8 | 30.4 | 237.3 ± 25.1 | 57.5 | 137.1 ± 25.1 | 12.1 |
| 11-18-2010 | 4531 ± 1719 | 355.8 ± 24.0 | 55.7 | 248.0 ± 26.8 | 14.6 | 140.2 ± 24.8 | 29.7 |
The analysis of the collector installed at the time point when the larval presence was recorded and retrieved after 28 days of submersion indicated the fixation of both A. ater and Ch. chorus.
The amount of fixed A. ater spat reached 4183 ± 1725 indiv. * 600 cm2 collector−1 and showed a size structure that allowed the discrimination of the two microcohorts, whose sizes varied between 985.9 and 457.7 μm (Table 2). In turn, Ch. chorus reached an average amount of 11 400 ± 2610 * 600 cm2 collector−1 with a size structure that allowed the detection of three microcohorts with average sizes varying between 1382.5 and 403.6 μm (Table 2). The same size structure analysis conducted on the collectors retrieved 131 days later indicated the existence of 6 microcohorts of Ch. chorus with average sizes ranging between 31.6 and 3.6 mm (Table 2). For A. ater, the existence of four microcohorts was detected with average sizes ranging between 22.2 and 7.9 mm (Table 2).
| C 1 | C 2 | C 3 | C 4 | C 5 | C 6 | ||
|---|---|---|---|---|---|---|---|
| A. ater | |||||||
| T1 = 28 days | μ (μm) | 985.9 ± 0.15 | 457.7 ± 75.1 | ||||
| π (%) | 12.7 | 87.3 | |||||
| T2 = 133 days | μ (mm) | 22.2 ± 2.3 | 17.7 ± 2.0 | 12.8 ± 2.1 | 7.9 ± 2.1 | ||
| π (%) | 4.4 | 28.7 | 46.3 | 20.6 | |||
| Ch. chorus | |||||||
| T1 = 28 days | μ (μm) | 1382.5 ± 181.2 | 758.5 ± 105.8 | 403.6 ± 61.2 | |||
| π (%) | 5.5 | 77.4 | 17.1 | ||||
| T2 = 133 days | μ (mm) | 31.6 ± 2.3 | 26.3 ± 2.2 | 16.2 ± 2.3 | 12.2 ± 2.3 | 8.5 ± 1.5 | 3.6 ± 0.9 |
| π (%) | 3.4 | 4.6 | 37.3 | 31.8 | 19.8 | 3.1 | |
- Tn = immersion time; μ = average size in μm and mm; π = percentage proportion of the cohort.
The separation of the spat collected from the collectors and transported to Errázuriz Cove in March 2011, at the species level indicated that 90.6% corresponded to Ch. chorus and had a mean length of 13.5 ± 6.1 mm. The remaining 9.4% belonged to A. ater and had a mean length of 13.6 ± 4.3 mm. Once the sowing started, each rope of both species held a biomass of 0.78 kg of spat, which was equivalent to an average of 900 specimens. Seeding was conducted on 290 ropes for Ch. chorus and 30 ropes for A. ater.
Growth and mortality
Table 3 shows the statistics calculated on the unimodal distributions of the length structures of the specimens cultured over 29 months. The modelled growth curves for both species (Fig. 2) showed adjustments that were consistent with the mean observed values based on the low dispersion in lengths around the estimated length. The estimated parameters had a L∞ = 136 mm for Ch. chorus, with an annual growth coefficient of k = 0.58 and a L∞ = 107 mm for A. ater, with an annual growth coefficient of k = 0.66. While L∞ and K varied between the species, resulting in a similar growth performance index (Φ′ = 4.03 for Ch. chorus and Φ′ = 3.88 for A. ater). Both species showed similar levels of growth during the first three months of culture; afterwards, Ch. chorus showed higher increases in mean length compared to A. ater for the same age. These increases became more significant with deltas above 1 cm after 16 months of culture.
| Date | n | Ch. chorus | A. ater | |||
|---|---|---|---|---|---|---|
| length (mm) | SD | n | Length (mm) | SD | ||
| Mar 11 | 286 | 13.5 | 6.1 | 383 | 13.6 | 4.3 |
| Aug 11 | 366 | 34.3 | 7.3 | 168 | 29.2 | 4.7 |
| Nov 11 | 304 | 46.7 | 9 | 224 | 40 | 4.8 |
| Jan 12 | 281 | 57.4 | 8 | 297 | 49.8 | 5.1 |
| May 12 | 125 | 74.6 | 5.3 | 205 | 59.8 | 5.1 |
| Jul 12 | 310 | 75.4 | 6.2 | 356 | 63.3 | 5.6 |
| Oct 12 | 181 | 76.6 | 5.4 | 189 | 71.3 | 4.5 |
| Jan 13 | 186 | 90.7 | 7.7 | 112 | 78.9 | 4.9 |
| Aug 13 | 110 | 107.1 | 7.2 | 97 | 85.8 | 5.3 |
- Date = date of sampling, n = sample size, average length (mm), and SD = standard deviation.
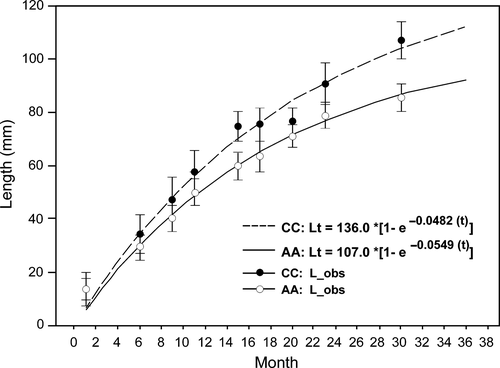
The total length–weight and length–soft part weight ratios for both species showed good isometric and allometric fits respectively (Fig. 3). From lengths of 60 mm onwards, A. ater showed higher total and soft part weights compared with Ch. chorus specimens of the same length. This trend was maintained to lengths up to 90 mm when the highest weights of Ch. chorus were found as a result of the greater lengths reached by this species.

The results obtained from the final analysis of harvested ropes after 29 months of cultivation yielded an average amount of 623.7 ± 62.2 specimens of A. ater per rope, which corresponded to 69.3% of the sowed spat, and 575.3 ± 49.3 specimens of Ch. chorus, which accounted for 63.9% of the amount sowed (Table 4). The average gross weight of the ropes of A. ater was 51.3 ± 2.1 kg, which yielded 44.0 ± 3.0 kg (85.8%) of the product. The average gross weight of the Ch. chorus ropes reached 62.0 ± 4.4 kg and generated 52.7 ± 3.5 kg of the product (85%). These results were mirrored in the higher mortality estimated for Ch. chorus (M = 0.448) compared to A. ater (M = 0.367) for the entire cultivation period (Table 4).
| Species | Ni | Nf | T (months) | Total mortality (e) | Monthly mortality |
|---|---|---|---|---|---|
| A. ater | 900 | 623.7 | 29 | 0.367 | 0.0126 |
| C. chorus | 900 | 575.3 | 29 | 0.448 | 0.0154 |
- Ni = average number of organisms at the beginning of the culture, Nf = average number of organisms at the end of the culture period, T = time of culture.
Time – optimal harvest length
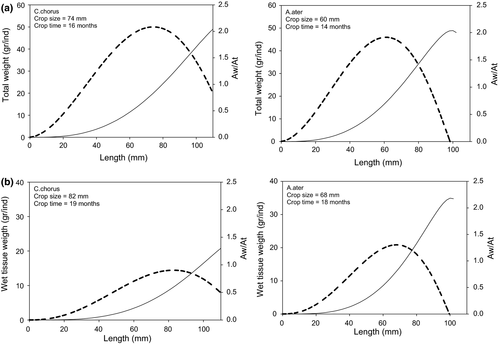
The optimal age–harvest length estimation as a function of the biomass (Fig. 4) indicated that Ch. chorus reached an optimal biomass at 74 mm, which corresponded to an age of 16 months. Aulacomya ater presented the highest biomass at 60 mm, which corresponded to an age of 14 months. When only the soft parts were considered, the differences between the species persisted; however, the harvest length increased to 19 months for Ch. chorus (82 mm) and 18 months for A. ater (68 mm). Due to the greater size at the age reached by Ch. chorus compared with A. ater, the latter presented higher yields at 90 mm.
Environmental parameters
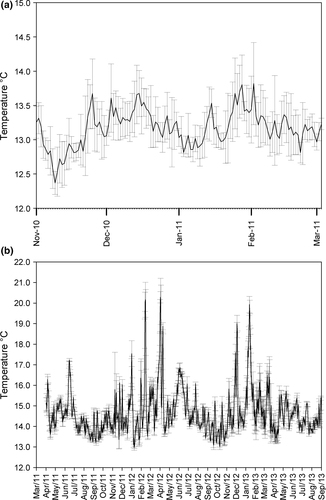
The temperature recorded at Punta Arenas Cove showed daily averages during the period of spat collection that ranged from 14.5 to 12.7°C (Fig. 5). Substantial decreases (>1°C) were observed on November 9th and December 1st and 24th 2010, and January 3rd and 19th 2011. On these dates, the average temperatures reached values of 12.7 and 13.4°C. In contrast, the daily mean temperatures at Errázuriz Cove during the cultivation period varied between 20.3 and 12.9°C. Continuous cold water upwelling events were recorded that were similar to the collection area. The largest variations (6.65 and 4.21°C respectively) occurred in February, April and December 2012, and January 2013 (Fig. 5).
Discussion
The presence of A. ater larvae at different developmental stages and the appearance of a new cohort in its initial stage after 14 days considerably increased the larval count of this species at the collection site and confirmed the significance of this species' spawning during November at this location (Avendaño & Cantillánez 2013). At the same time, the presence of different larval stages during both surveys, including postlarval stages of both A. ater and Ch. Chorus, helped to categorize this location as a larval retention site because both species metamorphosed at approximately 260 μm (Ramorino & Campos 1983). The presence of areas where currents concentrate larvae not only contributes to the study of the larval and postlarval dynamics of bivalve mollusks by generating important information regarding their spatial, temporal and larval settling intensity patterns and the growth and survival of the attached spat (Avendaño, Cantillánez & Peña 2006; Cantillánez et al. 2007) but may also be decisive in deciding where to locate the collectors supplying larvae to farms (Tremblay & Sinclair 1991).
The capacity for larval retention at the site selected for spat supply was established in this study based on the detection of the attachment of two postlarval cohorts of A. ater and Ch. chorus in the collectors 28 days after the first larval sampling was conducted. The analysis of this attachment showed that the Ch. chorus fixation was 172% higher than that of A. ater. Moreover, the Ch. chorus cohorts presented greater mean lengths than A. ater, which was in agreement with the results of Avendaño and Cantillánez (2013). However, the greater lengths shown by Ch. chorus in the collectors and its higher abundance had a significant negative impact on A. ater's survival as evidenced by the decrease in the number of specimens harvested 133 days later when only 9.4% of the spat was collected. Several authors have indicated spatial-trophic competition as the reason for the survival and growth of the target species (Ventilla 1982; Mason 1983; Thouzeau 1995; Narvarte, Félix-Pico & Ysla-Chee 2001; Avendaño et al. 2006, 2007; Filgueira, Peteiro, Labarta & Fernández-Reiriz 2007). The efficiency of the competition observed for Ch. chorus in this study was also detected in the re-established natural beds in northern Chile, where A. ater was displaced to depths beyond 15 m when occupying the shallow (4–13 m) strata (Avendaño & Cantillánez 2011).
The pronounced differences in the mean lengths of the specimens from the first and last cohorts of Ch. chorus and A. ater in the collectors after 131 days indicated that the attachments continued as long as the collectors were kept in the water, thereby confirming this area as a larval retention site. This finding enables the implementation of massive spat supply programmes in the area. Increases in pectinid spat collection during prolonged periods of collector submersion have been reported for Pecten maximus (Linnaeus), Aequipecten opercularis (Linnaeus), Chlamys varia (Linnaeus) and A. purpuratus (Román & Cano 1987; Avendaño, Cantillánez & Thouzeau 2008).
The growth parameters recorded during the culture of both mytilidae in this study showed that A. ater grew faster than Ch. chorus based on their growth coefficients (0.66 and 0.58 respectively), although the latter reached greater lengths than A. ater (L∞ = 136 mm and L∞ = 107 respectively). Pauly and Munro (1984) indicate that species of the same family have close values of growth performance index (Φ′), indicating a similar growth pattern for both species studied.
These results also indicated that the average length of Ch. chorus (70 mm) after 15 months of cultivation was noticeably greater than that attained by A. ater at the same age (60 mm). These results differed from those reported in suspended Ch. chorus cultures in southern Chile, where 60 mm lengths were reached after 24 months (Osorio 2002), which was double the time required in the present study, and from the growth rates found for natural populations of A. ater in Antofagasta (k = 0.25 and L∞ = 103 mm) reported by Solis and Lozada (1971). These authors also indicated similar growth rates between natural populations of A. ater in Antofagasta and those located in the south (Chiloé), although here they reached greater lengths (L∞ = 173). Different growth rates between Choromytilus meridionalis (Krauss) and A. ater have also been reported for populations from the Malgas and Marcus islands (South Africa), with growth coefficients varying between 0.58 and 0.98 for the first species and between 0.1 and 0.31 for the second (Barkai & Branch 1989).
Variations in bivalve growth rates between sites, seasons and depth ranges have been primarily attributed to variations in temperature and food availability (Bricelj & Shumway 1991; Emerson, Grant, Mallet & Carver 1994; Lodeiros, Maeda-Martínez, Freites, Uribe, Lluch-Cota & Sicard 2001). Accordingly, the constant upwelling phenomena affecting the study area (Escribano et al. 1995), the development of phytoplankton throughout the year (Cantillánez 2000; Herrera & Escribano 2006) and the plankton retention in the area as a result of coastal water confinement favoured by upwelling systems (Graham, Field & Potts 1992; Graham & Largier 1997; Escribano & Hidalgo 2001) would be responsible for the higher growth obtained in our study. Permanent cold water upwellings that bring nutrients to the upper levels that allow the phytoplankton to develop during the spring, summer and sometimes autumn (Avaria & Muñoz 1982; Rodriguez & Escribano 1996; Cantillánez et al. 2007) have been established by temperature recordings from the fattening locations. At the same time, the five cold water upwelling episodes recorded during the time period when the collectors were submerged at the collection site (which were used in this study as indicators of upwelling) strengthened the aforementioned arguments regarding their larval retention capacity. This capacity would be favoured by the north-east current predominating at the microscale that encountered the barrier set-up by the beach. This set-up would further contribute to the permanent presence of larvae throughout the year and their continuous settling in the collectors reported by Avendaño and Cantillánez (2014). However, a study to determine the retention time of particulate matter at this site is still lacking.
The results obtained regarding the length–total weight and length–soft part weight relationships showed that A. ater showed higher weights at lengths greater than 60 mm compared to Ch. chorus. However, the harvest time as a function of their soft part weight yields indicated that the optimum value was reached at 68 mm (i.e. at 18 months of age). In contrast, due to its lower soft part productivity after 60 mm, Ch. Chorus reached its optimum harvest time at 19 months of age and 82 mm in length. These results differed from the 60 mm in length at which Ch. chorus was harvested in farms in southern Chile and from the 70 mm indicated for A. ater (Winter, Toro, Navarro, Valenzuela & Chaparro 1984; Navarro & Gutiérrez 1990). The optimum harvest times estimated in the present study suggested that the commercial farming of both species in northern Chile should be established at 18 months, which was 36% less than the 29 months assessed in this study. This incubation time would have a positive effect on both cultures by reducing hand production costs and the losses recorded for these species in this study (30.7% for A. ater and 36.1% for Ch. chorus during the 29 months of the experiment). Moreover, the development of this activity in the Humboldt Current upwelling system of northern Chile would not only profit from the higher productivity of its waters but also expand bivalve aquaculture in the country, thereby ameliorating some of the current problems affecting southern Chile such as shorter cultivation times.
The results point to the viability of initiating A. ater and Ch. chorus cultivation in northern Chile due to the feasibility of obtaining spat through natural collections. These findings indicate that this activity can be performed on a commercial scale because it satisfies fundamental criteria for establishing a market, such as the continuous and sufficient supply of raw matter to sustain the bivalve farming productive chain. However, given the predominance of Ch. chorus during the collection of both species, it is necessary to conduct new studies to optimize the survival of the A. ater spat attached to the collectors. These studies will contribute to innovation in collection technologies or the implementation of new studies to define different attachment depths based on their stratification in natural beds.
Acknowledgments
The present study was conducted under the INNOVA Project Code 07CT91DM-56.




