Anaesthetic efficacy of eugenol on Flowerhorn (Amphilophus labiatus × Amphilophus trimaculatus)
Abstract
Anaesthetic efficacy of eugenol was investigated on Flowerhorn (Amphilophus labiatus × Amphilophus trimaculatus). A total of 104 fish with average weights of 12 ± 2.5, 28 ± 5 and 53 ±5.1 g were subjected to 25–200 mg L−1 eugenol and behavioural responses as well as induction and recovery times were recorded. Induction and recovery times were significantly affected by eugenol concentration as well as fish weight (P < 0.05). Generally, 49.9–127.3 s after exposure to 50–200 mg L−1 eugenol, fish reached stage 3 anaesthesia (suitable for general handling). Fish entered stage 4 anaesthesia (suitable for surgery and blood sampling) over 57.3–140.4 s post exposure to such concentrations. Recovery time was 91.7–312 s in all weight classes for all eugenol concentrations. Mortality (23%) was only observed in 12-g fish when were subjected to 200 mg L−1 eugenol. This study showed the behavioural response of Flowerhorn to anaesthesia and eugenol efficacy as an anaesthetic in this important ornamental species. The general quadratic equation revealed that concentrations of eugenol and fish size along with their interactive effects have significantly contributed to the model, with concentration recording the highest beta value in all models (β = −0.809, −0.818 and −0.909, P = 0.000). According to the results, minimum eugenol concentration to induce anaesthesia in less than 3 min was 50 mg L−1.
Introduction
Anaesthesia may be used to attenuate stress or physical impairments arisen from common practices in fisheries management and aquaculture enterprise, namely crowding, capture, handling (marking, counting, injection, stripping, weighing, etc.) and release (Ross & Ross 1999; Palic, Herolt, Andreasen, Menzel & Roth 2006). The necessity of live fish transport in various fish enhancement programmes, commercial fisheries and pisciculture requires anaesthetizing fish without upsetting their health or commercial value (Yildiz, Kayim & Akin 2013). Such conditions involve investigating species-specific concentration and exposure time of an anaesthetic (Summerfelt & Smith 1990).The various stages of anaesthesia in different fish species were already described (McFarland 1959; Jolly, Mawdesley-Thomas & Bucke 1972; Hikasa, Takase, Ogasawara & Ogasawara 1986). These stages are identified according to the behavioural response to anaesthetic including response to stimuli, opercular rate and fish equilibrium (Hoseini, Rajabiesterabadi & Tarkhani 2013). In this regard, time to reach rapid anaesthesia, loss of equilibrium and deep anaesthesia should be considered due to their consequences in blood collection, handling and surgery respectively (Auperin, Baroiller, Ricordel, Fostier & Prunet 1997; Roubach, Gomes, Fonseca & Val 2005; Hoseini & Jafar Nodeh 2011). There are many anaesthetics available for aquatic animals, such as metomidate, 2-phenoxyethanol, quinaldine, tricaine methanesulfonate (MS-222), benzocaine, clove oil and Aqui-STM (Iversen, Finstad, McKinley & Eliassen 2003; Pirhonen & Schreck 2003; Coyle, Durborow & Tidwell 2004). However, few anaesthetics are permitted for use in aquaculture. MS-222, the only anaesthetic approved by the Food and Drug Administration of the United States of America (USFDA), is expensive and less efficient in controlling plasma cortisol level (Coyle, Dasgupta, Tidwell, Beavers, Bright & Yasharian 2005). In addition, a 21-day withdrawal period is required if the fish is intended for human consumption, leading to an enthusiasm in less persistent and natural anaesthetics, such as clove oil. Eugenol (2-methoxy-4-(2-propenyl) phenol), ‘generally regarded as safe’ by FDA, is the major component of clove oil (70–90% by weight) (Ross & Ross 1999). Because of its efficacy, low price, no withdrawal period and lack of side effects on fish appetite, eugenol and isoeugenol have been considered as future anaesthetics of choice in the aquaculture industry (Cupp, Hartleb, Fredricks & Gaikowski 2014). Its anaesthetic properties were documented in different fish species such as medaka, Oryzias latipes, goldfish, Carassius auratus, and crucian carp, Carassius carassius (Endo, Ohshima, Ogishima & Tanaka 1972), common carp, Cyprinus carpio (Hikasa et al. 1986), rabbitfish, Siganus lineatus (Soto & Burhanuddin 1995), rainbow trout, Oncorhynchus mykiss (Keene, Noakes, Moccia & Soto 1998), tambaqui, Colossoma macropomum (Roubach et al. 2005). Iversen, Økland, Thorstad and Finstad (2013) concluded that Aqui-S vet. (iso-eugenol) is a promisingly suitable stress-reducing anaesthetic agent for European eel, Anguilla anguilla L., with the ability of improving animal welfare and survival during and after common aquaculture practices. On the other hand, safety of human and the target animal is amongst the main criteria of choosing the right chemicals, especially when anaesthetics are used in large quantities by unskilled labours and also discharged into natural water bodies (Javahery, Nekoubin & Haji-Moradlu 2012). According to USFDA (1978), eugenol is safe to people up to 1500 mg L−1 with a rapid hepatic metabolism (Fischer, Von Unruh & Dengler 1990).
Flowerhorn fish is a genetically selected/improved hybrid of Amphilophus labiatus and Amphilophus trimaculatus (Sornsupharp, Lomthaisong, Dahms & Sanoamuang 2013) and is an important fish species in ornamental fish industries especially in South-East Asia (Sandford 2007). According to Kegley, Conlisk and Moses (2010), there is an abrupt time-related and species-specific variation between exposures that cause effective anaesthesia and those which cause mortality for clove oil and its constituents. Despite its worldwide aquacultural importance, there is a dearth of information on the response of Flowerhorn to anaesthetic chemicals. Consequently, the aim of this study was to verify the anaesthesia properties of eugenol in different size classes of Flowerhorn.
Materials and methods
Fish and experimental conditions
Flowerhorn (N = 104) were purchased from a local farm. Fish were transferred to laboratory and stocked in a 1000-L tank for 24 h. Then, they were sorted in three different weight classes: 12 ± 2.5 g (7 ± 2 cm), 28 ± 5 g (11 ± 2.2 cm) and 53 ± 5.1 g (15 ± 3.4 cm). Each weight class contained 100 individuals. Fish were stocked in 100- to 550-L tanks according to their weight (density of 10 g L−1) for next 7 days and fed commercial diet (BioMar, Nersac, France) equivalent to 1.5% of their respective body weight. Daily water exchange rate was 80%. All tanks were continuously aerated. Water quality parameters during acclimatization period and throughout the experiments were as follows: water temperature 28 ± 1°C, pH 8.0–8.4, dissolved oxygen 7 ± 1 mg L−1 and total ammonia nitrogen (TAN) <0.5 mg L−1.
Anaesthetic preparation
A stock solution was prepared by mixing eugenol (Sigma, St. Louis, MO, USA, 99% purity) and ethanol (Razi, Iran, with 96% purity) with respective volumetric ratio of 1:2. Solutions were freshly prepared right before experimentation. Plastic 2-L containers with continuous aeration were used (Hoseini et al. 2013). Exposure of fish to ethanol did not bring about anaesthesia or any apparent modifications in fish behaviour including opercular ventilation rate, indicating that the concentration of ethanol used had no effects on the fish during the experiment.
Behavioural observations during anaesthetic exposure
Fish of different sizes were placed into the anaesthetic containers and their behavioural responses were recorded. Fish were hyperactive promptly after anaesthetic exposure. Then, fish remained relaxed and calm in the anaesthetic bath without any reactivity to tactile touch. Time to reach stage 1 was recorded at this point. Afterwards, imbalance swimming suggestive of disequilibrium and disorientation was noticeable, implying stage 2 of anaesthesia. When imbalance swimming brought to the end, fish laid down on one side without any reactivity to external stimuli. However, they showed slow respiration corresponding to stage 3 anaesthesia. When opercular rate dropped and became irregular, stage 4 anaesthesia commenced. Irregular opercular movement continued until it ceased known as stage 5, indicative of irreversible stage or death (Table 1).
| Stage | Behaviour of fish |
|---|---|
| 0 | Normal |
| I | Relaxation and no response to stimuli: fish were calmed and did not respond to tactile touch |
| II | Imbalance swimming: fish loss their equilibrium and show imbalance swimming |
| III | Total loss of equilibrium: fish laid on lateral side, slightly depressed but regular opercular movement |
| IV | Deep anaesthesia: slow and irregular opercular movement |
| V | Death: opercular movement ceased |
| Recovery stage | Fish regained its equilibrium |
Anaesthesia experiments
Fish were fasted for 24 h before conducting experiment. To evaluate the anaesthetic properties of eugenol in different weight classes, fish from each class were subjected to different concentrations of eugenol including 25, 50, 100, 150, 200 mg L−1. Ten fish from each group were subjected to each eugenol concentration. Fish were gently scoop-netted and placed in the anaesthetic bath. After anaesthetic exposure, elapsed time to reach each anaesthetic stage was recorded and when reaching the stage 4, fish weight and length were quickly recorded prior to transfer to recovery tank (containing clean and well aerated water). Recovery time was recorded from transferring the fish to recovery container until equilibrium regained. Finally, fish were transferred to freshwater aquaria to monitor potential mortality over a 24-h period (Javahery et al. 2012).
Statistical analyses
The homoscedasticity of the variance of the dependent variables was evaluated with the Levene's test. Standard normality test of Kolmogorov–Smirnov was applied to determine normality of data set. Two-way anova was used to elucidate whether or not there were significant differences amongst different experimental groups. The general quadratic equation applied to test for relationships using polynomial regression was Z = b0 + b1X + b2Y +b3X2 + b4XY + b5Y2 + ɛ, where Z is the response (time elapsed to reach each anaesthetic stage) and X and Y are the independent variables (e.g. fish size and eugenol concentration). Before running the regression, predictors were centred on their respective means to ensure there were no violations of the assumptions of normality, linearity, multicollinearity and homoscedasticity. With the use of a P < 0.001 criterion for Mahalanobis distance, no outliers were found (Steel, Torrie & Dickey 1997; Shanock, Baran, Gentry, Pattison & Heggestad 2010). Model validation analysis was also carried out by conducting a cross-validation, which requires that the regression model for the training sample replicates the pattern of the full data set (Osborne 2000). All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, NY, USA) at the significance level of P < 0.05. Results were reported as mean ± SD.
Results
Fish with an average weight of 12 g showed 23% mortality at 200 mg L−1 eugenol. However, no mortalities were recorded for larger fish in the course of the test. After 24 h, no mortality was observed in all experimental groups and the fish were feeding well within 1 day after treatment.
Two-way anova revealed a statistically significant interaction between eugenol concentration and average fish body weight with regard to anaesthetic efficacy of eugenol on Flowerhorn (Table 2). The fish sequentially entered different anaesthetic stages. In all stages, any increase in eugenol concentration led to a significant decrease in the induction time with a subsequent increase in the recovery time (Table 3). Inconsistently, any increase in fish weight resulted in a significant increment of the induction time with a subsequent decrease in the recovery time. All size classes showed all anaesthetic stages at 50 mg L−1 eugenol; 25 mg L−1 eugenol could not induce stages 3 and 4 in all size classes even over a 15-min period, which is far more than 3 min, a desired time to induce anaesthesia in fish. The stages 3 and 4 and recovery period for 53 g fish were 70.5–131.6, 80.1–140.4 and 91.7–212.6 s respectively. In all fish sizes, 200 mg L−1 eugenol effectively induced stages 3 and 4 very rapidly in comparison with other concentrations. At the same time, fish anaesthetized by the highest eugenol concentration significantly required more time to recover (Table 3).
| Source | Dependent variable | Type III sum of squares | d.f. | Mean square | F | Sig. |
|---|---|---|---|---|---|---|
| Size | Stage 3 | 20553.95 | 2 | 10276.98 | 1346.98 | 0.000 |
| Stage 4 | 19385.42 | 2 | 9692.71 | 1054.12 | 0.000 | |
| Recovery | 55385.47 | 2 | 27692.74 | 6348.67 | 0.000 | |
| Concentration | Stage 3 | 50600.96 | 3 | 16866.99 | 2210.72 | 0.000 |
| Stage 4 | 52622.39 | 3 | 17540.79 | 1907.63 | 0.000 | |
| Recovery | 381994.28 | 3 | 127331.43 | 29191.36 | 0.000 | |
| Size × Concentration | Stage3 | 1687.31 | 6 | 281.22 | 36.86 | 0.000 |
| Stage 4 | 1354.07 | 6 | 225.68 | 24.54 | 0.000 | |
| Recovery | 11850.91 | 6 | 1975.15 | 452.81 | 0.000 | |
| Error | Stage 3 | 701.92 | 92 | 7.63 | ||
| Stage 4 | 845.95 | 92 | 9.19 | |||
| Recovery | 401.30 | 92 | 4.36 | |||
| Total | Stage 3 | 1024141.00 | 104 | |||
| Stage 4 | 1227036.00 | 104 | ||||
| Recovery | 3308918.00 | 104 |
| Size | Anaesthetic concentration (mg L−1) | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Recovery time (s) |
|---|---|---|---|---|---|---|---|
| 12 g | 25 | 145.7 ± 3.5ba | |||||
| 50 | 82.9 ± 2.7f | 90.9 ± 3.3e | 98.6 ± 2.7d | 112.3 ± 2.6d | 127.9 ± 3.9h | ||
| 100 | 70.2 ± 1.7h | 80 ± 1.8f | 89.4 ± 2.3e | 98.5 ± 1.7f | 146.4 ± 2.6f | ||
| 150 | 50.2 ± 1.8i | 61.5 ± 1.7g | 72.8 ± 2.7f | 83.4 ± 2.3g | 192.2 ± 3.2d | ||
| 200 | 31.4 ± 2k | 40.2 ± 1.9i | 49.9 ± 2.1h | 57.3 ± 2.9i | 127.5 ± 2 | 312.1 ± 3.7a | |
| 28 g | 25 | 176.4 ± 2.1a | |||||
| 50 | 101.5 ± 3.9d | 111.5 ± 2.7c | 127.3 ± 3.2a | 136.7 ± 3.7a | 103.2 ± 3.2j | ||
| 100 | 93.4 ± 2.4e | 101.9 ± 1.9d | 110.9 ± 2.1c | 119.3 ± 2.8c | 125.2 ± 3.5h | ||
| 150 | 77.2 ± 1.7g | 87.6 ± 1.9e | 96.4 ± 2.6d | 105.1 ± 1.1e | 161.1 ± 2.7e | ||
| 200 | 40 ± 1.6j | 49.6 ± 1.5h | 61 ± 1.7g | 71.3 ± 2.4h | 272.6 ± 1.7b | ||
| 53 g | 25 | ||||||
| 50 | 111.2 ± 2.4c | 122.5 ± 1.5a | 131.6 ± 1.7a | 140.4 ± 181.5a | 91.7 ± 2.4k | ||
| 100 | 99.4 ± 1.9d | 117.2 ± 4.6b | 127.6 ± 4.3a | 137.7 ± 5.6a | 106.6 ± 4.2i | ||
| 150 | 91.7 ± 1.9e | 101.5 ± 1.3d | 115.7 ± 4b | 125 ± 3.6b | 142.1 ± 5.1g | ||
| 200 | 51.2 ± 2i | 61.6 ± 1.7g | 70.5 ± 2.5f | 80.1 ± 2g | 212.6 ± 1.7c |
- a Values with different superscripts in each column are significantly different at P < 0.05.
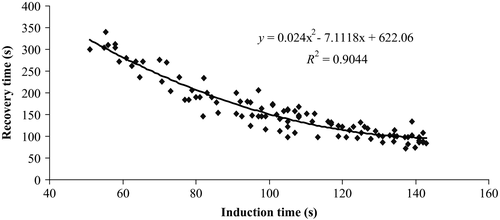
Results from stepwise multiple regression analyses yielded the following regression equations representing the description of time elapsed to reach stages 3 and 4 and full recovery from anaesthesia; Stage 3 = 2.040 − 0.002 × (concentration) + 0.004 × (size) −1.500 × 10−5 × (concentration)2 + 1.060 × 10−4 × (size)2 + 6.440 × 10−6× (concentration× size), Stage 4 = 2.074 − 0.002 × (concentration) + 0.004 × (size) −1.307 × 10−5 × (concentration)2 − 8.860 × 10−5 × (size)2 + 8.790 × 10−6 × (concentration× size), Full recovery from anaesthesia = 2.135 + 0.003 × (concentration) − 0.004 × (size) +1.353 × 10−5× (concentration)2 + 4.467 × 10−5 × (size)2 − 3.440 × 10−6 × (concentration × size). The total variance explained by the model as a whole was 97.6%, F (5, 98) = 808.117, P = 0.000 for stage 3; 97.4%, F (5, 98) = 741.322, P = 0.000 for stage 4; and 99.5%, F (5, 98) = 3756.208, P = 0.000 for full recovery from anaesthesia. Both independent variables and also their interactive effects have significantly contributed to the model, with concentration recording the highest beta value (β = −0.809, −0.818 and −0.909, P = 0.000 respectively). The quadratic regression of recovery time for all size classes was also significantly correlated with induction time to stage 4 (Fig. 1, 95%, F (2, 101) = 789.58, P < 0.001).
In the analysis of the training samples, the relationships between the predictors and the response variables were statistically significant, 97.9%, F (5, 67) = 632.910, P = 0.000 for stage 3; 97.5%, F (5, 67) = 514.041, P = 0.000 for stage 4; 99.5% and F (5, 67) = 2628.388, P = 0.000 for full recovery from anaesthesia and the pattern of significance for independent variables in training sample matched the pattern for full data set. The shrinkage in R² (1.184, 0.394 and 0.000 for stages 3 and 4 and recovery, respectively) was smaller than the 2% criterion of threshold shrinkage conferring that the regression models would be effective in predicting time required to get each anaesthetic stage for other experiments on the species using eugenol as the anaesthetic agent.
Discussion
Effective concentration of eugenol as an anaesthetic has been investigated in various aquatic species including rabbitfish (Siganus lineatus) (Soto & Burhanuddin 1995), fathead minnow (Pimephalespromelas Rafinesque, 1820) (Palic et al. 2006), iridescent shark (Pangasiushypophthalmus) (Hoseini et al. 2013), common snook (Centropomusundecimalis Bloch, 1792) (Bernardes-Júnior, Nakagome, Mello, Garcia & Amaral Júnior 2013), Mulgrave goby (Glossogobius bellendenensis, Gobiidae), empire gudgeon (Hypseleotris compressa), sleepy cod (Oxyeleotris lineolatus), Eastern rainbow fish (Melanotaenia splendida, Melanotaeniidae), Pacific blue-eye (Pseudomugil signifer, Pseudomugilidae), and eel-tailed catfish (Tandanus tandanus, Plotosidae), spotted tilapia (Pelmatolapia mariae, Cichlidae) and guppy (Poecilia reticulata, Poeciliidae) (Kroon 2015) and prawns (Macrobrachium rosenbergii) (Coyle et al. 2005; Saydmohammed & Pal 2009). It seems that the effective concentration varied from 30 to 100 mg L−1, depending on fish/shellfish species and body size with the most frequent concentration range of 40–50 mg L−1.
Our results showed that higher eugenol concentrations resulted in a decreased induction time and a longer recovery period. At the same time, recovery time was negatively correlated with induction period. The findings are well in agreement with previous studies on the efficacy of eugenol on other species (Hikasa et al. 1986; Soto & Burhanuddin 1995; Keene et al. 1998; Cunha & Rosa 2006; Park, Im, Seol & Park 2009). However, contrasting results have been reported for European sea bass, Dicentrarchus labrax and gilthead sea bream, Sparus aurata using eugenol and 2 phenoxyethanol (Mylonas, Cardinaletti, Sigelaki & Polzonetti-Magni 2005). However, the authors hypothesized that longer exposure to the anaesthetic might lead to more anaesthetic absorption by fish, which in turn lengthened the recovery time. If the statement is correct even for lower anaesthetic concentrations, the contrary might also be true. However, Weyl, Kaiser and Hecht (1996) stated that compared with exposure time, anaesthetic concentration imposes a significant effect on the recovery time. Independence of the recovery time from the anaesthesia duration is mainly due to the dose-dependent absorption of anaesthetic through the gills and it is conceivable that as soon as equilibrium level is achieved, no further anaesthetic will be taken up by the fish. During recovery, the anaesthetic agent is leaked via such gradient so that the recovery time is regulated by the anaesthetic concentration rather than by duration of anaesthesia (Weyl et al. 1996). It is yet to be clarified whether or not either induction time or anaesthetic concentration affects recovery time. One possible solution might be to determine the absorption and excretion of eugenol in fish tissues after short and long induction times as well as its washout rate during the recovery period.
Present study revealed that fish weight significantly resulted in increased induction and decreased recovery time, which is in good concordance with the results on white sea bream, D. sargus, sharp snout sea bream, Diplodus puntazzo (Tsantilas, Galatos, Athanassopoulou, Prassinos & Kousoulaki 2006) and channel catfish Ictalurus punctatus (Small 2005). Although in tench (Tinca tinca) (Myszkowski, Kamiński & Wolnicki 2003) and gold lined sea bream, Sparus sarba (Hseu, Yeh, Chu & Ting 1994) induction time increased in concomitant with any increase in fish weight, recovery time remained unaffected. However, degrees of weak correlations were also reported between body weight and induction or recovery time (Weyl et al. 1996; Stehly & Gingerich 1999; Roubach et al. 2005). Such contrasting results might be due to interspecies differences (Weyl et al.1996; Kegley et al. 2010). As weight increment could be accompanied by age, lipid content, sexual maturity, scale development and gill surface area to body weight ratio (Wootton1990), it is difficult to conceive that weight increment is responsible for such differences in induction and recovery time per se. Small fish have larger gill or body surface/body volume ratios than large fish, which may consequently lead to faster anaesthetic absorption. Accordingly, it is possible to speculate that induction occurs within a shorter time in small fish in comparison with large ones. The only problem is that why such a speculation does not imply shorter recovery times in small fish according to anaesthesia leakage through fish tissue by concentration gradient (Weyl et al. 1996). One might consider that the rate of anaesthetic clearance from fish body could be a function of such gradient and gill surface area to body volume ratio, which was to some extent supported by the presence of statistically significant interaction between fish size (i.e. body weight) and anaesthetic agent concentration in Flowerhorn. This implies that there should be a positive correlation between induction and recovery time, which was not the case in preceding studies. Such contradictions may indicate that determination of the exact behaviour of the anaesthetic agents requires thorough investigation. As other factors such as health state and stocking density, pH, water temperature, salinity, dissolved oxygen and water mineral content may also affect the efficacy of anaesthetic agents in aquatic animals (Jolly et al. 1972; Sylvester & Holland 1982; Josa, Espinosa, Cruz, Gil, Falceto & Lozano 1992; Weyl et al. 1996), it is difficult to compare all studies or even to draw a definite conclusion. Some degrees of mortality might be likely if inappropriate protocol is applied (channel catfish, I. punctatus, (Waterstrat 1999) and white sea bream, D. sargus and sharp snout sea bream, D. puntazzo, Tsantilas et al. (2006). As eugenol is oil soluble, it could cover gill surface and cause less oxygen diffusion with a consequent hypoxia (Sladky, Swanson, Stoskopf, Loomis & Lewbart 2001). The situation may lead to medullary collapse and death (i.e. euthanasia) (Kegley et al. 2010).
According to beta values, a criterion of total variance explained by the variable after controlling contribution of others, the effect of eugenol concentration was more considerable than that of fish size. Therefore, one might infer that fish would be rapidly anaesthetized in higher eugenol concentration. However, higher eugenol concentrations confer longer times for full recovery from anaesthesia. Hoseini et al. (2013) also reported a significantly negative trend between eugenol concentration and induction times for stages 3 and 4 in iridescent shark (Pangasius hypophthalmus). Meanwhile, they observed that it takes longer time for fish to recover at higher eugenol concentrations. Similarly, Bernardes-Júnior et al. (2013) reported a strong relationship between eugenol concentration and induction time (R² = 0.899, IT = 4785C−0.9190) in juvenile common snook (Centropomus undecimalis). However, they did not observe any consistent relationship between recovery time and anaesthetic concentration may be due to a high variability in response time observed in all treatments during recovery. A significant relationship was also found between the recovery times for all size classes and induction time to stage 4 in the present study. In another word, longer induction time was coincided with shorter recovery time, since longer induction times were recorded for lower eugenol concentrations, which might cause lower amounts of anaesthetic uptake via gill epithelia and consequently shorter time would be required to wash out the agent irrespective of fish size and species (Javahery et al. 2012).
Our results showed that 200 mg L−1 eugenol is not applicable to 12 g Flowerhorn; however, there was no mortality in the other weight classes. Eugenol concentration of 50 mg L−1 was capable to induce all stages of anaesthesia in 12–53 g Flowerhorn. Marking and Meyer (1985) suggested that a suitable anaesthetic for use in fish culture and research should has an induction time of less than 15 min and preferably less than 3 min as well as recovery time of 5 min or shorter. Accordingly, 50 mg L−1 eugenol was suitable to induce stage 3 (suitable for general handling) and 4 in a desired time of less than 3 min in all size classes of Flowerhorn. Results showed that 25 mg L−1 eugenol is appropriate for live fish transport especially for 12 ± 2.5 and 28 ± 5 g Flowerhorn as they would not go beyond anaesthesia stage 1 (McFarland 1960). At stage 1 of anaesthesia, activity and interaction with conspecifics are reduced and fish are able to maintain equilibrium and swimming capacity and avoid physical damage from collision with the container walls (Cooke, Suski, Ostrand, Tufts & Wahl 2004). However, for larger Flowerhorns (53 ± 5.1 g) it seems that the effective eugenol concentration to induce stage 1 might be between 25 and 50 mg L−1, which requires further research.




