Effects of salinity and temperature on artificial cultivation and early ontogeny of giant kokopu, Galaxias argenteus (Gmelin 1789)
Abstract
Attempts at artificial propagation and rearing of juvenile galaxiids (‘whitebait’) have been hindered by low fecundities and survival. To circumvent these issues, we subjected wild and captivity-acclimated giant kokopu (Galaxias argenteus), a hitherto unexplored galaxiid, to captive breeding protocols and evaluated the effects of salinity and temperature on egg fertilisation, incubation duration and hatching. Eggs sourced over several spawning seasons were subjected to different salinity (0, 9, 15, 20 and 30–35 PSU) and temperature (4, 10, 15 and 17°C) combinations. Average fertilisation rates were highest (95%) when gametes were activated in fresh water (0 PSU) and declined as salinity increased. Average hatch rates were highest (71–68%) when eggs were incubated in fresh water (0 PSU) at 10°C and decreased as salinity and temperature increased. Sequential stages of embryonic development were documented and several year classes of offspring were produced to form a captive breeding colony. We conclude that the giant kokopu has notable reproduction-related qualities that may be useful for future aquaculture exploitation of this species.
Introduction
Advances in captive breeding conventions can facilitate aquaculture and captive rearing of threatened species. This, in turn, opens avenues for genetic preservation (Fisch, Ivy, Burton & May 2013), for trait selection (Symonds, Walker, van de Ven, Marchant, Irvine, Pether, Gublin, Bruce, Anderson & McEwan 2012), for conservation physiology or for insights into the optimal environmental conditions for ex situ preservation or cultivation of the desired species (Cooke, Sack, Franklin, Farrell, Beardall, Wikelski & Chown 2013).
The whitebait fishery is a distinctive freshwater fishery that targets shoals of slender translucent juvenile fish of the genus Galaxias (Order Osmeriformes). These juveniles are caught during spring in close proximity to river mouths while migrating upstream into adult freshwater habitats (McDowall 1964). Inanga, also known as the common jollytail, puye, oa or puyen Galaxias maculatus (Jenyns 1842) (Pollard 1971; Mardones & Ríos-Escalante 2012), is the most frequently encountered whitebait species (Chateris, Allibone & Death 2003). Four additional amphidromous species, the koaro Galaxias brevipinnis (Günther 1866), banded kokopu Galaxias fasciatus (Gray 1842), shortjaw kokopu Galaxias postvectis (Clarke 1899) and giant kokopu Galaxias argenteus (Gmelin 1789), contribute to the whitebait fishery in New Zealand (Chateris et al. 2003).
Not only does whitebait have high cultural significance as a recreational fishery or as a treasured species and a traditional food source for indigenous Māori (McDowall 1964; Haggerty 2007), it also has considerable aquaculture potential, as it is regarded a luxury product in a suite of Southern Hemisphere countries. For this reason, the cultivation of whitebait, particularly that of inanga, has received attention in Chile (Mardones, Vega & Encina 2008; Mardones & Ríos-Escalante 2012) and to a lesser extent in New Zealand (Mitchell 1989). Attempts at artificially rearing whitebait has met with limited success (Mitchell 1989; Jeffs 2003; Mardones et al. 2008; Mardones & Ríos-Escalante 2012), partly due to technical obstacles associated with low fecundity and larval survival of inanga (Jeffs 2003; Mardones et al. 2008; Mardones & Ríos-Escalante 2012). Accordingly, the use of a different galaxiid, such as the giant kokopu, seems worthwhile. This large (up to 58 cm and 2.7 kg; David 2002) and fecund species is likely to be more robust than the much smaller inanga (maximum length 17 cm; Mardones & Ríos-Escalante 2012).
Despite being collected by naturalists as early as 1773 (McDowall 2010), notable gaps in the understanding of giant kokopu reproductive biology remain. Thus, spawning activity of captivity-raised giant kokopu has been observed (O'Brien & Cooper 2013) and eggs have been located in the wild (Smith, Franklin, Barker & Bartels 2013), however, egg fertilization, embryonic development, hatching and culture of this species have not been described in any detail or yielded any practical rates of success. Furthermore, key aquaculture qualities, such as the species' amenability to artificial propagation, as well as optimum conditions for fertilisation (temperature, salinity) and egg incubation, remain unexplored; knowledge of these attributes is a prerequisite for large-scale production (Mihelakakis & Kitajima 1994), whether for commercial farming or for the augmentation of dwindling stocks (giant kokopu is ranked as declining in the New Zealand threat classification system; Allibone, David, Hitchmough, Jellyman, Ling & Ravenscroft 2010). Accordingly, in our study, giant kokopu were acclimated to captivity during early gonadal recrudescence and once ovulated eggs were obtained, we sought to expose the effects of salinity and temperature on artificial fertilisation, egg incubation and hatching. Observations from captive fish were complemented with those from wild-caught fish that had ovulated eggs or were spermiating at the time of capture.
Materials and methods
Animal capture and gamete collection
Giant kokopu were caught in unbaited fyke nets set overnight in a large body of fresh water on a rural private property in Eastern Southland, New Zealand, in the austral summer (i.e. during early gonadal recrudescence) of 2009 and the early winters (i.e. when fully mature) of 2010, 2011 and 2012. At the site where ripe fish were captured, temperatures averaged 4°C, dissolved oxygen content 10.4 mg L−1, salinity 0.05 PSU and pH 6.0 (Yellowstone Instruments, YSI Professional Plus). This study was approved by the Animal Ethics Committee of the University of Otago, Dunedin, New Zealand.
Six immature fish (200–350 g), captured in December 2009, were transported in 20 L buckets aerated by battery-operated air pumps to the laboratory and acclimated to captive conditions (‘captive fish’). They were maintained under a simulated natural photo-thermal regime in a partitioned 1000 L recirculating tank and fed on meal worms Tenebrio molitor, American cockroaches Periplaneta americana, blood worms Chironomus sp, frozen chinook salmon smolt Oncorhynchus tshawytscha and commercial salmon pellets (Reliance Stock Foods, Dunedin, New Zealand). Captive giant kokopu were biopsied and blood-sampled at near-monthly intervals as described by Wylie, Forbes and Lokman (2013), until June 2010 to track oocyte development; two fish died during the first 3 months of captivity (for details, see Wylie et al. 2013). In October 2010, ovulated eggs were retrieved by gentle stripping of the dried abdomen from each of the remaining four captive fish following anaesthesia in 100 mg L−1 benzocaine. Gametes were collected in petri dishes and stored on ice (up to 1 h) until dry fertilisation and incubation; only eggs from the first three fish were used for these trials due to lack of time.
Giant kokopu collected in June 2010 (‘wild fish’; 370–543 g) had fully extended abdomens, but had not yet ovulated at the time of capture; fish (8 females, 2 males) were therefore transported to the laboratory, where they were housed individually in 70 L plastic bins equipped with aquarium filters. Animals were fed daily on the same diet as captive fish and monitored for ovulation on alternate days. Ovulation was evident within 1 week of capture in seven females, whose eggs were collected and stored as described above for subsequent experimentation. Ovulation did not occur in one female.
On the basis of the findings from 2010, the timing of fish collections was fine-tuned to enable the capture of additional females with ovulated eggs from the wild in June 2011 and 2012. Accordingly, two (2011) and five (2012) females (157–489 g) were collected and following anaesthesia, ovulated eggs were retrieved in the field at the site of capture and stored on ice until use (see below). Total weight (g) of eggs was recorded. All fish were returned to the site of capture on completion of gamete collection.
Fertilisation
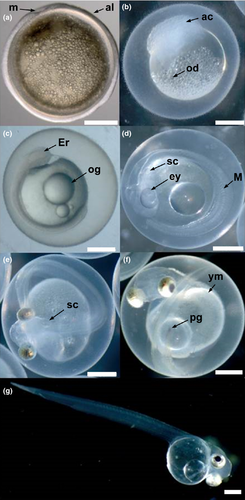
Eggs were dry-fertilised with pooled milt from two males for the trials in 2010 and with pooled milt from three males in both 2011 and 2012. Regardless of year, the method of dry fertilisation was kept consistent and milt from males with estimated sperm motility > 70% was used; pooled milt was added to the eggs and gametes were mixed gently by hand before activation. Ovulated and fertilised eggs were photographed through a dissecting microscope connected to an Olympus digital camera and diameters were measured to the nearest micrometre using Olympus DP2-BSW Application Software.
Trial I: wild and captive giant kokopu from 2010
The effects of salinity on fertilisation and hatching rates were assessed. For this purpose, water of different salinities was prepared by mixing fresh spring water (0 PSU) and full-strength seawater from the Otago coast (30-35 PSU) in different ratios. Salinities (0, 9, 15 or 30 PSU) were confirmed using a YSI Professional Plus meter.
Eggs coated in pooled milt were divided into roughly equal portions using a plastic teaspoon (ca. 400 eggs) and placed onto incubation trays consisting of 0.2 mm mesh netting. Fertilisation of eggs was initiated by adding water of different salinities (0, 9, 15 or 30 PSU). Thereafter, eggs were briefly washed with water of the same salinity and incubated semi-dry (simulated terrestrial oviposition), as terrestrial egg deposition in the riparian margins of water bodies during elevated water levels has been documented in all amphidromous species of galaxiid in New Zealand (McDowall & Charteris 2006; Smith et al. 2013). In addition, a fifth group of eggs was fertilised in 0 PSU and incubated fully submerged (simulated aquatic oviposition) in static 0 PSU water during incubation in petri dishes. To minimise evaporation, petri dishes were covered with loose-fitting plastic lids and incubated at 10 ± 1°C, under 100% humidity. Eggs incubated semi-dry were kept moist by spraying once daily with 0 PSU water. Three replicate incubations were run for each female at each salinity.
Due to consistent mass mortalities of all semi-dry incubated eggs from wild giant kokopu (June 2010), eggs from captive fish (October 2010) were only fertilised in 0 PSU and incubated fully submerged in static 0 PSU water at 10 ± 1°C, under 100% humidity.
Trial II: wild giant kokopu from 2011 and 2012
Eggs from individual females coated in pooled milt were divided into roughly equal portions and placed into petri dishes as described above. Fertilization of eggs was initiated as described above using water at 0, 9, 15, 20 or 35 PSU salinity. Thereafter, eggs fertilised at a given salinity were subdivided between three subgroups and incubated in duplicate at either 10 ± 1°C, 15 ± 1°C or 17 ± 1°C, under 100% humidity. In addition, surplus eggs from all five fish collected in 2012 were pooled together, fertilised and incubated submerged in 0 PSU in duplicate at 4 ± 1°C, under 100% humidity. Fertilization rate was calculated as described by Hicks, Barbee, Swearer and Downes (2010) once photos had been taken of all incubation trays.
Embryonic development
From 24 h post-fertilization until hatching, developing eggs from 2010 incubated submerged in 0 PSU at 10 ± 1°C were photographed through a dissecting microscope to document embryonic development. Water containing 1 mL L−1 of 1% methylene blue (Brooklands Pet Products, New Plymouth, New Zealand) was replaced daily. Rotting and unfertilised eggs were routinely removed from among free-flowing eggs incubated submerged but not from eggs incubated semi-dry, as these eggs adhered together.
Hatching and larval rearing
Developing eggs were checked daily. Free-swimming larvae were removed upon hatching and promptly transferred to 200 L larval rearing tanks containing aerated full-strength seawater. The period of incubation and elapsed time until hatching were recorded for each batch of eggs. Incubation period was defined as the number of days from fertilization till hatching of the first larva. Hatch rate was calculated by counting the total number of hatched eggs in each replicate and expressing it as a percentage of the total number of eggs.
Larvae were fed freshly hatched brine shrimp Artemia salina nauplii, before being weaned onto dry commercial salmon feed (Reliance Stock Foods, Dunedin, New Zealand). Juveniles were transferred to broodstock conditioning tanks (1000 L recirculating tanks of freshwater).
Statistical analysis
Unpaired independent two-tailed t-tests were used to compare mean egg sizes between captive and wild 2010 fish; pseudo-replication was avoided by averaging egg diameters within fish to yield a single egg diameter for each animal, which was then used for statistical analysis. Hatching rates from wild and captive fish in 2010 were analysed by univariate anova in IBM spss Statistics, Version 20, in which replicate incubations were nested within individual fish to evaluate the effect of source (wild vs. captive) on the response variable. Effects of salinity on fertilisation and hatching could not be determined due to widespread mortality of semi-dry incubated eggs in Trial I.
Data from Trial II were analysed combined for 2011 and 2012 to tease out salinity and temperature effects; for this purpose, we square-root arcsine-transformed fertilisation and hatching rates to generate approximately normally distributed data which were analysed by nested univariate anova in spss, using the following statistical model: /DESIGN=temp sal temp*sal fish(temp*sal) in which fish was defined as a random variable. Significant treatment effects were further analysed with multiple comparisons using Scheffé's procedure, a conservative test that minimises Type I error. For all analyses, treatment effects were considered significant for P < 0.05.
Results
Trial I: wild and captive giant kokopu from 2010
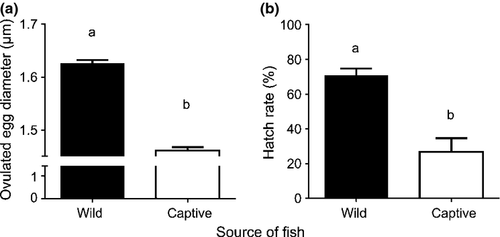
Giant kokopu sourced from the wild produced eggs with a significantly larger diameter than those from captive fish upon strip-spawning (Fig. 1a) (two-tailed t-test; t-statistic = 7.02, P < 0.001, d.f. = 9). Hatch rates were significantly higher in eggs sourced from wild fish (71%) compared to those sourced from captive fish (26%) (Fig. 1b) (F1, 8.12 = 5.29, P = 0.05).
Although embryonic development occurred in eggs incubated in semi-dry conditions, hatching was not observed due to the gradual decay and mortality of eggs by a progressively invasive microbe. As a result, the effects of salinity on fertilisation and hatch rates could not be determined accurately in 2010. Hatch rate could only be calculated from eggs fertilised and incubated submerged in 0 PSU at 10 ± 1°C. Embryonic development was successfully documented (Fig. 2).
Trial II: wild giant kokopu from 2011 and 2012
Fertilisation rate decreased significantly as salinity increased, with a mean fertilisation rate of 95% (98–99% in some replicates) when gametes were activated in freshwater (0 PSU) (Fig. 3a) (F4, 30 = 39.66, P < 0.001). Mean fertilisation rates halved (48%) as salinity increased above 9 PSU. Fertilisation was not observed when gametes were activated in salinities of 20 PSU or greater. The mean diameter of fertilised eggs was 2.1 mm.
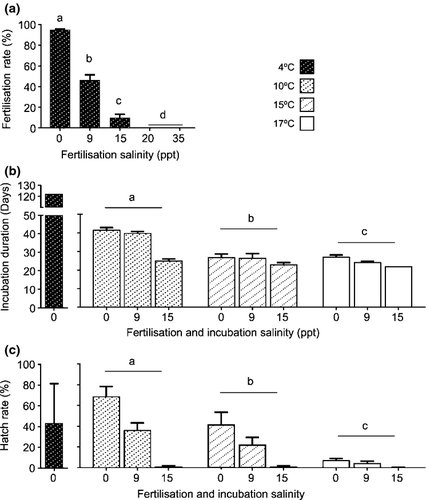
Significant differences in time until hatching among salinities (F4, 86.04 = 67.37, P < 0.001) (data not shown) and temperatures (F2, 85.74 = 7.28, P = 0.001) were observed, with incubation duration until first larva hatching decreasing as temperature and salinity increased (Fig. 3b). Eggs incubated at 4°C in 0 PSU water remained viable for 122 days (488 day degrees) before hatching. Incubation duration more than halved (mean = 41 days) at 10°C (410 day degrees) and only took an average of 27 days at 15 (405 day degrees) and 17°C (459 day degrees) in 0 PSU water. The small number of viable eggs incubated in 15 PSU water had shorter incubation durations (<25 days; <405 day degrees) than other salinity treatments in each temperature. A significant interaction between salinity and temperature on incubation duration was detected (F8, 85.74 = 2.73, P < 0.001).
Hatch rates varied significantly among salinities (F4, 80.66 = 10.31, P < 0.001) and temperatures (F2, 80.74 = 6.14, P < 0.05), decreasing as temperature and salinity increased. Highest hatch rates (68%) were observed at 10°C and 0 PSU, and the lowest (<1%) at 17°C and 15 PSU (Fig. 3c). There was also a significant interaction between salinity and temperature on hatch rate (F8, 80.61 = 2.52, P < 0.05). Hatch rates of eggs incubated at 4°C averaged 43%.
Larval feeding commenced approximately 5 days after hatching. At around 6 months of age, crystalline juveniles (whitebait) began consuming dry commercial salmon feed until reaching maturity.
Giant kokopu batch fecundity
There was considerable variation among the number of ovulated eggs stripped from each fish with estimated fecundities ranging from 2520–15820 eggs in wild fish and from 5850–9890 eggs in captive fish. On average, giant kokopu produced 8340 eggs. Relative fecundity was 36 500–45 800 eggs kg−1 of body weight when it could be calculated. The average ovulated egg diameter was 1.6 mm with diameters ranging from 1.5–1.8 mm in wild fish and from 1.3–1.7 mm in captive fish.
Discussion
The objective of our study was to develop methods to cultivate giant kokopu in captivity to aid species conservation and commercial production efforts. More specifically, we investigated the effects of salinity and temperature on fertilisation, incubation and hatching of eggs sourced from wild and captivity-acclimated giant kokopu that had spontaneously ovulated. Sequential stages of embryonic development were documented and several year classes of offspring were produced to form a captive breeding colony to support future aquaculture and conservation research endeavours. It is anticipated that developed culture methods will also be applicable to other species in the Galaxias genus that share similar reproductive strategies.
Giant kokopu sourced from the wild produced eggs that had a significantly larger diameter upon ovulation and that yielded significantly higher hatch rates in comparison to eggs sourced from captive fish. According to Brooks, Tyler and Sumpter (1997), hatch rates are the ultimate measures of egg quality, suggesting that eggs obtained from captive fish were of ‘poorer’ quality; despite egg quality being a major bottleneck in aquaculture settings, there is still little known about the factors that influence this (Migaud, Bell, Cabrita, Mc Andrew, Davie, Bobe, Herraez & Carrillo 2013). Some such factors include the diet and endocrine status of a brood female throughout oogenesis, the environmental conditions brood fish are exposed to during oogenesis, the intrinsic properties of the egg itself, genetics, the environment in which eggs are fertilised and subsequently incubated, and the husbandry practices to which captive brood fish are exposed during a reproductive season (e.g. stressors such as handling) (Brooks et al. 1997; Bobe & Labbé 2010; Migaud et al. 2013). In this context, it is relevant to emphasise that ripe wild fish were captured in water that was considerably cooler than that imposed on captive fish; moreover, the latter were also accidentally exposed to temperatures between 18 and 23 ± 1°C for a brief period prior to the onset of vitellogenesis due to technical difficulties. Furthermore, stress from repeated gonad sampling used to track gonadal development may have also contributed to lower hatch rates in captive fish as this manipulation involved fish handling, anaesthesia and the making of a surgical incision into the body cavity (for details, see Wylie et al. 2013).
In the 2011 and 2012 spawning seasons, fertilisation rates of eggs sourced from wild fish decreased significantly when gametes were activated in water of increasing salinities (i.e. 0, 9, 15, 20 or 35 PSU); thus, in fresh water, fertilisation rates were close to 100%, whereas rates dropped to 0% in salinities greater than 15 PSU. Hicks et al. (2010) reported a similar pattern for inanga across salinities ranging from 1.4 to 34 PSU. This trend suggests that giant kokopu, like inanga, requires water bodies of a low salinity for spawning, which is supported by the recent discovery of terrestrially deposited giant kokopu eggs in the vegetation of a small urban stream following high-flow events (Smith et al. 2013).
In our experiments, semi-dry incubated eggs failed to complete development due to microbial infection. In contrast, submerged eggs could be easily maintained, not in the least as they lost their gelatinous adhering properties and became free flowing, allowing for the removal of infected, dead or unfertilised eggs. Submerged incubations of giant kokopu eggs revealed an interaction between temperature and salinity. The average incubation duration of eggs incubated at 10°C extended from 25 days in 15 PSU water to 41 days in 0 PSU water, and took no longer than 26 days on average to hatch in any of the other temperature-salinity combinations. These observations are very comparable to those of inanga cultivated by Mardones et al. (2008) which took 25–30 days to hatch when incubated at 10–12°C and a salinity of 10–16 PSU. These results are also consistent with findings from McDowall and Kelly (1999) that by analysing whitebait otoliths, estimated giant kokopu development to take 28 days. Incubation at 4°C demonstrated the remarkable plasticity of giant kokopu eggs, with development proceeding for 122 days until hatching. Having the ability to advance or delay the incubation period has potential advantages, when cultivating whitebait to generate out of season products and improve responsiveness to market variables and timing (Pillay 1990). It could also have implications during the transportation of fertilised eggs destined for hatchery rearing.
Similar to the trends observed for incubation duration, hatch rates also decreased as salinity increased, this trend becoming more pronounced as temperature increased. Highest hatch rates (71%) from spawning in 2010, and from spawning events in 2011 and 2012 (68%) were achieved when eggs were fertilised and incubated in 0 PSU at 10°C. Hatch rates dropped to <7% in all salinities at 17°C, and regardless of incubation temperature, salinities of ≥15 PSU appeared to be detrimental to hatching, with hatch rates of <1%. Hatching rates of terrestrially deposited inanga eggs incubated at 10, 15 and 17°C were not specifically reported by Semmens and Swearer (2011). However, the authors observed that larvae incubated at lower temperatures were larger and had larger yolk sacks upon hatching than those incubated at warmer temperatures. Optimal temperatures described by Mardones et al. (2008) for the commercial production of whitebait using inanga are also within this range (10–12°C during incubation and 12–15°C during hatching). Hatching temperatures were further consistent with those described by Mitchell (1989). Despite high mortalities in temperature-salinity combinations greater than 10°C and 0 PSU, hatch rates observed at 10°C and 0 PSU were still more than double the 30% rate reported for giant kokopu by O'Brien and Cooper (2013).
To reinforce the need for future research on whitebait cultivation, Mardones et al. (2008) and more recently Mardones and Ríos-Escalante (2012) identified several areas that need to be investigated to optimise the current whitebait farming industry. These include increasing the production of high-quality domesticated broodstock, and identifying reproducers with high fecundities and high larval survival rates. Currently, the Chilean whitebait industry can manually spawn 96 000 female inanga over a thirty-day period, and to produce ten tonnes of whitebait, approximately 200 million eggs need to be collected (Mardones et al. 2008; Mardones & Ríos-Escalante 2012). With an average fecundity about 10-fold higher for giant kokopu than for inanga (Jellyman 1979; Rasmussen 1990; this study), such a target can be reached with a substantially smaller number of broodstock, thus potentially reducing space and labour costs. Ideally, future research would involve a feasibility study incorporating biological attributes of this species and a bio-economical assessment of various end products.
In conclusion, we have demonstrated that hatch rates were significantly higher in eggs sourced from wild than from captive giant kokopu. Hatching rates were further affected by incubation conditions, edging notably higher when eggs were maintained under submerged conditions at low temperatures and at low salinities. Accordingly, reproduction-related traits of giant kokopu seem superior to those of Galaxias maculatus, reinforcing the potential of this fish for aquaculture use.
Acknowledgments
The authors thank R and G Munro of Mokotua for access to their property to capture fish, A Hicks, S Divers, S Olliver and J Shelley for assistance with wild fish capture and Y Ozaki for his assistance during the fertilisation trial. This study was funded by the Foundation for Research, Science and Technology Te Tipu Pūtaiao Fellowship (contract number: UOOX0910).




