Non-invasive fast real-time PCR assay for detection of the enteric parasite Enteromyxum scophthalmi in cultured turbot (Scophthalmus maximus L.)
Abstract
Enteromyxum scophthalmi is a myxozoan parasite that causes severe parasitic diseases in cultured turbot affecting mainly the intestine of the host. It is characterized by producing acute enteritis, starvation and eventually death. Current diagnosis of E. scopthalmi use traditional techniques, based on the identification of the morphology of the parasite. These techniques take extended time to be carried out and do not favour the adoption of control measure at turbot farms and require the sacrifice of fish. This study develops a fast real-time PCR molecular tool for the detection of E. scophthalmi in infected farmed turbot. This methodology is applicable for routine controls on the farm at every stage of the parasite infection. Results of the study demonstrate the robustness, specificity, efficiency and reliability of the technique. In addition, this study also provides a non-invasive procedure of sampling through swaps. This allows control, prevention and diagnosis of the parasite infection at turbot farms while maintaining the welfare of the cultivated fish and avoiding sacrifice of the fish sampled.
Introduction
The turbot, Scophthalmus maximus, is one of the most farmed marine fish in Europe. Production of turbot has increased all around the world in the last decades. Flatfish production increased from 26 300 tonnes in 2000–148 800 tonnes in 2008 (FAO 2010). However, production of turbot is affected by multiple factors that limit its growth, including the infection by pathogens that limit the production and the profitability. Among the pathogens affecting turbot is the parasite Enteromyxum scophthalmi, described as being responsible for causing severe disease in turbot and mortality of up to 100% of affected fish in some cases (Branson, Riaza & Alvarez-Pellitero 1999).
E. scophthalmi is a myxozoan parasite described by Palenzuela, Redondo & Alvarez-Pellitero 2002; but the first report about the occurrence of this parasite was done by Branson et al. 1999. A more detailed morphological and ultrastructural study, complemented with molecular phylogenetic analyses, allowed Palenzuela et al. to explain the taxonomy of this pathogen. These authors established the new genus Enteromyxum to accommodate the new species E. scophthalmi. (Branson et al. 1999; Palenzuela et al. 2002).
Further research provided increased knowledge about E. scophthalmi. Studies on experimental infections confirmed the direct fish-to-fish transmission through cohabitation or waterborne contamination (Redondo, Palenzuela, Riaza, Macias & Alvarez-Pellitero 2002). In vitro cultures were tested to obtain a source of material for subsequent studies. However, the culture process was unsuccessful and only intestinal turbot explants resulted in a useful model to study the interaction of this pathogen (Redondo, Palenzuela & Alvarez-Pellitero 2003; Redondo & Alvarez-Pellitero 2010). In addition, ultra-structural and immunological studies of the different stages of the parasite in the fish clarified the routes of invasion and dispersion of the parasite, part of life cycle of the parasite, as well as the innate and acquired immunity of fish (Bermudez, Losada, Vazquez, Redondo, Alvarez-Pellitero & Quiroga 2010; Bermudez, Vigliano, Marcaccini, Sitja-Bobadilla, Quiroga & Nieto 2006; Bermudez, Vigliano, Quiroga, Nieto, Bosi & Domeneghini 2007; Redondo & Alvarez-Pellitero 2010; Redondo, Cortadellas, Palenzuela & Alvarez-Pellitero 2008; Sitja-Bobadilla, Redondo, Bermudez, Palenzuela, Ferreiro, Riaza, Quiroga, Nieto & Alvarez-Pellitero 2006; Sitja-Bobadilla, Redondo, Macias, Ferreiro, Riaza & Alvarez-Pellitero 2004). This opened the possibility of considering turbot as an alternate and not a targeted host of this parasite (Redondo, Quiroga, Palenzuela, Nieto & Alvarez-Pellitero 2003; Redondo, Palenzuela & Alvarez-Pellitero 2004).
To avoid the great losses caused by E. scophthalmi, its early detection and identification is essential for establishing control and prevention measures at turbot farms. The identification of the parasite in infected turbot is carried out through traditional techniques; or by scraping the caecum (beginning of the large intestine) and looking through a microscope for parasite structures. However, in these cases, personnel must be trained to identify the parasite microscopically, and parasites must be in sufficient numbers to be detected. Detection of the parasite at the beginning of the infection is vital to avoid the spreading at the farm and to provide a fast control reaction. A delay in the detection could result in a severe infestation at the farm.
Another important reason for the detection of parasitosis at a farm is to carry out routine controls while maintaining the welfare of the fish. The development of a detection assay using a non-lethal sampling method would allow high frequency of testing without compromising the health of the fish or the profitability of the production. The combination of molecular methodologies of detection with a non-lethal sampling will allow a fast, specific and harmless diagnosis (Fox, Palenzuela & Bartholomew 2000; Yanagida, Freeman, Nomura, Takami, Sugihara, Yokoyama & Ogawa 2005; Estensoro, Redondo, Álvarez-Pellitero & Sitja-Bobadilla 2010).
Molecular techniques have been applied for many years for the detection and identification of pathogen organisms in fish. However, only recently has the rapid technique of Real-Time PCR (RT_PCR) been applied for parasitological detection focused on food security (Alonso, Herrero, Vieites & Espiñeira 2011; Herrero, Vieites & Espiñeira 2011). The technique allows monitoring the results of the assay in real time and combines the specificity and sensitivity offered by the PCR technique. These characteristics led to strengthen its development and application in aquaculture parasitology (Hallett & Bartholomew 2006; Funk, Raap, Sojonky, Jones, Robinson, Falkenberg & Miller 2007; Alonso, Lago, Gomez-Reino, Fernandez, Martin, Vieites & Espineira 2013).
Only one recent study describes a molecular methodology for detection of E. scophthalmi by RT-PCR (Piazzon, Mallo, Martin, Fernandez-Casal, Sanmartin, Lamas & Leiro 2012), but their authors does not reflect the use of inhibitor controls, allowing the occurrence of false-negative results (Hoorfar, Cook, Malorny, Wagner, De Medici, Abdulmawjood & Fach 2003; Hoorfar, Malorny, Abdulmawjood, Cook, Wagner & Fach 2004; Apfalter, Reischl & Hammerschlag 2005; Hoffmann, Beer, Reid, Mertens, Oura, Van Rijn, Slomka, Banks, Brown & Alexander 2009). They also amplified a large fragment, greater than 800 bp, which is less efficient than a small fragment (Bustin 2000; Dorak 2007; Huggett & Bustin 2011). Furthermore, the detection is based on SYBR Green chemistry, with low specificity, instead of using a more specific internal probe (Yin, Shackel, Zekry, McGuinness, Richards, Van Der Putten & Bishop 2001; Bustin 2004; Bustin & Nolan 2004; Bustin, Benes, Nolan & Pfaffl 2005; Mackay & Landt 2007; Bustin, Benes, Garson, Hellemans, Huggett, Kubista, Mueller, Nolan, Pfaffl, Shipley, Vandesompele & Wittwer 2009; Kumar, Kumar, Rajput, Daga, Singh & Khanna 2012). In addition, the methodology does not consider the welfare of the fish, producing stress or death at the time the sample is taken (Fox et al. 2000; Yanagida et al. 2005; Estensoro et al. 2010). Conditions like lack of inhibitor control, fluorescence chemistry carried out by SYBR Green, fragment size being too large and invasive or lethal sampling reflect non-optimal conditions for Real-Time PCR creating the necessity for an alternative methodology.
In this study, a molecular methodology for the detection of E. scophthalmi in farmed turbot has been developed and applied, assuring a trustworthy result through a non-lethal sampling method. The fast real-time PCR methodology developed in this study is applicable in routine control and is effective at all stages of infection. The results show that the technique developed is robust and has a high specificity, efficiency and reliability.
Materials and methods
Sample collection, storage and DNA extraction
Whole juvenile turbot, S. maximus, and turbot caecum tissue infected by E. scophthalmi, were provided from a local farm in the north of Galicia (Insuíña S.L, Spain). In both cases, the parasite was previously identified through ultrastructural studies by experts at the farm (Palenzuela et al. 2002). These samples were preserved and transported under refrigerated conditions until the isolation of the parasite at the laboratory.
Other parasites which can affect S. maximus were provided from the same local farm. Tetramicra brevifilum and Philasterides dicentrarchi were used to support the specificity of the developed methodology. These parasites were microscopically identified by the pathologist of the farm before the DNA extraction. These DNA were frozen at −80°C until used.
Different tissues samples from whole infected fish, such as fin, gills, intestine, kidney, liver, spleen tissues and the mucus obtained by scraping the skin of the fish, were analyzed to evaluate the presence of the parasite. Also, non-invasive samples were taken due to the importance of developing a method that allows detecting the parasite at the farm which does not imply sacrificing the fish. These samples consisted of blood, faeces and rectal swabs. Blood samples were obtained by puncturing the caudal vessels, and faeces samples were obtained from the tanks of the farm directly. To obtain the rectal samples, the fish were anaesthetized with benzocaine and a sterile swab was inserted into the anogenital pore by a slight motion. This procedure was performed assuring that no harm was caused to the fish. After sampling, fish was allowed to recover from the anaesthesia and were monitored in the subsequent days.
Furthermore, different methods of storage [dry, in 95% ethanol and in RNAlater® (Ambion, Life Technologies S.A., Madrid, Spain)], temperatures (at 4°C and −20°C) and shipment times (0, 7 and 21 days) were assessed to determine the cheapest and easiest way (while keeping the appropriate preservation of the sample) of sending samples to be analyzed from the farm to the laboratory. The methods of storing and sending samples are shown in Table 1.
| Storage time | Storage temperature | |
|---|---|---|
| 4°C | −20°C | |
| 0 days | Dry | Dry |
| Ethanol 95% | Ethanol 95% | |
| RNA later | RNA later | |
| 7 days | Dry | Dry |
| Ethanol 95% | Ethanol 95% | |
| RNA later | RNA later | |
| 21 days | Dry | Dry |
| Ethanol 95% | Ethanol 95% | |
| RNA later | RNA later | |
DNA extractions from infected tissues, faeces and rectal swab were performed according to the protocol described by Lago, Herrero, Madriñán, Vieites and Espiñeira (2011) with slight modifications. The modifications are as follows: extractions were carrying out from 500 mg of tissues and faeces, and for rectal samples, the swap tip was cut and directly used. UltraClean BloodSpin DNA isolation kit (MoBio Laboratories, Carlsbad, CA, USA) was employed for the DNA isolation from blood samples.
The quality and quantity of the DNA extractions were determined by measuring the absorbance at 260 nm and at 260/280 and at 234/260 ratios using a NanoDrop™ ND-1000 spectrophotometer (Thermo Scientific, Madrid, Spain) (Winfrey, Rott & Wortman 1997). DNA extractions were stored at −80°C for subsequent tasks.
Design of a specific Real-Time PCR method to detect E. scophthalmi
From the ssrRNA (18S rRNA) sequences obtained from the NCBI database (National Center for Biotechnology Information (NCBI) 2013) AF411335 (E. scophthalmi), and EF126038 (S. maximus), two internal primers and a probe set specific for E. scophthalmi were designed with the Primer Express v 3.0 software (Applied Biosystems, Life Technologies S.A.): ENTEROMYX F (5′-GGC TTA ATT TGA CTC AAC A- 3′), ENTEROMYX R (5′-CTC CAC CAA CTA AGA ACG- 3′); and a labelled probe, ENTEROMYX PROBE (5′- 6-FAM-CCA TGC ACC ACC AAT CAT TGT ATC AT-BHQ-1 -3′).
The same region was selected to be used as an inhibitor control. This DNA extraction control allows the amplification of S. maximus with the same primers and a new probe (CONTROL ROD 1 5′- 6-FAM-CAC CAC CAC CCA CAG AAT CG-BHQ-1 -3′) to check the correct extraction and the absence of inhibitor compounds in the sample. This probe was designed in the same way as described above and used with the same primer pair designed for the detection of E. scophthalmi.
For both primers/probe sets designed, PCR was performed in a total reaction volume of 20 μL containing 200 ng DNA template, 10 μL TaqMan Fast Advanced Master Mix (Applied Biosystems), the optimized amount of primers and probe, and molecular biology grade water (Eppendorf, Ibérica, Madrid, Spain) adjusted up to the final volume. Optimal amounts of primers and probe were evaluated by preparing a dilution series to determine the minimum concentrations giving the maximum ΔRn (normalized reporter, defined as emission intensity of reporter/Emission intensity of passive reference). First, forward and reverse primer concentrations of 50, 300, 500 and 900 nM were evaluated with a constant probe concentration of 50 nM. Following primer optimization, probe optimization was initiated using concentrations of 50–250 nM. The two primers/probe sets, were optimized independently. Independent optimization was necessary because they were used in different reactions.
The reaction was performed in triplicate on DNA samples in MicroAmp Optical 96-well reaction plates (Applied Biosystems) with MicroAmp optical caps (Applied Biosystems) in the ViiA™ 7 Real Time PCR System (Applied Biosystems). Amplification was carried out with the following cycling protocol: 95°C for 20 s, and 40 cycles each of 95°C for 1 s and 60°C for 20 s.
Analytical specificity, detection limit (LOD) and reproducibility of the Real-Time PCR assay
Specificity was calculated by testing the specific primers/probe set for E. scophthalmi on DNA extracts from some of the most important parasites affecting turbot (T. brevifilum and P. dicentrarchi) and on its host (S. maximus). Also, phylogenetic analyses were carried out to evaluate relationship between E. scophthalmi and other parasites, determined by 18S rRNA. The analyses included sequences from mixozoan species, typical intestinal parasites in fish (Cryptosporidium) and parasites affecting the turbot. Parasites' sequences used for analysis were obtained from the NCBI database with accession number: AY38260, JX090294, AB643794, AY382606, AY152747, AB553293, AY152750, AY152749, AB710384, AY078428, JX090293, AF414692, AY078429, JX090295, JQ974029, AF378347, AY152748, AB693043, FJ204246, EU440379, HM037787, HM037789, HM037789, FJ848877, AJ581916, DQ851568, U13829, AJ582061, AY688957, JX271832, AF411334, DQ448298, DQ139796, DQ127230, AF001579, DQ301509, and AF411335. The phylogenetic tree was created by the software Mega 4.0 using the Tamura–Nei model to calculate the genetic distances between sequences (Tamura, Dudley, Nei & Kumar 2007). The inference of the phylogenetic tree was carried out with the Neighbour– Joining method (Saitou & Nei 1987). The reliability of the clades formed at the species level in the tree was evaluated by means of a bootstrap test with 2000 replications.
Due to technical limitations that currently exist in purifying the parasite, the calculation of the detection limit of the methodology was carried out from dilutions made by scraping the content of the caecum of infected turbots. The dilutions were made in water and corresponded to 500, 100, 50, 25, 10, 5, 2, 1, 0.5 and 0.1 μL of the scraping of the caecum contents in a final volume of 500 μL. These preparations were mixed by vortex. All the dilutions were done in duplicate, and the methodology developed here was applied to a complete series.
To infer the limit of detection (LOD), parasites from the duplicate of the last tube of the series that tested positive were counted under a microscope using a Neubauer counting chamber. This quantification is necessary because the degree of infestation in the fish is highly variable and will determine the concentration of parasites in the dilutions and therefore the LOD reached by the methodology developed. The parasites were visualized by examination under oil immersion microscopy objective (1000×). The number of parasites counted in five squares of the chamber, and the total parasites in the suspension were calculated according to general instructions of Neubauer chamber to recount of microorganisms. This procedure was repeated three times and the data presented as mean ± standard error.
The reproducibility of the RT-PCR assay was evaluated by testing multiple replicates of the standard control material. Each intra- and inter-assay was performed to evaluate its reproducibility: two analysts; three samples (with two replicates in each assay); three different days. The mean (M), standard deviation (SD), and coefficient of variation (CV) of the cycle threshold values were calculated. The CV values were calculated as the standard deviation (SD) divided by the mean (M) × 100% (CV = SD/M × 100%).
Application of the developed RT-PCR method in cultured turbot
The method was validated in infected and non infected fish samples provided from a local farm in the north of Spain. All samples were analyzed by means of classical histological techniques carried out by the pathologist of this farm. Subsequently, they were transported to the laboratory under refrigerated conditions (4°C) and were analyzed with the described methodology.
Results and Discussion
Enteromyxum scophthalmi is among the most dangerous parasites in turbot farms producing high mortality, which can reach up to 100% in affected stock in cases of severe infection. Currently, diagnosis of E. scophthalmi is done by traditional and ultrastructural studies (Redondo, Quiroga et al. 2003). Traditional techniques need extended time for preparation and specialized personal to carry them out. In addition, the long process can delay the adoption of control measures and result in severe infestation at the farm. Molecular techniques are a commendable alternative to the traditional techniques and are being applied to parasitological diagnosis. They can provide specificity, sensitivity and fast results.
The development of identification and detection techniques, such as metabolic and biochemical study on E. scophthalmi is limited by the inability to produce in vitro culture (Redondo, Palenzuela et al. 2003). This fact complicates the isolation of the parasite and limits its availability for the study. Maintaining this organism in vivo is the only way of isolating the parasite. Currently, experimental sustenance of this parasite is possible by direct transmission from fish to fish or by its culture in intestinal explants of turbot (Redondo & Alvarez-Pellitero 2010; Redondo et al. 2002; Redondo, Palenzuela et al. 2003).
One molecular methodology to detect this parasite was developed (Piazzon et al. 2012). However, this test did not include controls for assay inhibitors that can affect DNA amplification and lead to false-negative results or to decreased reliability and specificity of the method (Hoorfar et al. 2003, 2004; Apfalter et al. 2005; Hoffmann et al. 2009). In addition, the assay of Piazzon et al. was developed using SYBR green chemistry, which use a non-specific DNA binding dye that joins to any double stranded DNA, including primer dimmers, thus is not as specific as other approaches such as probe-based assays, In this study, a higher level of detection specificity is provided by using an internal probe to detect only the RT- PCR product of interest (Bustin 2004; Bustin et al. 2005, 2009; Bustin & Nolan 2004; Kumar et al. 2012; Mackay & Landt 2007; Yin et al. 2001). Our assay also amplifies a shorter fragment for more efficient amplification and increased tolerance of reaction conditions (Bustin 2000; Dorak 2007; Huggett & Bustin 2011). Conditions such as lack of inhibitor control, fluorescence chemistry being carried out by SYBR Green and fragment size being too large, reflect non-optimal conditions for RT-PCR. Therefore, creating the necessity for the development of an alternative methodology, with optimal conditions and which can be applied to aquaculture farms, where the fish welfare is protected. In this study, a fast real-time PCR technique for the detection and identification of E. scophthalmi has been designed, and its specificity, sensitivity, robustness and reliability have been verified.
Evaluation of sample collection, storage and DNA extraction
In this study, different tissue preparation, preservation and shipping methods were evaluated. This evaluation was conducted based on the quantity and quality of the isolated DNA (Table 1), and reflects the real alternatives for tissue storage and transport from the farm to the laboratory. In this sense, RNA later, besides being a reagent for the stabilization of RNA, is used typically as a stabilization reagent to store tissue (Nsubuga, Robbins, Roeder, Morin, Boesch & Vigilant 2004; Bent & Taylor 2010; Michaud & Foran 2011).
All DNA extractions obtained from the different variables showed good results because of the high quantity and quality of DNA obtained, according to spectrophotometric measurements (data not shown). Only dry samples, which were send without any refrigeration or preservation conditions, and in conditions of ordinary dispatch, showed low DNA quality. It is reflected in the low purity of the extractions (< 1.6), probably due to the degree of decomposition of the sample. When samples arrived within a few hours, the quality of extracted DNA was high regardless of the transport method (dry, without refrigeration or preservation). In all these cases, the inhibitor control test was always negative, eliminating the possibility of false-negative results, unlike the method published by Piazzon et al. (Piazzon et al. 2012).
Therefore, we concluded that the best way to send samples to reduce costs would be shipping the samples by ordinary shipment in 95% ethanol, regardless of the time that has elapsed before and during shipment. Thus, some extra costs such as different preservatives as RNAlater® (Ambion), or hiring special shipping conditions such as refrigerated shipping or dry ice, can be avoided.
Regarding the evaluation of the presence of E. scophthalmi in other tissues different from caecum or intestine tissues, it was concluded that the parasite was only present in target tissues such as caecum and intestine tissues, and in the rectal samples obtained by non-lethal sampling by rectal swab (Cq <35). E. scophthalmi was not detected in muscle, fin, gills, kidney, liver, spleen or in the mucus, or in blood and faeces, regardless of the degree of infestation in the analyzed fish.
These results agree with the study of Redondo et al., which showed that, from different areas of infection, parasites were detected in blood and intestine. Only when infection was severe the parasite was observed in other organs as spleen, kidney, pancreas, gills and muscular tissue (Redondo et al. 2004). In this study, the presence of the parasite in blood was tested. But contrary to what Redondo et al. reported in their study, the results for the blood samples from fish infected by the parasite were all negative and their controls were correct, discarding a false-negative result. On the basis of these findings, we highly recommend that the sampling for E. scophthalmi is carried out on the intestine and with rectal swabs to avoid the sacrifice of fish.
Design of a specific fast real-time PCR method for the detection of E. scophthalmi
In this study, a specific Fast RT-PCR method for authentication of E. scophthalmi and another probe for detection of S. maximus with the same primer pair were designed. From a selected internal region of the 18S rRNA, these sets generate PCR products of 120 bp in both cases. The design of an inhibitor control was used to indicate the appropriate development of the analytical procedure. The inhibitor control assured a correct DNA extraction at quantity, quality and purity level. Even more, it assured the absence of amplification inhibitors and avoided the diagnosis of false-negative results.
The combination of two probes with the same primers allows testing for parasites and amplification inhibitors in the same sample without the need of designing other primer set in a fast and reliable assay. An inhibitor control to avoid false-negative results and assure the correct DNA extraction process is essential in PCR methodologies. The inhibitor control is a non-target sequence of DNA amplified in the sample and can be an endogenous gene in sample or an incorporated plasmid with a gene control (Arkush, Miller, Leutenegger, Gardner, Packham, Heckeroth, Tenter, Barr & Conrad 2003; Hoorfar et al. 2003, 2004; Apfalter et al. 2005; Hoffmann et al. 2009). In this case, the selected gene is the same of the parasite, but the signal in the host is provided by the primer/probe specific set from S. maximus.
When the inhibitor control shows absence of amplification, the result is negative. In this case, the complete extraction process should be repeated or, in order to eliminate impurities in the DNA extraction, this could be purified by commercial purification kits (by magnetic systems, e.g. Wizard magnetic DNA purification system-Promega, Madrid, Spain, or by column systems, e.g. NucleoSpin tissue Genomic DNA purification system- MACHEREY-NAGEL, Düren, Germany).
The optimization of the reaction in terms of specificity, sensitivity, efficiency and reproducibility of the fast real-time PCR for the detection of E. scophthalmi is possible through a mixture of different concentrations of primers and probe. Concentrations of 900 nM for both primers and 250 nM for probe yielded the highest endpoint fluorescence and the lowest threshold cycle. The same optimal concentrations of primers and probe for the control set of S. maximus were obtained.
The specificity of the assay was tested using DNA from some of the most important parasites which affect the turbot (T. brevifilum and P. dicentrarchi) and with its host (S. maximus) to verify the suitability and reliability of the method. A BLAST analysis, specifically the MEGABLAS search with the sequences of the E. scophthalmi primer/probe set was carried out to investigate specificity and non-target affinities with other submissions in the database. Each primer and the probe in separate mode showed an homology of 100% with a lot of species but all three in combination, two primers and the probe, show only 100% of homology with E. scophthalmi. There were no other findings regarding homologies with some other parasite that affects turbot based on the affinity of the primers and the probe.
For the rectal swabs, the negative control test was taken with a sterile swab to avoid collecting material that could interfere with the reaction, giving false-positives. It is important to mention that the inhibition controls have not been tested on other host species. This means that the inhibition controls should be previously tested on other fish species so that the methodology can be applied to these species. If the inhibition control works with the new host species, the test could be used right away, and if it does not work, a new control would have to be designed. No cross-reactivity was observed with any of the tested samples using the methodology herein developed.
A methodology for the detection of pathogens must be specific and must be able to differentiate with all security whether the species of interest are present or not. This security is supplied by the specificity of the assay. The application of the developed methodology to different related species, genetically close or that are sharing the same host, must assure the specificity of the method.
The assay described in this manuscript has been designed for specific conditions in which the host is S.maximus and only for application in aquaculture. Within this framework, the specificity evaluation has been reduced to the main parasites in the culture of S. maximus. This renders the evaluation of many parasites obsolete which do not affect the turbot in culture conditions, although they are closely related with the target parasite (E. scophthalmi). It is also unnecessary to evaluate the specificity with other hosts, because the framework is specific for a single host (S. maximus). The only variable that plays a role in specificity is the parasite, and its variability is determined by two constant parameters, the host and farm cultivation conditions. In this study, the specificity was tested empirically for species host-related to avoid interferences or cross-reactivity by the presence of different parasites.
Also, phylogenetic analyses have been performed to evaluate the relation, determined by 18S rRNA, between E. scophthalmi and other related parasites (host-related, taxonomic-related and location-related). Figure 1 shows the phylogenetic tree obtained where all the sequences belonging to individuals of the same species were grouped in the same cluster, allowing their identification. The bootstrap method can be used to obtain the support of the different groups obtained in the phylogenetic tree. It has been calculated that bootstrap values higher or equal to 70 usually correspond to a probability higher or equal to 95% that the corresponding cluster is real (Hillis & Bull 1993), giving a quantitative measurement of the certainty of the assignment of a sample to a particular species.
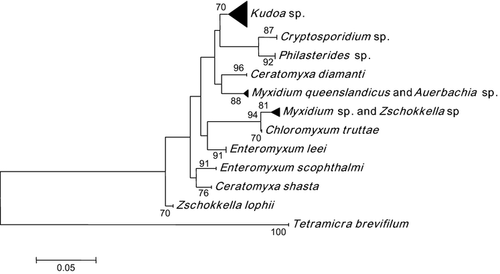
The phylogenetic tree shows that E. scophthalmi is differentiated from all parasites with bootstrap values higher than 90. This shows that the variable positions inside the selected fragment of 120 bp allows the differentiation of the parasites included in the analysis. There is a noticeable and clear differentiation between E. leei and E. scophthalmi, which are included in two separated clades strongly supported and based on this DNA sequence. For all these reasons, we conclude that the selected fragment provides the necessary information to develop this molecular tool with high specificity and robustness.
The threshold cycle (Cq) is a basic parameter of the RT-PCR and it is an essential component that produces accurate and reproducible data. It is defined as the fractional PCR cycle number at which the reporter fluorescence is greater than the threshold. The Cq depends on the starting template copy number, the efficiency of PCR amplification, the efficiency of separation or hybridization of the fluorogenic probe and the sensitivity of detection (Bustin 2004; Bustin et al. 2009). The aim of the data analysis is to determinate when the target amplification is sufficiently above the background signal, by facilitating more accurate measurement of fluorescence.
In all samples analyzed, when 200 ng of template were used, the Cq values obtained were <35 and 17 ± 2, for E. scophthalmi and S. maximus primer/probe sets respectively.
For E. scophthalmi primer/probe set, a particular Cq cannot be determined. It depends on the degree of infection of the fish in question. The DNA added to the reaction (200 ng) contains DNA from both the turbot and the parasite. For this reason, the concentration was determined by manual cell counting using a Neubauer chamber. The LOD, regardless of the degree of infection, was two parasites for 500 μL. This limit was obtained with DNA extractions from real contaminated fish, in contrast with Piazzon, who obtained a very low limit from an ideal situation with pure DNA from E. scophthalmi (Piazzon et al. 2012). The real situation in turbot aquaculture is that the DNA extraction from infected fish contains a mixture of turbot and parasite DNA, or only turbot DNA in DNA extraction from non-infected fish. For this reason, a low LOD from pure DNA of the parasite, although it is a signal of a sensitive methodology, is not a real indicator of the limit reach in detection of the pathology.
The sensitivity obtained is very important at the moment of the detection because sensitivity allows detection of the presence of E. scophthalmi at early stages of infection, when the infection intensity is low, and regardless of the life cycle stage of the parasite. The method allows the adoption of early control measures and the avoidance of propagation of the pathogen.
For the E. scophthalmi primer/probe set, UND (undetected) results corresponded to the values of non-target species (the most important parasites affecting the turbot) and negative controls. Also, in the cross-reactivity analysis, no false positive results have been observed under the most severe conditions used in the test.
The accuracy of the fast real-time PCR is highly dependent on the PCR efficiency. In the developed method, it was calculated based on the slope of the standard curve obtained using DNA serial dilutions with a template of known concentration (from 250 ng to 25 pg) as templates for RT-PCR. The efficiency (E) of the reaction was calculated according to the equation E = (10(−1/slope) − 1) × 100 (Bustin 2004) (Fig. 2a).
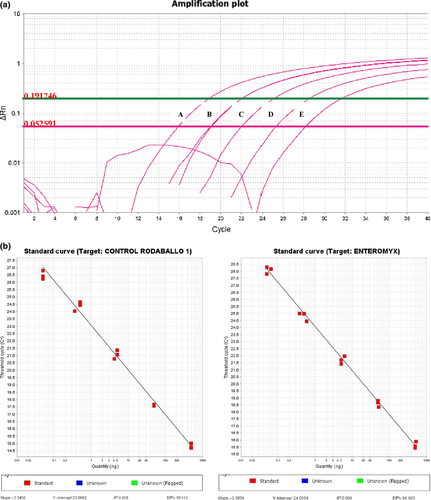
The amplification plot of the experiment with the primer/probe set E. scophthalmi and S. maximus generated a slope of −3.38 and −3.35, an efficiency of 97% and 99%, and a correlation coefficient of 0.999 and 0.998 respectively (Fig. 2b). These values of Cq and efficiency have demonstrated the utility of the fast real-time PCR system to detect E. scophthalmi and S. maximus, which is used for the control of the methodology developed, where the efficiency is nearly 100%.
Moreover, within the reproducibility of the assay, intra-assay variation (CV) was 3.18%, while inter-assay variation (CV) was 4.21%, thereby indicating that the RT-PCR was highly reproducible (data not shown). This confirms that RT-PCR is a robust methodology to detect the presence of E. scophthalmi in cultured turbot.
Application of the developed real-time PCR in cultured turbot
Extracted DNA showed a good yield and optimal purity in all products analyzed (data not shown). Both molecular and classical histological techniques showed concordant results for the detection of E. scophthalmi in the analyzed samples.
This concordance in the result indicates the reliability of the methodology; the results are in accord with histological results. In addition, it is applicable in early stages of infection, and shows high specificity, efficiency and reliability.
Conclusions
The developed methodology allows the specific detection of the parasite E. scophthalmi in turbot, regardless of the life cycle stage of the parasite and of the infection intensity. The fast real-time PCR assay developed is a robust and fast methodology and it provides an effective tool for the control and prevention of the spreading of this pathogen in cultivated turbot. The methodology described in this manuscript is reliable for its application in samples of aquaculture industry. It eliminates the possibility of false-negative results and can be applied to live fish and avoids the sacrifice of them. Furthermore, the developed methodology allows specific detection of E. scophthalmi in early stages of infection; this makes it possible to adopt the necessary measures in time to avoid the spread of infection. The early control of pathologies improves the health and welfare of cultivated fish. It also may lead to the reduction in disinfecting chemicals and antibiotics used in aquaculture, contributing to environmental sustainability and at the same time increasing the profitability of the sector.
This study shows, in agreement to Redondo et al. 2004, the localization of the infection in the intestine, as the target tissue, and its absence in other tissues such as kidney, gills, liver and muscle. The target tissue identification allows locating and establishing the area of optimal sampling to be defined in the routine control at farms.
Cost reduction is of big importance for the implementation of a methodology as routine tool for the control and prevention of pathologies at the farms. This cost includes, beside the technique itself, preservation and sending of samples. This study also provides the appropriate method for sending infected samples to the laboratory to be analyzed and the appropriate way of preserving the samples (in terms of quality and cost) until the moment of analysis. This optimization allows avoiding unnecessary extra costs and assuring good results for DNA extraction.
Additional research is necessary in order to apply the developed methodology to detect the parasite in water at aquaculture farms (Hallett & Bartholomew 2006; Griffin, Pote, Camus, Mauel, Greenway & Wise 2009). This application is conditioned to the short-term survival of E. scophthalmi in seawater, to the transmission pathway from fish to fish, and to the scarcity of spores even in advanced infections, as is reflected in studies of Redondo et al. (2002); Redondo, Palenzuela et al. (2003) Despite this, water detection could provide a necessary tool to avoid sacrifice of fish or stress during the sampling in routine controls, improving the health and welfare of turbot at aquaculture farms (Redondo et al. 2002; Redondo, Palenzuela et al. 2003).
Acknowledgments
This study was funded by the 10MMA006E grant from the Consellería de Innovación e Industria de la Xunta de Galicia. We thank Jacobo Docal and Martín Gómez for their helpful assistance and for kindly supplying the fish and parasites samples. Finally, we thank Henlo Matthee for her helpful review of the English manuscript.




