Leucocyte profile and growth rates as indicators of crowding stress in pejerrey fingerlings (Odontesthes bonariensis)
Abstract
Crowding is one of the most common stressors found in intensive aquaculture, compromising growth rates and immune function. Plasmatic cortisol is a classic stress biomarker for fish, but its quantification is expensive and demands blood volumes that small individuals do not provide, constraining the usage of this technique to assess stress in fingerlings. The leucocyte profile is an alternative methodology to quantify stress with reduced costs and volumes of blood. Stress conditions promote neutrophilia and lymphocytopenia as response to elevated glucocorticoids levels. Considering the difficulties to assess stress imposed by intensive fish farming using measurement of glucocorticoid hormones, this study aimed to evaluate the stress-induced changes in leucocyte profiles and growth rates imposed by crowding in fingerlings of Odontesthes bonariensis, a promising South-American candidate for freshwater aquaculture. To meet these objectives, fingerlings (initial weight 0.05 ± 0.06 g and length 1.68 ± 0.13 cm) were reared for 45 days under three rearing densities (1, 5 and 10 fingerlings L−1). At the end of this period, fish were anaesthetized and euthanized to obtain the leucocyte profile, neutrophil:lymphocyte ratio (N:L) and growth rate. Increasing density promoted: significant reduction in growth (final length, weight and specific growth rate); neutrophilia and lymphocytopenia; and increased N:L ratio. Concluding, the tested rearing densities imposed distinct levels of stress characterized by different N:L ratio, demonstrating that the leucocyte profile is a reliable alternative to measure stress levels in O. bonariensis fingerlings and probably in other fish species.
Introduction
The stress imposed by farming practices has received considerable attention over the years (Pickering 1993; Wendelaar Bonga 1997; Tsuzuki, Ogawa, Strussmann, Maita & Takashima 2001) and the stressors found in fish production are varied, such as capture, transport, temperature variations, noise and stocking density (Schram, Van Der Heul, Kamstra & Verdegem 2006; Anderson, Berzins, Fogarty, Hamlin & Guillette 2011). These factors compromise several physiological and behavioural processes such as growth rates (Hatziathanasiou, Paspatis, Houbart, Kestemont, Stefanakis & Kentouri 2002), reproduction (Iwama, Pickering, Sumpter & Schreck 1997), haematological aspects (Clauss, Dove & Arnold 2008) and immune function (Tort 2011). Therefore, stress imposed by farming practices compromises the efficiency of intensive fish production (Iwama et al. 1997). Among these aspects, high stocking densities are one of the most common stressors found in aquaculture and can be related with chronically elevated cortisol levels (Barcellos, Kreutz, de Souza, Rodrigues, Fioreze, Quevedo, Cericato, Soso, Fagundes, Conrad, da Lacerd & Terra 2004a; Barcellos, Kreutz, Quevedo, Fioreze, Cericato, Soso, Fagundes, Conrad, Baldissera, Bruschi & Ritter 2004b; Ramsay, Feist, Varga, Westerfield, Kent & Schreck 2006; Brown, Watson, Bourhill & Wall 2010).
Glucocorticoids release is controlled by hypothalamus–pituitary–interrenal axis and coordinates the primary stress response (Wendelaar Bonga 1997; Montero, Izquierdo, Tort, Robaina & Vergara 1999; Ruane, Carballo & Komen 2002; Ramsay et al. 2006). Plasma cortisol is a classic stress biomarker for fish (Anderson et al. 2011), but its quantification is expensive and demands blood volumes that small individuals do not provide, limiting the utilization of this technique to quantify stress in larvae and small juveniles. Thus, alternative methodologies to assess stress, such as leucocyte profiles, are becoming more popular (Davis, Maney & Maerz 2000; Rijn & Reina 2010; Anderson et al. 2011; Bacchetta, Cazenave & Parma 2011). The stress-induced changes in leucocyte profiles (increase in circulating neutrophils and decrease in circulating lymphocytes) have been shown to be related to elevated glucocorticoids, both endogenous and exogenous (Harris & Bird 2000; Wojtaszek, Dziewulska-Szwajkowska, Lozinska-Gabska, Adamowicz & Dzugaj 2002; Anderson et al. 2011). Moreover, the relative proportion of neutrophils to lymphocytes (N:L ratio) is considered to be a composite measure of a secondary stress response, since both leucocyte types are affected by stress in opposite directions (Davis et al. 2000). Cortisol is also known to cause reduced growth rates by direct action or by suppression of food intake (Gregory & Wood 1999), thus, growth rates are often used as a tertiary stress index (Anderson et al. 2011).
The pejerrey Odontesthes bonariensis belongs to the Atherinopsidae family and has been introduced into several countries (Peru, Bolivia and even Japan) as a promising candidate for freshwater aquaculture (Tsuzuki et al. 2001; Somoza, Miranda, Berasain, Colautti, Lenicov & Strussmann 2008) as a result of the high quality of its flesh combined with its attractiveness as a game fish (Somoza et al. 2008; Colautti, Garcia de Souza, Balboni & Baigún 2010). Many pejerrey species have been proved to be sensitive to stress imposed by intensive farming such as handling and long-term captivity (Somoza et al. 2008), with indicated rearing densities (for O. bonariensis juveniles) ranging from 1 to 2 individuals L−1, demonstrating that O. bonariensis is sensitive to crowding (Berasain, Velasco & Coulatti 2001).
Considering the difficulties to assess stress using measurement of glucocorticoid hormones in small individuals, such as high cost and the blood volumes that are required, the aim of this study was to evaluate the stress-induced changes in leucocyte profiles and growth rates imposed by crowding in O. bonariensis fingerlings.
Materials and methods
Experimental animal and rearing
Three thousand eight hundred O. bonariensis fingerlings (60 days old) hatched and reared at the Chasqueiro hatchery (Arroio Grande Town, Brazil) were used in the experiment. Fish were fed twice a day ad libitum with commercial food powder (Supra Alevins®; 56% PB; 10% lipids; Alisul, Brazil).
Experimental design and necropsy
First, initial weight (0.05 ± 0.06 g) and length (1.68 ± 0.13 cm) were measured in a sample of 60 fingerlings. Following initial biometrics, fish were randomly distributed among each experimental cage, which consist of three different rearing densities: 1, 5 and 10 fingerlings L−1. All rearing densities were made in triplicate, with exception for the 10 fingerlings L−1 treatment, made in duplicate. The cage nets were made from nylon (2 × 2 mm) and had 100 L volume. For running the experiment, all cages were immersed in an earthen pond (200 m2 × 1 m depth) under natural photoperiod and temperature. Water pH (6.86 ± 0.83) and NH3 (<0.1 mg L−1; by Nutrafin NH3/NH4 test – Rolf C. Hagen Corp., Mansfield, MA, USA) were measured once a week at 9:00 hours and 14:00 hours. Dissolved oxygen (8.82 ± 1.63 mg L−1) and water temperatures (22.74 ± 3.52°C) were measured twice a day at the same times. All parameters were statistically equal among treatments. At the end of the experiment (past 45 days), 15 fish from each rearing density (exception for 10 fingerlings L−1 in which 10 fish were used) were anaesthetized (benzocaine, 50 ppm) for blood smears preparation after caudal peduncle section, according to Anderson et al. (2011). Finally, fish were weighed and measured to obtain final weight and length. The specific growth rate (SGR) was calculated by: SGR = 100 (ln Wf – ln Wi)/t; where Wf and Wi are the final and initial weights or lengths of the fish, respectively, and t is the number of experimental days.
Haematological analysis
Blood smears obtained from fingerlings were air-dried and then submerged in methanol for fixation. Smears were stained with Giemsa 5% according Tavares-dias and Moraes (2004). Leucocyte profiles (white blood cell differentials) were obtained by light microscopy examination of 100 leucocytes. The quantified cells were as follows: lymphocytes, monocytes, neutrophils and other granulocytes. The neutrophil:lymphocyte (N:L) ratio was used to measure the stress response displayed by juveniles.
Statistical analysis
Data (leucocyte profiles, weight and length) from different treatments were tested by anova, followed by unequal N HSD post hoc tests. Correlations were tested by Pearson product-moment correlation coefficient.
Results
Haematology
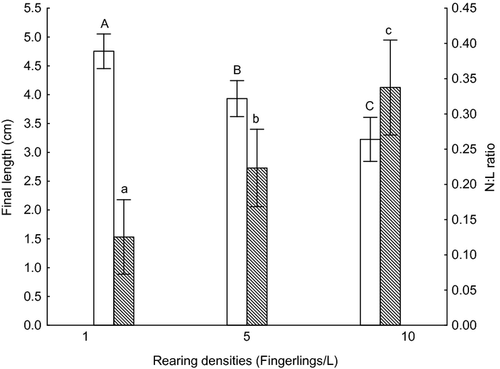
The number of neutrophils was significantly different among all rearing densities (Table 1) with significant positive correlation (Pearson's correlation: r2 = 0.47; r = 0.68; P < 0.001). Significant differences in the number of lymphocytes were only observed between 1 and 10 fingerlings L−1 treatments (Table 1) with negative correlation (Pearson's correlation: r2 = 0.29; r = −0.54; P < 0.001). Other leucocytes numbers showed no significant differences. As consequence for the correlations between neutrophils and lymphocytes with the rearing densities, the N:L ratio was significantly different among all rearing densities (Fig. 1) with significant positive correlations (Pearson's correlation: r2 = 0.46; r = 0.68; P < 0.001).
| Physiological measure | One fish L−1 Mean ± SD | Five fish L−1 Mean ± SD | Ten fish L−1 Mean ± SD |
|---|---|---|---|
| Final weight (g) | 0.67 ± 0.27a | 0.38 ± 0.09b | 0.18 ± 0.06b |
| Final length (cm) | 4.85 ± 0.63a | 4.00 ± 0.43b | 3.17 ± 0.35c |
| Specific growth rate in weight (%) | 5.74 ± 0.44a | 4.51 ± 0.29b | 2.88 ± 0.63c |
| Specific growth rate in length (%) | 2.34 ± 0.12a | 1.92 ± 0.15b | 1.41 ± 0.17c |
| Lymphocytes (%) | 75.3 ± 6.5a | 68.9 ± 8ab | 64.0 ± 6.9b |
| Monocytes (%) | 13.3 ± 5.6a | 16.0 ± 7.5a | 15.0 ± 3.7a |
| Neutrophils (%) | 9.6 ± 3.0a | 14.8 ± 6.4b | 20.9 ± 4.4c |
| N:L ratio | 0.12 ± 0.05a | 0.22 ± 0.12b | 0.33 ± 0.09c |
Growth
Fingerlings final weight had a negative correlation with the rearing densities (Pearson's correlation: r2 = 0.52; r = −0.72; P < 0.001) and fish from one fingerling L−1 treatment were statistically heavier than the other densities (Table 1). For the final length, negative correlations were observed (Pearson's correlation: r2 = 0.63; r = −0.80; P < 0.001) and fish from all the rearing densities had statistically different final lengths (Fig. 1). The SGR index, both in weight and in length, showed significant differences among all treatments (1≠10, 1≠5; 5≠10 fingerlings L−1; Table 1) and had negative correlations between rearing densities (Pearson's correlation: r2 = 0.91; r = −0.95; P < 0.001; Pearson's correlation: r2 = 0.90; r = −0.95; P < 0.001) respectively.
Discussion
Haematology
The WBC counts might be a useful parameter to evaluate fish welfare, but the lack of information about basal percentage of these cells in the blood of different species, reared in diverse culture conditions, constrains the use of this parameter to evaluate physiological status (Davis et al. 2000). Thus, determining the basal concentrations of leucocytes in species under different stress conditions might be of great importance to provide comparable data that can be used to monitor the health status of fish and other vertebrate species.
Lymphocytopenia and/or neutrophilia are classic haematological responses to stress and can be modulated by glicocorticoid hormones through redistribution of these cells from the blood to other body compartments (Dhabhar 2002). Following stress, lymphocytes undergo transmigration from circulation into other tissues, e.g. lymph nodes, spleen, bone and skin, leading to a significant reduction in their circulating numbers (Fauci 1975; Ottaway & Husband 1994). In contrast, glicocorticoids also stimulate the exodus of neutrophils from leucopoetic organs into the blood and mitigate the egress of neutrophils from the blood vessels to other body compartments (Bishop, Athens, Boggs, Warner, Cartwrig & Wintrobe 1968; Dhabhar, Miller, McEwen & Spencer 1996). In fact, studies demonstrate these stress-induced patterns of change in leucocyte profiles of fish, i.e. lymphocytopenia (Ghazaly 1992; Barcellos et al. 2004a,b) accompanied or not by neutrophilia (Ellsaesser & Clem 1986; Khangarot, Rathore & Tripathi 1999; Valenzuela, Silva & Klempau 2008; Rijn & Reina 2010). In accordance with the dichotomous response observed in leucocyte profiles after stress, the N:L ratio can be used as an index of a secondary stress response (Davis et al. 2000), but just a few studies use this parameter to assess stress in fish (Rijn & Reina 2010; Anderson et al., 2011) despite the extensive body of evidence that sustain the N:L ratio (or Hetephils:Lymphocytes) as a measure of stress for other vertebrate groups (Gross & Siegel 1983; Hansen & Damgaard 1993; Fisher, Crowe, Ó Nuallàin, Monaghan, Prendiville, O'Kiely & Enright 1997; Davis, Anderson & Carroll 2008; Parga, Pendl & Forbes 2001; Moreno, Merino, Martinez, Sanz & Arriero 2002; Case, Lewbart & Doerr 2005; Obernier & Baldwin 2006; Chen, Niu & Pu 2007; López-Olvera, Marco, Montané, Casas-Díaz & Lavín 2007; Noda, Akiyoshi, Aoki, Shimada & Ohashi 2007).
WBC counts can also be of great interest to assess stress in small fish. Actually, a small volume of blood incapacitates the measurement of circulating cortisol. Thus, the measurement of this hormone in fish with little amount of blood, e.g. juvenile forms and small species, relies on the quantification of whole-body cortisol (Barry, Malison, Held & Parrish 1995; Pottinger & Calder 1995; Feist & Schreck 2002; Ramsay et al. 2006), a relatively expensive and complex procedure. In this regard, blood smears can provide a reliable assessment of stress with a simple, practical and relatively inexpensive procedure.
Growth
The different rearing densities tested in this experiment promoted distinct growth rates, which can be attributed to imposition of stress at different intensities (Ruane et al. 2002). The final length had a stronger correlation with the rearing densities than the final weight, proving to be a more stress-sensitive parameter for O. bonariensis fingerlings. The SGR observed in our experiments was very similar with those reported in the literature. Indeed, Berasain et al. (2001) reported SGR's of 2.2%/day for 60-days-old O. bonariensis fingerlings reared in stoking densities ranging from 1 to 5 fish L−1, the same SGR observed in the one fingerling L−1 treatment of our experiment. This means that fish grew at the expected rates, and that the reduced growth presented by fish exposed to higher densities (i.e. 5 and 10 fingerlings L−1) could be associated with a stress condition (Hatziathanasiou et al. 2002; Schram et al. 2006).
Conclusions
The distinct patterns of leucocyte profiles and growth rates obtained for each rearing density indicate that O. bonariensis fingerlings are sensitive to crowding. Moreover, when compared to the SGR reported by literature, the growth rates obtained in our experiment demonstrate that the higher rearing densities tested promoted stress conditions for the fingerlings. Furthermore, we were able to assess a secondary stress response in small fish, as fingerlings, using blood smears. Thus, we indicate this methodology to quantify stress in small vertebrates, such as fish fingerlings.
Acknowledgments
All work was conducted in adequation with the Universidade Federal de Pelotas Animal Ethics Commission (Protocol 5.06.03.023) and was supported by grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). R. Robaldo is a research fellow of CNPq (307478/2012-2).




