Comparative study on immunomodulatory and growth enhancing effects of three prebiotics (galactooligosaccharide, fructooligosaccharide and inulin) in common carp (Cyprinus carpio)
Abstract
This study investigated the effects of different prebiotics, including galactooligosaccharide (GOS), fructooligosaccharide (FOS) and inulin (INL), on skin mucosal immune parameters, humoral immune responses as well as performance of common carp (Cyprinus carpio). Two hundred and forty specimens (13.85 ± 0.85 g) were stocked in 12 fibreglass tanks assigned into three treatments and a control group. The experimental diets were formulated to have equal level (2%) of the prebiotics. At the end of the feeding trial, the highest skin mucus lysozyme activities and total immunoglobulin (total Ig) were observed in GOS-fed group (P < 0.05). However, skin mucus protease activity showed no significant difference among different dietary groups (P < 0.05). Blood respiratory burst activity was significantly increased in all prebiotic-fed fish compared to the control group (P < 0.05); the highest activity was observed in GOS treatment. Furthermore, evaluation of humoral immune response revealed that feeding with GOS-supplemented diet significantly increased lysozyme and alternative complement (ACH50) activity as well as total Ig compared to the control and other prebiotic groups. While no significant difference was observed between FOS and INL groups, common carps fed GOS-supplemented displayed improved (P < 0.05) growth performance, including final weight, weight gain, specific growth rate (SGR) and feed conversion ratio (FCR), compared to the control treatment. These results revealed that different prebiotics modulate carp growth and immune response in different manner, and GOS seems to be the most suitable prebiotic.
Introduction
It is now well established that gut microbiota composition strongly affects the health status of an organism (Gómez & Balcázar 2008; Nayak 2010a; Pérez, Balcázar, Ruiz-Zarzuela, Halaihel, Vendrell, De Blas & Múezquiz 2010; Llewellyn, Boutin, Hoseinifar & Derome 2014; Pohlenz & Gatlin 2014; Song, Beck, Kim, Park, Kim, Kim & Ringø 2014; Hoseinifar, Esteban, Cuesta & Sun 2015). Recently, extensive researches have been conducted to shift gut microbiota towards an ideal composition for improvement of host health (Dimitroglou, Merrifield, Carnevali, Picchietti, Avella, Daniels, Guroy & Davies 2011; Daniels & Hoseinifar 2014; Hoseinifar, Ringø, Shenavar Masouleh & Esteban 2014; Lauzon, Dimitroglou, Merrifield, Ringø & Davies 2014; Ringø, Dimitroglou, Hoseinifar & Davies 2014). Dietary administrations of various probiotics, prebiotics and their combination (synbiotics) have been practiced for this purpose (Daniels & Hoseinifar 2014; Hoseinifar, Ringø et al. 2014; Lauzon et al. 2014; Ringø, Dimitroglou et al. 2014). Probiotics are desired bacteria species that can be incorporated in diet to deliver and colonize in host gut; however, their viability during passage through gastrointestinal tract and after the supplemented diet cease is not always supported (Robertson, O'Dowd, Burrells, Williams & Austin 2000; Panigrahi, Kiron, Puangkaew, Kobayashi, Satoh & Sugita 2005). Prebiotics are non-digestible material for fish but metabolizable by gut microbiota (Ringø, Dimitroglou et al. 2014); therefore, dietary prebiotic supplementation is used to increase gut beneficial bacteria populations. A variety of prebiotics have been studied for their beneficial effects in human and domestic animals suggesting that each bacterium potentiates to metabolize certain prebiotics (Biedrzycka & Bielecka 2004). Fish gut microbiota is different of those of terrestrial animals and human (Merrifield, Balcázar, Daniels, Zhou, Carnevali, Sun, Hoseinifar & Ringø 2014; Romero, Ringø & Merrifield 2014); therefore, they may need prebiotics other than those needed by human and other animals. In this regard, numerous studies showed beneficial effects of prebiotics on growth performance, stress resistance, immune stimulation and disease resistance (Kiron 2012; Oliva-Teles 2012; Daniels & Hoseinifar 2014; Hoseinifar, Ringø et al. 2014; Lauzon et al. 2014; Ringø, Dimitroglou et al. 2014; Song et al. 2014; Dawood, Koshio, Ishikawa & Yokoyama, 2015; Dawood, Koshio, Ishikawa, Yokoyama, El Basuini, Hossain, Nhu, Moss, Dossou & Wei, 2015). However, some negative effects of dietary prebiotic were also reported in different fish species (Olsen, Myklebust, Kryvi, Mayhew & Ringø 2001; Akrami, Hajimoradloo, Matinfar & Abedian Kinari 2009; Hoseinifar, Mirvaghefi, Mojazi Amiri, Rostami & Merrifield 2011), suggesting that the effect of prebiotics on fish is case-dependent. Different prebiotics are metabolized by different bacteria with different extends. Prebiotics with higher bacterial metabolization rate provide more feed for the bacteria, thus extend their colony in the host gut. Therefore, the beneficial effects of a prebiotic in fish depend on species and specific gut microbiota.
Common carp (Cyprinus carpio) is an important aquaculture species in many countries (FAO, 2014). The effects of some prebiotics were studied in common carp, including chitosan, mannan, glucans, FOS, short-chain FOS (Sc-FOS) and INL (Gopalakannan & Arul 2010; Ebrahimi, Ouraji, Khalesi, Sudagar, Barari, Zarei Dangesaraki & Jani Khalili 2012; Lin, Mao, Guan, Luo, Luo & Pan 2012; Kühlwein, Emery, Rawling, Harper, Merrifield & Davies 2013; Gupta, Pal, Sahu, Saharan, Mandal, Prakash, Akhtar & Prusty 2014; Hoseinifar, Soleimani & Ringø 2014; Ringø, Dimitroglou et al. 2014; Eshaghzadeh, Hoseinifar, Vahabzadeh & Ringø 2015; Hoseinifar, Eshaghzadeh, Vahabzadeh & Peykaran Mana, 2015). Readers with special interest on prebiotic administration in carp aquaculture refer to a recently published paper in this field (Dawood & Koshio 2016). To the best of our knowledge, there is no study on the effect of dietary GOS on growth performance and immune status in common carp. Also, as mentioned above, the effects of prebiotics on fish are case-dependent; thus, for comparison of different prebiotics, it is necessary to test them under similar experimental conditions. In this study, three prebiotics, FOS, GOS and INL, were incorporated into carp diet, at the same levels, and were fed to the fish under similar experimental conditions. The aim of this study was to determine the effects of these different prebiotics on cutaneous mucosal immune response and serum non-specific immune parameters as well as performance in common carp.
Material and Methods
Fish culture and feeding trial
A total number of 240 common carps with an average weight of 13.85 ± 0.85 g were randomly distributed into 12 fibreglass tanks (20 fish per tank). The tanks dimensions were 0.8 × 0.6 ×0.5 m, filled with 144 L dechlorinated tap water. The fish were allowed acclimatizing to the experimental conditions for 10 days. During the acclimation period, the fish were fed control diet (Table 1) twice a day at 2.5% of body weight. After acclimation, the tanks were divided into four triplicated experimental treatments: control, FOS, GOS and INL. Each treatment was fed with its corresponding diet (Table 1) for further 60-day growth trial. Treatments were investigated under static aerated water conditions with a 50% water change every day (tanks were siphoned to remove faeces). The water quality parameters including temperature, dissolved oxygen and pH were monitored daily and maintained at optimum levels.
| Ingredients | Experimental diets | |||
|---|---|---|---|---|
| Control | FOS | GOS | INL | |
| Soybean meal | 20.5 | 20.5 | 20.5 | 20.5 |
| Fish meal | 36.2 | 36.2 | 36.2 | 36.2 |
| Wheat meal | 33.8 | 33.8 | 33.8 | 33.8 |
| Soybean oil | 6.8 | 6.8 | 6.8 | 6.8 |
| Mineral mix | 0.29 | 0.29 | 0.29 | 0.29 |
| Vitamin mix | 0.19 | 0.19 | 0.19 | 0.19 |
| Cellulose | 2 | – | – | – |
| FOS | – | 2 | – | – |
| GOS | – | – | 2 | – |
| INL | – | – | – | 2 |
| Proximate composition (%) | ||||
| Moisture | 10 | 10 | 10 | 10 |
| Crude protein | 38.8 | 38.8 | 38.8 | 38.8 |
| Crude lipid | 8.82 | 8.82 | 8.82 | 8.82 |
| Crude ash | 8.5 | 8.5 | 8.5 | 8.5 |
Prebiotics and experimental diets
The prebiotics used in this study were non-digestible, but fermentable carbohydrates include galactooligosaccharide or GOS, fructooligosaccharide or FOS and inulin or INL. FOS and INL were kindly provided by Orafti (Raffinerie Tirlemontoise, Tienen, Belgium), and GOS was kindly supplied by Friesland Foods Domo Company (Zwolle, The Netherlands). According to Orafti fact sheets, INL is extracted from the chicory root and FOS is derived from INL through partial enzymatic hydrolysis. Based on Domo Company fact sheet, GOS was obtained from high-quality lactose using a proprietary enzymatic production technology. Experimental diets were prepared by supplementing a basal formulated diet with the same level (2%) of above-mentioned prebiotics. Experimental diets were kept in plastic bags at 4°C until use. The proximate composition was determined according to AOAC (1995). Fish were hand-fed to apparent satiation twice a day (09:00 and 15:00) for 8 weeks. Utmost care was taken to avoid feed losses.
Cutaneous mucosal immune response and serum non-specific immune parameters
At the end of the trial, skin mucus samples were taken from three fish per tank (nine fish for each treatment) according to (Hoseinifar, Roosta, Hajimoradloo & Vakili, 2015). Each fish was anesthetized (100 ppm clove oil), placed in a polyethylene bag containing 10 mL of 50 mM NaCl. After 2 min, the fish was removed from the bag. The mucus samples were transferred to 15-mL sterile centrifuge tubes and centrifuged (1500 g for 10 min at 4°C), and supernatant was stored at −80°C for further analysis. Also, fish were blood-sampled for serum immunological parameter studies. Three samples were taken from each tank. The fish were anesthetized with 100 ppm clove oil. Blood was taken by caudal puncture using heparinized syringe. For serum isolation, blood samples were aliquoted into non-heparinized tubes and left to clot for 12 h (at 4°C), prior to centrifugation at 5000 g for 5 min in a clinical centrifuge (Hettich-D7200, Tuttlingen, Germany). Blood respiratory burst activity was measured by chemiluminescent assay (measurement of light emission) based on the modified protocol of Mathews, Warinner and Weeks (1990) as described by Hoseinifar, Soleimani et al. (2014) using an automated system (Luminoskan Ascent T392; Thermo Fisher Scientific, Waltham, MA, USA) for chemiluminescent analysis. Serum and mucus lysozyme activity was estimated by turbidimetric assay according to the method explained by (Ellis 1990) with Micrococcus luteus (Sigma, St Louis, MO, USA) as target in 0.05 M phosphate buffer (pH = 6.2). Serum alternative complement activity (ACH50) was determined according to Yano (1992) using sheep RBC as target in EGTA–Mg++–gelatin–veronal buffer. Serum total protein levels were determined according to Lowry, Rosebrough, Farr and Randall (1951). The levels of serum and mucus total immunoglobulin (total Ig) were determined after polyethylene glycol precipitation according to Siwicki and Anderson (1993). Azocasein hydrolysis assay was used to determine mucus protease activity as described by Ross, Firth, Wang, Burka and Johnson (2000). Briefly, an equal volume of mucus was incubated with 100 mM ammonium bicarbonate (pH 7.8) buffer containing 0.7% azocasein for 19 h at 30°C on shaker. Trichloroacetic acid (4.6% final concentration) was used to stop the reaction, and the solution was cooled on ice. The reaction mixture was centrifuged at 6000 g for 10 min, and 100 μL of supernatant was mixed to equal volume of 0.5 M NaOH. The protease activity was measured as the increase in the OD values at 450 nm.
Growth performance
- Weight gain percentage (WG) = 100 × (final weight − initial weight)/initial weight
- Food conversion ratio (FCR) = food intake/weight gain
- Specific growth rate (SGR) = 100 × (ln final weight − ln initial weight)/days of rearing
Statistical analysis
Normality of data was confirmed by Shapiro–Wilk test. The data were analysed by one-way anova and Duncan test. The probability level for rejection of the null hypotheses was 0.05. All analyses were performed in spss v.22 (SPSS, Chicago, IL, USA). Data are presented as mean ± SD.
Results
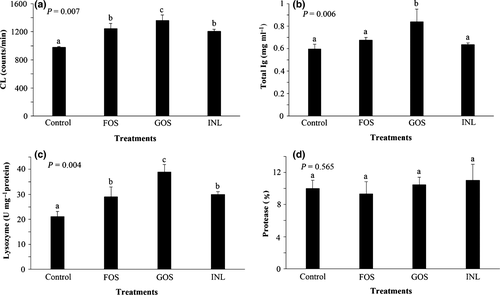
Figure 1 represents the cutaneous mucosal immune response as well as blood respiratory burst activity of common carp fed experimental diets supplemented with different prebiotics. The lowest blood respiratory burst activity was observed in the control treatment. FOS and INL had similar blood respiratory burst activity being significantly lower than GOS treatment (Fig. 1a; P < 0.05). Cutaneous mucus total Ig levels in the control, FOS and INL treatments were similar (P > 0.05), and common carps fed GOS-supplemented diet showed significantly elevated total Ig levels (Fig. 1b; P < 0.05). The cutaneous mucus lysozyme activity of the control treatment was significantly lower than those in prebiotic-fed common carps. The highest cutaneous mucus lysozyme activity was observed in GOS-fed group (P < 0.05). No significant difference observed between FOS and INL treatments regarding mucus lysozyme activities (P > 0.05) (Fig. 1c). Moreover, feeding on different prebiotics had no significant impact on cutaneous mucus protease activity (Fig. 1d; P > 0.05).
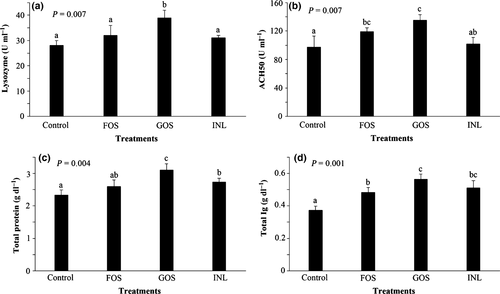
The effects of dietary administration of different prebiotics on serum non-specific immune parameters are shown in Fig. 2. Serum lysozyme activity in GOS treatment was significantly higher than the other treatments (P < 0.05); however, there was no significant difference among the other treatments (P > 0.05) (Fig. 2a). Serum ACH50 in GOS and FOS treatments was significantly higher than those of the control (P < 0.05). However, no significant difference was noticed between control and INL groups regarding serum ACH50 level (P > 0.05) (Fig. 2b). Serum total protein levels in the control group were similar to that of FOS group, but lower than those of the other group; serum total protein of FOS and INL was similar; however, GOS group had significantly higher total protein compared to the other groups (Fig. 2c). Serum total Ig levels were elevated in prebiotic-fed carps; the highest level was observed in GOS-fed group (P < 0.05) (Fig. 2d).
Growth performance parameters of common carp fed experimental diets supplemented with prebiotics with different degree of polymerization are presented in Table 2. Compared to the control treatment, common carps fed GOS supplemented displayed improved (P < 0.05) growth performance, including final weight, weight gain, specific growth rate (SGR) and feed conversion ratio (FCR). However, there were no statistically significant differences between INL, FOS and control group regarding growth performance (P > 0.05). Also, no mortality was recorded during feeding trial and survival rate was 100% in all treatments (Table 2).
| Control | FOS | GOS | INL | P value | |
|---|---|---|---|---|---|
| Initial weight (g) | 13.8 ± 0.44a | 14.0 ± 0.32a | 13.9 ± 0.07a | 13.7 ± 0.19a | 0.614 |
| Final weight (g) | 35.2 ± 0.54a | 35.7 ± 1.51a | 38.8 ± 0.17b | 35.9 ± 1.01a | 0.007 |
| Food conversion ratio | 2.10 ± 0.05b | 2.07 ± 0.20b | 1.81 ± 0.02a | 2.03 ± 0.08b | 0.046 |
| Weight gain (%) | 156 ± 7.25a | 154 ± 16.4a | 179 ± 2.13b | 161 ± 6.15a | 0.041 |
| Specific growth rate (% per day) | 1.56 ± 0.05a | 1.55 ± 0.11a | 1.71 ± 0.01b | 1.60 ± 0.04ab | 0.049 |
| Survival rate (%) | 100 | 100 | 100 | 100 | 1.000 |
- Values in a row with different superscripts denote a significant difference (P < 0.05).
Discussion
Dietary administrations of prebiotics have been increased to meet the demands for a substitute for antibiotics. Indeed, recent studies showed that prebiotics have the potential to be used in intensive aquaculture as anti-stress and immunostimulant dietary supplement (Nayak 2010b; Dimitroglou et al. 2011; Ringø, Olsen, Jensen, Romero & Lauzon 2014; Song et al. 2014; Gatlin 2015; Hoseinifar, Esteban et al. 2015). However, there is limited studies regarding comparison of immunomodulatory effects of prebiotics (Grisdale-Helland, Helland & Gatlin Iii 2008; Buentello, Neill & Gatlin 2010; Zhou, Buentello & Gatlin, 2010; Anguiano, Pohlenz, Buentello & Gatlin 2013; Talpur, Munir, Mary & Hashim 2014; Carbone & Faggio 2016; Guerreiro, Couto, Machado, Castro, Pousão-Ferreira, Oliva-Teles & Enes 2016; Munir, Hashim, Chai, Marsh & Nor 2016).
Nutritional modulation of mucosal immune response through administration of dietary pre- or probiotics received increasing attention in recent years (Lazado & Caipang 2014; Caipang & Lazado 2015; Merrifield & Rodiles 2015). There are limited published study regarding the effects of prebiotics on skin-associated lymphoid tissues (SALT) and cutaneous mucus immune response (Rodrigues-Estrada, Satoh, Haga, Fushimi & Sweetman 2008; Torrecillas, Makol, Caballero, Montero, Ginés, Sweetman & Izquierdo, 2011; Sheikhzadeh, Heidarieh, Karimi Pashaki, Nofouzi, Ahrab Farshbafi & Akbari 2012; Hoseinifar, Khalili, Khoshbavar Rostami & Esteban 2013; Hoseinifar, Sharifian, Vesaghi, Khalili & Esteban 2014; Hoseinifar, Mirvaghefi, Amoozegar, Sharifian & Esteban, 2015; Torrecillas, Montero, Caballero, Robaina, Zamorano, Sweetman & Izquierdo 2015; Adorian, Goulart, Mombach, de Menezes Lovatto, Dalcin, Molinari, Lazzari & da Silva 2016; Azimirad, Meshkini, Ahmadifard & Hoseinifar 2016; Carbone & Faggio 2016; Safari, Hoseinifar, Nejadmoghadam & Jafar 2016). The results of the present study revealed that fish fed GOS had remarkably higher skin mucus lysozyme activity and total Ig levels compared to FOS, INL and control group, although the effects on protease activity was not noticeable. Indeed, better results obtained when fish fed GOS. However, there is no published study regarding comparison different prebiotics on mucosal immune response. In agreement with this finding, Hoseinifar, Khalili et al. (2013) and Hoseinifar, Sharifian et al. (2014) reported improved skin mucus immune response in Caspian white fish (Rutilus frisii kutum) and Caspian roach (Rutilus caspicus) fry fed dietary XOS and GOS respectively. Furthermore, dietary administration of MOS affects cutaneous mucosal parameters of rainbow trout, although this finding was not corroborated by (Torrecillas, Makol, Benítez-Santana, Caballero, Montero, Sweetman & Izquierdo (2011) study on European sea bass (Dicentrarchus labrax). In spite of the above-mentioned improvements, the mechanisms by which dietary prebiotics affect SALT as well as the effectiveness of different prebiotics are not clear and merit further research.
In line with the results obtained about different prebiotics on mucosal immune parameters, the highest serum Ig and lysozyme levels as well as respiratory burst and alternative complement activity were observed in fish fed GOS-supplemented diet. Similar to this finding, by comparing GOS, INL and MOS effects on innate immune parameters of red drum (S. ocellatus), Zhou et al. (2010) reported significantly higher lysozyme activity and neutrophil oxidative radical production in fish fed GOS prebiotic. However, in a sister study, contradictory results obtained when GOS, FOS and MOS effects were compared on red drum immune response (Buentello et al. 2010); while disease resistance was significantly higher in GOS-fed fish, there were no noticeable difference between lysozyme activity and neutrophil oxidative radical production of GOS and FOS group. Furthermore, comparison of GOS, FOS and MOS revealed no remarkable difference regarding changes in lysozyme activity and neutrophil oxidative radical production (Grisdale-Helland et al. 2008).
It has been suggested that fermentation of prebiotics by beneficial component of intestinal microbiota produces short-chain fatty acids (typically, acetic acid, propionic acid and butyric acid) which contribute to host immune modulation (Scheppach 1994; Cummings & Macfarlane 2002; Caipang & Lazado 2015; Merrifield & Rodiles 2015). Moreover, Maslowski & Mackay (2010) stated that butyrate is the main energy source for colonic epithelial cells and thus associated with maintenance of the epithelium and stimulation of the immune system. Interestingly, in vitro studies revealed that butyrate production was significantly higher by probiotic and intestinal bacteria when GOS used as substrate compared to FOS, INL, MOS, XOS substrates (Burr, Hume, Ricke, Nisbet & Gatlin 2010; Hoseinifar, Mirvaghefi, Amoozegar, Merrifield & Ringø, 2015), a fact that supports the results of the present study regarding higher immune response and mucosal immune parameters in GOS-fed carp.
The growth performance data showed that only GOS had positive effect on growth performance; however, FOS had no significant effects on growth performance. It seems that GOS can be suitable prebiotic for improving C. carpio growth performance. Likewise, Burr, Hume, Neill & Gatlin Iii (2008) reported that red drum fed GOS-supplemented diet showed better performance and nutrient digestibility compared to INL and control groups. Similar to this finding, juvenile red drum fed dietary GOS showed improved intestinal morphology compared to those fed MOS, FOS and control diet (Anguiano et al. 2013). However, in contrast to above-mentioned results, no significant difference was observed between GOS, FOS and MOS (Buentello et al. 2010) as well as between GOS, INL and MOS in juvenile red drum (Zhou et al. 2010) regarding growth enhancement. These results clearly suggest that type of prebiotic is not the sole feature affecting the efficacy of prebiotic on fish growth performance. Indeed, several factors including prebiotic dosage, intestinal bacterial communities and intestinal structure species as well as life stages may affect fermentability of prebiotics and subsequent effects on growth performance.
In conclusion, the results of the present study revealed that as a prebiotic, GOS has the potential to be more effective in growth enhancement and immune modulation (mucosal and serum innate immune response) compared FOS and INL.
Acknowledgment
The present study is co-funded by Golestan University of Medical Sciences (79%) and Gorgan University of Agricultural Sciences and Natural Resources (21%). We would like to thanks financial supports of Research Affairs. The funder had no role in the design, analysis or writing of this article. The authors would like to thank the kind helps of the staff at Aquaculture Lab of GUASNR.




