Analysis of viral load between different tissues and rate of progression of white spot syndrome virus (WSSV) in Penaeus monodon
Abstract
At present the most common and most devastating disease of shrimp is caused by the white spot syndrome virus (WSSV), which has spread throughout the world mainly by different species of crustaceans carrying the virus. After experimental injection of Penaeus monodon with a known copy number of WSSV in the abdominal muscle, the rate of viral progression in different tissues at 12, 24, 36 and 48 hpi (hours post infection) was assessed using quantitative real-time PCR. At 12 hpi the viral load was highest in haemocytes followed by pleopod, muscle and gills whereas at 48 hpi, the gills, the main target of WSSV, showed the highest viral load followed by pleopod, muscle and haemocytes. Viral copy number in the haemocytes was the lowest beyond 12 hpi indicating a remarkable reduction in the rate of viral replication in haemocytes compared with other tissues. The viral load in haemocytes, though increased again beyond 36 hpi, never surpassed the load in the other tissues. The real-time PCR assay with its high sensitivity and wide dynamic range make it ideal for detecting low-level WSSV infections that can occur in apparently healthy P. monodon.
Introduction
Worldwide, viral diseases are a major reoccurring problem in the shrimp aquaculture industry. Serious viral outbreaks often cause catastrophic losses in shrimp harvest (Lightner, Redman, Poulos, Nunan, Mari & Hasson 1996). Under farming conditions, white spot syndrome virus (WSSV) infection can result in total crop losses within 3–10 days (Lightner et al. 1996). Besides shrimp, a range of crustaceans, including lobsters (Chang, Chen & Wang 1998; Wang, Hassan, Shariff, Zamri & Chen 1999) and crabs (Lo, Ho, Peng, Chen, Hsu, Chiu, Chang, Liu, Su, Wang & Kou 1996; Supamattaya, Hoffmann, Boonyaratpalin & Kanchanaphum 1998; Otta, Shubha, Joseph, Chakraborty, Karunasagar & Karunasagar 1999) have been found to exhibit varying degrees of vulnerability to the virus, whereas other species, such as copepods, krill (Supamattaya et al. 1998) and brine shrimp Artemia spp. (Lo, Ho et al. 1996) may be considered viral vectors contributing to the dissemination of the virus.
Immersion and cannibalism are the routes by which the virus enters the host in the wild and in culture ponds. Both administration methods will inadvertently show a high variability in infectivity. During infection by immersion, the virus needs to cross the outer defence barrier, the cuticle, which may be compromised in individual shrimps by injuries or at moulting (Corteel, Dantas-Lima, Wille, Alday-Sanz, Pensaert, Sorgeloos & Nauwynck 2009). During infection by cannibalism, the virus has to pass the gastrointestinal tract where partial inactivation of the virus will occur through pH changes and digestive enzymes. In addition, infectivity depends on the individual's feeding habit. Intramuscular injections bypassing such natural barriers are mostly used in experimental infections. Here the virus enters the haemolymph sooner and disseminates to organs of ectodermal and mesodermal origin (Escobedo-Bonilla, Audoorn, Wille, Alday-Sanz, Sorgeloos, Pensaert & Nauwynck 2006).
Changes in pond environmental conditions such as salinity (Liu, Yu, Song, Guan, Jian & He 2006; Joseph & Philip 2007), temperature (Guan, Yu & Li 2003; Rahman, Escobedo-Bonilla, Corteel, Dantas-Lima, Wille, Alday-Sanz, Pensaert, Sorgeloos & Nauwynck 2006) and dissolved oxygen (Kautsky, Rönnbäck, Tedengren & Troell 2000) can trigger the WSSV incidence in shrimp. Stress exerted on shrimp by changes in these environmental factors increases their susceptibility to pathogens. Although the initial viral load in the infected shrimp tissues is considered to be one of the most important factors in the progression and transmission of the disease, the unavailability of immortal cell lines hampers the standardization of infectivity parameters. Recently a shrimp minimum infectious dose (SID50) was defined in viral-free Litopenaeus vannamei (Escobedo-Bonilla, Wille, Alday-Sanz, Sorgeloos, Pensaert & Nauwynck 2005). This in vivo parameter is not biased by non-infective DNA segments that tend to overestimate viral load using DNA amplification methods, however, a correlation between the SID50 and the viral load as determined by DNA amplification has not been established.
The competitive PCR and the real-time PCR allow the determination of the number of copies of a DNA segment in a particular tissue sample. These methods have been applied to penaeid shrimp virology and to develop assays for WSSV and IHHNV (Tang & Lightner 2000, 2001). For the management of shrimp farms in areas endemic with WSSV, quantitative detection methods would definitely be a more valuable diagnostic tool than purely qualitative detection methods (Tan, Soon, Lee, Shariff, Hassan & Omar 2001). By quantitative methods, the severity of a WSSV infection in a culture pond can be easily assessed and used to decide whether to harvest (if the infection is severe) or to persist with remedial approaches if the infection is light (Lo, Chang, Cheng & Kou 1998). Quantitative detection techniques are equally important in research studies involving chronological investigation of WSSV infection (Durand, Lightner, Redman & Bonami 1997). In recent years, reports based on real-time PCR indicated that a disease outbreak is triggered once the critical concentration of virus is reached (Dhar, Roux & Klimpel 2001; Durand & Lightner 2002; Durand, Redman, Mohney, Tang-Nelson, Bonami & Lightner 2003). These studies suggest that viral loads differ with different culture conditions; hence, by assessing the viral loads in commercial ponds, effective measures and precautions can be taken against outbreaks of WSSV disease.
This study aims to reveal the time course of the WSSV progression in different tissues and provide knowledge regarding the infectivity stages at different hours after infection. This information can be helpful in predicting WSSV outbreaks and in finding the minimal viral load at which shrimp culture can be sustained. This will help farmers to carry out mariculture operations through scientific management strategies and thereby avoid severe economic losses.
Materials and methods
Experimental animals
Adult Penaeus monodon (20.2 ± 0.5 g mean body weight) were collected from local farms (Chearai, Cochin). Shrimps tested to be negative to WSSV through PCR (Kimura, Yamano, Nakano, Momoyama, Hiraoka & Inouye 1996) were maintained at 28 ± 1°C and salinity of 20 g L−1 with continuous aeration in marine hatchery. Shrimps were acclimatized for 1 week. Animals in the intermoult stage were used for the study.
Viral inoculum preparation and WSSV challenge
White spot syndrome virus inocula were prepared by homogenizing the infected shrimp muscle tissue in phosphate-buffered saline (PBS) (0.1 mol L−1, pH 7.4). The homogenate was centrifuged at 5000 g for 10 min and the supernatant collected. This supernatant was used to inoculate an animal from which fresh inoculum was prepared to be injected into the experimental shrimp. Prior to the injection, the viral load was determined using real-time PCR. In vivo titration revealed that the injection of 6 × 107 copies of virion particles into shrimp resulted in 100% mortality within 4 days post infection. Animals were injected with the viral inoculum (50 μL, 6 × 107 copies) between the second and third tergal plates of the lateral side of the tail using a 1 mL syringe (30-gauge) and were housed individually in tanks.
Tissue collection
Five infected shrimps were selected for each time point. Gill, muscle, pleopod and haemocyte were collected from each shrimp at 12, 24, 36 and 48 h post infection (hpi), flash frozen at −80°C and stored in a deep freezer. Haemolymph (100 μL) was collected using a 30-gauge needle fitted to a 1-mL syringe prefilled with 100 μL anticoagulant (Alsevers solution) and centrifuged at 700 g for 10 min to collect the haemocyte pellet.
Isolation of nucleic acids
Tissues from five animals belonging to the same time point were pooled and DNA was extracted separately from gills, muscle, pleopod and haemocytes using Qiagen DNeasy Kit (Qiagen, Valencia, CA, USA). The DNA thus extracted is expected to contain both the host DNA and viral DNA. All DNA isolation procedures utilized aerosol-resistant barrier pipette tips and were undertaken in biological safety cabinet in rooms segregated from the diagnostic PCR laboratory. The DNA concentration and quality were estimated by UV absorbance at 260 nm and 280 nm using a BioPhotometer (Eppendorf Scientific, Hamburg, Germany).
Design of SYBR Green PCR primers
The primers used for the PCR amplification of the viral DNA were based on WSSV genomic sequence in the GenBank database (AY870344). The primers were designed using the Beacon designer version 7.51 (Premier Biosoft International, CA, USA). The sense and antisense primers for WSSV were 5′-CTGCTGATATGATAAGAGA-3′ and 5′-TCACTGTAATTGCTTGTA-3′, which generate a 171 bp amplicon.
Construction of positive control vectors and standards for quantification
A WSSV fragment containing a 171 bp target amplicon for real-time PCR was ligated into the pCR4 vector (TOPO TA Cloning Kit, Invitrogen, Germany) and cloned into Escherichia coli (TOP10). The target segment in the recombinant plasmid, pWSSV-171, was confirmed by DNA sequencing. The copy number of the target amplicon in the plasmid was estimated and tenfold serial dilutions were used as absolute standards for quantification using real-time PCR.
Real-time PCR amplification
Real-time PCR assays of DNA extracted from infected shrimp tissues, at different time points, using WSSV specific primers were carried out to determine the viral copy number. The SYBR Green based real-time PCR assay was carried out in an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The amplifications were performed in 96-well plates with 25 μL reaction volume containing 12.5 μL of 2 × Power SYBR Green PCR Master Mix (Applied Biosystems), 0.5 μL (each) of the forward and reverse primers (5 μM), 1 μL of template and 10.5 μL of water. The thermal profile for the SYBR Green real-time PCR was 50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. In a 96-well plate, each sample was run in triplicate. DEPC-water replacing the template was used as the negative control. A series of dilutions of WSSV recombinant plasmid from 1.32 × 101–1.32 × 106 were used for generating a standard curve. Data were analysed with SDS 2.4 software (Applied Biosystems).
Analysis of variance (anova) test was used to compare the mean viral copy number at different time points. A Tukey post-hoc test was used to determine significant difference in the viral copy number among tissues at a particular time. All statistical tests were performed using the program spss 16 (SPSS Inc., Chicago, IL, USA).
Results
Pathogenesis of infection
In preliminary experiments, it was determined that a dose of 6 × 107 viruses was sufficient to effectively kill shrimps within 96 h post injection. Animals injected with the WSSV inoculum showed the following signs of infection. At 24 hpi, the shrimps significantly reduced food intake and showed a faded or reddish body colour. By 55 hpi, some of the infected shrimps were dead, whereas the remaining ones appeared weak to moribund. Before 96 hpi all the infected shrimps had died.
Standard curve
A plasmid clone harbouring a 171 bp sequence of WSSV was used as the standard template to evaluate the performance of the selected primers and conditions for real-time SYBR Green PCR. The optimized PCR conditions were applied to develop a standard curve using tenfold serial dilutions of the purified plasmid. A representative amplification of the plasmid standard (ranging from 1.3 × 106 to 1.3 × 101 copies per reaction) is shown in Fig. 1; each dilution for this experiment was assessed in triplicate. The reproducibility of the data is shown by the narrow distribution of the amplification curves at each dilution step. No signal was detected in non-template controls.
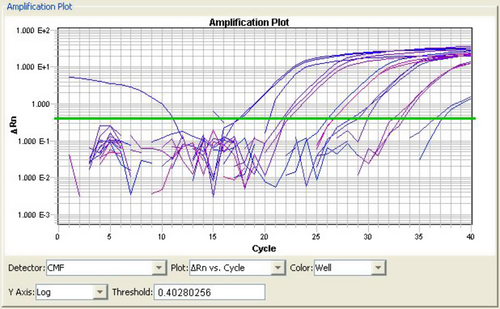
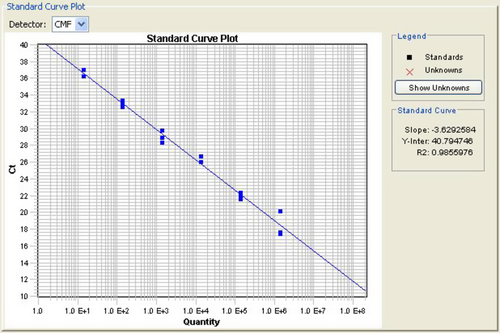
The melting curve analysis revealed a single peak for the amplified product with a melting temperature (Tm) of 79.6°C; non-specific amplification products, including primer–dimers, were not discernible (data not shown). A strong linear correlation (R2 = 0.985) was obtained between threshold cycles (CTs), indicating a detection limit of 10 copies. The slope of M = −3.6 is indicative of high reproducibility and precision (Fig. 2).
Viral load in WSSV-infected tissues
White spot syndrome virus DNA was detected in all the P. monodon tissues under study using real-time PCR. The sample copy numbers were determined against a standard curve (Fig. 2). The viral load in the tissues increased as the time progressed (Table 1).
| Sample | Copy number μg−1 of total DNA | |||
|---|---|---|---|---|
| 12 hpi | 24 hpi | 36 hpi | 48 hpi | |
| Gills | 8.8 × 102 | 6.28 × 105 | 3.25 × 106 | 6.45 × 108 |
| Pleopod | 2.6 × 103 | 1.35 × 105 | 2.7 × 106 | 3.2 × 108 |
| Muscle | 2.56 × 103 | 3.9 × 104 | 8.55 × 105 | 7.8 × 106 |
| Haemocytes | 4.3 × 103 | 9.5 × 103 | 2 × 104 | 1.8 × 106 |
- hpi, hours post infection.
In the gill, 8.8 × 102 WSSV DNA copies μg−1 of total DNA were detected at 12 hpi and levels continued to increase to 6.28 × 105, 3.25 × 106 and 6.45 × 108 at 24, 36 and 48 hpi. Compared with gills, WSSV DNA levels in pleopod showed a threefold increase at the first sampling (2.6 × 103 WSSV DNA copies μg−1 of total DNA at 12 hpi), though at the later stages, the increase was slower (1.35 × 105, 2.7 × 106 and 3.2 × 108 WSSV DNA copies μg−1 of total DNA at 24, 36 and 48 hpi respectively). Though the WSSV DNA copies μg−1 of total DNA in the muscle was similar to pleopod at 12 hpi (2.56 × 103) it was about tenfold lower at 24 and 36 hpi (3.9 × 104 and 8.55 × 105), and about 100-fold lower at 48 hpi (7.8 × 106). In the haemocytes the WSSV DNA level was 4.3 × 103 at 12 hpi and a marginal increase in progression was observed over time (9.5 × 103, 2 × 104 and 1.8 × 106 WSSV DNA copies μg−1 of total DNA at 24, 36 and 48 hpi respectively).
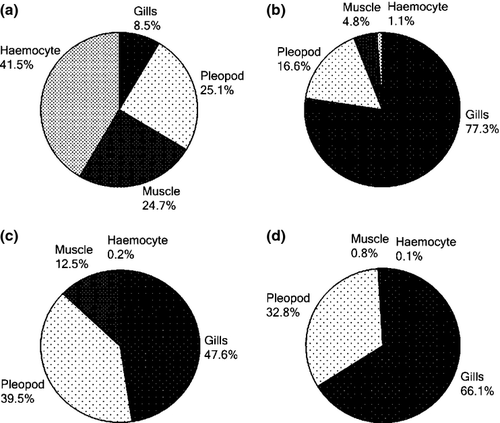
The changes in viral copy number for each tissue was recorded at different time points and graphically represented in Fig. 3-6. The tissue-wise distribution of viral copies at defined time points is represented by pie graphs (Fig. 7).
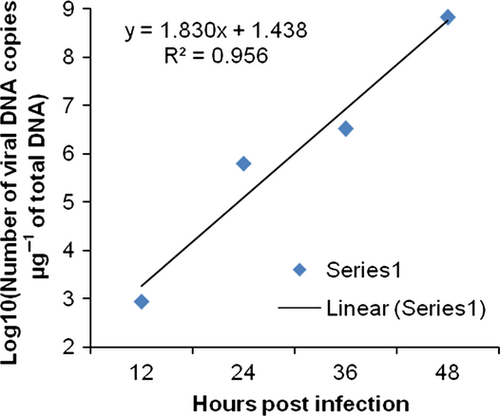
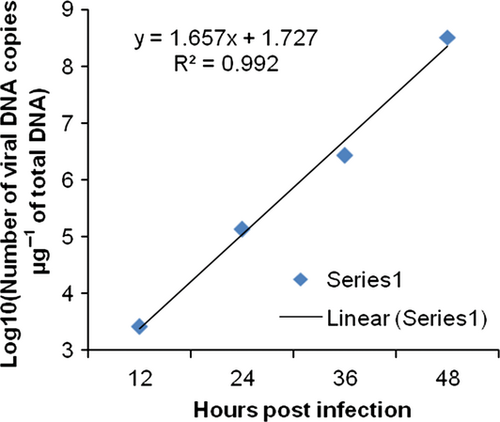
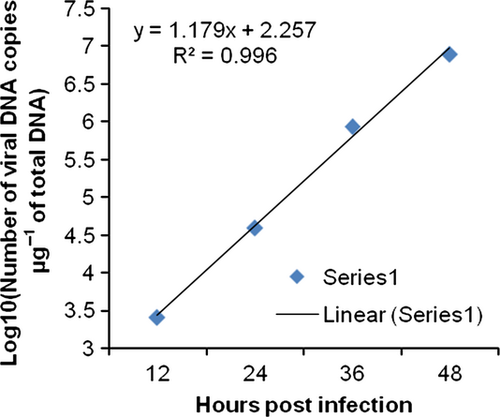
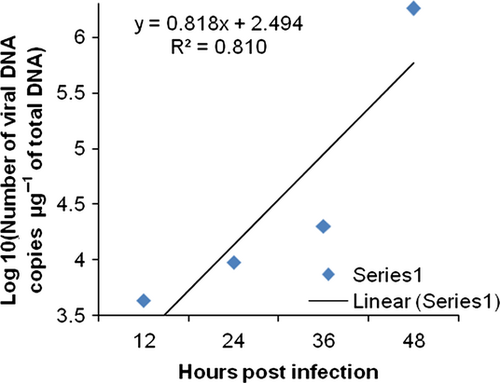
The rate of viral proliferation based on the 12 hpi data points was calculated. In all tissues, the viral load increased as time progressed, however, at different degrees. In haemocytes the viral proliferation at 24, 36 and 48 hpi was found as 2-, 4-, 418-fold, in gills it was 7.13 × 102-, 3.69 × 103- and 7.32 × 105-fold, in pleopod it was 51-, 1.03 × 103- and 1.23 × 105-fold, whereas in muscle it was 15, 3.33 × 102- and 3.04 × 103-fold.
The viral copy number of tissues at different time intervals, analysed using one-way analysis of variance (anova), showed a significant difference between tissues at all time points (P < 0.001). Subsequent pairwise analysis of WSSV copy number in different tissues at a particular time point using Tukey post-hoc test revealed high levels of significance (P < 0.001). The difference in copy number during progressing hours in each tissue is described in Table 2.
| hpi | Mean copy number | |||
|---|---|---|---|---|
| Gills | Pleopod | Muscle | Haemocyte | |
| 12–24 | 6.27 × 105 | 1.32 × 105 | 3.64 × 104 | 5.19 × 103 |
| 24–36 | 2.62 × 106 | 2.56 × 106 | 8.16 × 105 | 1.00 × 104 |
| 36–48 | 6.41 × 108 | 3.17 × 108 | 6.94 × 106 | 1.78 × 106 |
- hpi, hours post infection.
Discussion
In this study real-time PCR was used to determine the time-dependent viral load in defined tissues and cells of P. monodon experimentally infected with a known copy number of WSSV. A sensitive SYBR green real-time PCR assay was developed which has a large dynamic detection range that can detect as few as 10 copies of WSSV genomes per reaction.
The ability of the real-time PCR to accurately quantify WSSV load in infected shrimp was assessed using different tissues of experimentally infected P. monodon. To date, few studies on tissue distribution of WSSV in infected penaeid shrimps has been reported. Durand and Lightner (2002) studied the WSSV load in various tissues of different shrimp species using moribund juveniles. Moribund juveniles of Penaeus vannamei showed 2.5 × 109, 1.6 × 109, 1.2 × 109 and 1.9 × 108 copies of WSSV μg−1 of total DNA from haemolymph, pleopod, gills and muscle respectively. The muscle tissue contained almost 10 times less WSSV genome copies than gills, pleopods and haemolymph. Moribund juveniles of Penaeus stylirostris and P. monodon showed 3 × 1010 and 2.1 × 106 WSSV copies μg−1 of DNA in pleopods. This implies that large variations in viral load can be observed between different tissues in the same species and between the same tissues in different shrimp species.
This study reveals the differential viral progression in each of the tissues during the course of viral replication over a period of time. At 12 hpi, haemocytes showed relative high copy number followed by pleopod, muscle and gills (Fig. 7a). Over time, a rapid increase in viral load is observed in gills. At 24, 36 and 48 hpi, gills showed the highest viral load among all tissues analysed. According to Escobedo-Bonilla, Wille, Alday-Sanz, Sorgeloos, Pensaert and Nauwynck (2007), the mechanism behind such change in viral distribution is that after primary replication (12 or 18 hpi), newly produced WSSV would have been released from epithelial cells and crossed the basal membrane to reach the underlying connective tissues and associated haemal sinuses. Through the circulating haemolymph the virus would have reached other organs, so that WSSV-infected cells would be apparent in various organs throughout the body.
There is a marked increase in viral load in haemocytes after 36–48 hpi which might be due to the haemocytes infiltrating infected tissues carrying the virus back into circulation and simultaneously increasing the viral load in the haemolymph. Shrimp possess an open circulation system that allows haemocytes to infiltrate and adhere to many tissues. Immuno-histochemical observations by Van de Braak, Botterblom, Huisman, Rombout and Van der Knaap (2002) indicated that the haemocytes in the tissues exceeded the circulation especially when P. monodon was challenged. An invasion by haemocytes in the gills was observed in P. monodon upon WSSV infection (Arts 2006). The circulating haemolymph spreads the virus to tissues of ectodermal and mesodermal origin. This infection mechanism is in agreement with the current findings that after an initial spike in haemocytes, the highest viral load was seen in gills and followed by pleopod, muscle and haemocytes.
There are reports that early in infection, circulating haemocytes are refractory to WSSV infection and that WSSV spread in a cell-free form via haemolymph. The absence of infected circulating haemocytes early in infection was also noticed in other WSSV pathogenesis studies in P. monodon inoculated orally (Van de Braak et al. 2002) and crayfish inoculated intramuscularly (Shi, Huang, Zhang, Chen & Bonami 2000; Shi, Wang, Zhang, Xie, Li, Chen, Edgerton & Bonami 2005). In contrast, Di Leonardo, Bonnichon, Roch, Parrinello and Bonami (2005) showed that in the nucleus of circulating haemocytes, virions are present at different stages of morphogenesis, indicating that viral replication must be occurring in granular haemocytes, and even suggesting that this may be the first step of the systemic phase of infection. Thus, haemocytes carrying virions are dispersed in the haemocoel through haemolymph circulation and are rapidly distributed in different tissues (Di Leonardo et al. 2005). Although in our study we observed substantial increase in copy numbers of WSSV in haemocytes, in particular between 36 and 48 hpi, we did not differentiate whether the virus has infected the cells or is loosely attached to the cell surface.
In this study, experimental shrimp were housed individually to prevent viral reintroduction through cannibalism. The methodology used for viral delivery (injection) in the present study was designed to ensure that each challenged shrimp received an equivalent dose of the virus. Although reproducible infection of Litopenaeus vannamei with WSSV was reported using both, intra muscular injection and oral intubation, (Escobedo-Bonilla et al. 2005, 2006) we have chosen the intramuscular route because it is technically less demanding. We have not defined an SID50 as a reference point for infection but have set a time limit of 96 h to achieve mortality. As size and age are known to affect the susceptibility of challenged animals to various pathogens (Laramore, Scarpa, Laramore & Lin 2009), adult animals with 20 g body weight were used in this experiment and all were injected with equal volume of viral inoculum (50 μL). Hence, the data from different individuals were comparable.
The susceptibility of shrimp to WSSV varies with the shrimp species. It has been suggested that P. monodon is more susceptible compared with many other species. For instance, Durand and Lightner (2002) found that moribund juveniles of P. monodon collected from ponds have as few as 2.1 × 106 WSSV copies μg−1 of DNA, whereas moribund juveniles of L. vannamei contained 7.5 × 108 to 2.5 × 109 copies μg−1 of DNA. Dhar et al. (2001) reported that juveniles of Litopenaeus stylirostris carrying 8.09 × 106–2.72 × 108 WSSV copies μg−1 of DNA showed no sign of infection, thus indicating that L. stylirostris is less susceptible to WSSV than P. monodon. Similarly, brood stocks of Fenneropenaeus chinensis infected by WSSV at a level of 2.28 × 109 copies μg−1 of DNA, showed no signs of infection. Hence, in order to improve predictability of WSSV infection and its consequences to aquafarming in this study we investigated the time-dependent accumulation of WSSV in different organs.
Lo, Leu, Ho, Chen, Peng, Chen, Chou, Yeh, Huang, Chou, Wang and Kou (1996) suggested that most of the PCR-positive P. monodon were evidently in latent or pre-patent stages of WSSV infection, because no apparent pathological signs were observed. Use of a virus-resistant line would be of great benefit to the shrimp farming industry, however, lack of a sensitive quantification method for the virus is the major obstacle. The use of virus-free brood stock and postlarvae is another alternative way of preventing a viral epidemic in commercial shrimp farming. In recent years, conventional two-step PCR has been used to screen brood stock and postlarvae before stocking the ponds (Hsu, Lo, Lin, Liu, Peng, Chang, Chen, Liu & Kou 1999). Even two-step PCR frequently provides false-negative results because the virus level is beyond the detection limits of conventional PCR (Lo & Kou 1998; Hsu et al. 1999). The high sensitivity of real-time PCR is ideal for detecting low-level WSSV infections that might be present in apparently healthy P. monodon.
Cultivation in commercial shrimp ponds can last about four to 5 months. The incubation period for WSSV in ponds is 40–45 days, if the initial viral infection is low (Withyachumnarnkul 1999). During this period, shrimp can endure more variable conditions, in particular less controlled temperature fluctuations. As the WSSV-infected individual cannot be cured, we suggest that, by assessing health status and viral load of the animals at regular intervals, one can improve the culture conditions and delay the harvest time. Real-time PCR assays could be used in defining WSSV threshold levels that are associated with the transition from chronic to acute infection states and therefore assisting in predicting impending disease and mortalities in cultured P. monodon. Increased viral replication is directly linked with the presence of stressors such as bacteria, pH changes etc. in the pond. Hence, controlling pond conditions and periodic monitoring of WSSV using quantitative real-time PCR will help farmers to carry out shrimp farming under optimized culture conditions and reduce heavy economic loss from the unexpected outbreak of the disease. SYBR green PCR assay including DNA isolation can be performed within 6 h and can be an effective control measure for hatcheries.
This study demonstrates the time-dependent WSSV progression in different tissues and provides knowledge regarding the infectivity within the early time periods following infection. The general outcome of the study will help farmers and other entrepreneurs to adopt new strategies, incorporating scientific inputs and thereby minimizing the economic loss associated with WSSV incidence in mariculture. This will help to manage the farming operations even after the detection of WSSV by providing options of early harvest before a catastrophic outbreak occurs.
Acknowledgments
This study was financially supported by Indian Council for Agricultural Research (ICAR) and Central Marine Fisheries Research Institute (CMFRI). We greatly acknowledge Dr Erika E. Büllesbach, Department of Biochemistry and Molecular Biology, Medical University of South Carolina for kindly reading the manuscript and for valuable comments.




