Dietary L-tryptophan modulates growth and immuno-metabolic status of Labeo rohita juveniles exposed to nitrite
Abstract
Effect of nitrite exposure on growth and immuno-metabolic responses of Labeo rohita fed L-tryptophan (TRP) was studied. Fish previously fed normal and elevated levels of tryptophan for 60 days were exposed to nitrite (2.0 mg L−1) for another 45 days with same feeding regime. There were four treatment groups, viz., TRP0-N (control), TRP0+N, TRP0.75+N (0.75% supplemental tryptophan in the diet) and TRP1.5+N (1.5% supplemental tryptophan in the diet). Highest weight gain% and SGR were observed in control and lowest in TRP0+N. Dietary supplementation of elevated levels of tryptophan augmented weight gain% and SGR. Nitrite exposed groups recorded higher catalase, SOD, LDH, AST and ALT activities compared with control. However, activities reduced with additional levels of tryptophan supplementation. Nitrite exposure reduced WBC count, total protein, albumin, globulin and lysozyme activity compared with unexposed group but groups which were fed additional amounts of tryptophan restored total protein, albumin and globulin similar to TRP0-N. In conclusion, nitrite exposure had adversely affected growth, increased activities of LDH, AST, ALT, catalase, but decreased WBC, serum protein, lysozyme and acetylcholine esterase activity of L. rohita. Normal requirement of tryptophan was unable to combat nitrite stress. However, dietary fortification with tryptophan (minimum 0.75% of diet + normal requirement) found effective in combating nitrite induced stress.
Introduction
Aquatic organisms are usually exposed to various environmental pollutants during their life span. Nitrite is a commonly encountered pollutant in different types of aquatic ecosystems. Nitrite build-up in aquatic habitat occurs as a result of imbalances in bacterial nitrification and denitrification processes (Jensen 2003). The use of excessive proteinaceous feed and nitrogenous fertilizers along with higher stocking densities can also lead to nitrite accumulation in intensive culture systems. Fish are susceptible to methemoglobinemia (Brown Blood Disease) when exposed to nitrite. The nitrite toxicity to fish is attributed to its ability to oxidize haemoglobin to methaemoglobin, a form incapable of binding oxygen (Tomasso 1986; Urrutia & Tomasso 1987). Toxicity studies in fish models have revealed inconsistency in response to different nitrite concentrations (Palachek & Tomasso 1984; Das, Ayyappan, Das & Jena 2004a). Aquatic animals including fish are highly susceptible to elevated nitrite concentrations than terrestrial animals. This is mainly challenging in freshwater fish, due to the ability of nitrite to compete with active chloride uptake across the gills (Tomasso & Grosell 2005), resulting in nitrite accumulation in blood plasma, affecting acid base balance (Margiocco, Arillo, Mensi & Schenone 1983; Tomasso 1986; Huang & Chen 2002).
Nitrite exposure has been shown to induce tissue damage in fish (Margiocco et al. 1983). Changes in the activities of various tissue specific enzymes are reported to be correlated with cell damage (Casillas, Meyers & Ames 1983; Das, Ayyappan, Jena & Das 2004b). Increased activities of transaminases have been considered as biomarkers of cell injuries (Rajyasree & Neeraja 1989; Oluah 1999). Many studies in different fish species revealed elevated LDH activity under various stress (Das et al. 2004b; Tejpal, Pal, Sahu, Jha, Muthappa, Sagar & Rajan 2009; Akhtar, Pal, Sahu, Alexander, Gupta, Choudhary, Jha & Rajan 2010). Therefore, assessment of these enzymatic activities is considered as indicators of stress in fish. Similarly, albumin and globulin levels in the serum are used as primary indices of fish health (Buchanan, Sarac, Poppy & Cowan 1997).
Fish reared in aquaculture systems are predisposed to multiple stressors, which may impart adverse effects including reduction in growth and increased susceptibility to diseases (Akhtar et al. 2010). Nitrite has been reported to cause immunosuppression in fish (Carballo, Munoz, Cuellar & Tarazona 1995; Ciji, Sahu, Pal, Akhtar & Meena 2013). Fish that are exposed to even low levels of nitrite for long periods of time suffer damage to their immune system and are predisposed to diseases. The stress mitigation through the application of drugs and anaesthetics has been a common practice in aquaculture despite its harmful effects. In this context, nutritional manipulation with natural amino acids or vitamins, which are less likely to have side effects, seems to be a better option to get rid of stress (Tejpal et al. 2009; Akhtar, Pal, Sahu, Ciji & Gupta 2012); Akhtar, Pal, Sahu, Ciji, Meena & Das 2013;. Tryptophan, a precursor of neurotransmitter serotonin (5-hydroxytryptamine, 5-HT), has been reported to reduce stress by lowering corticosteroid production (Lepage, Tottmar & Winberg 2002; Hseu, Lu, Su, Wang, Tsai & Hwang 2003). Previous studies (Lepage et al. 2002; Tejpal et al. 2009; Hoseini & Hosseini 2010) showed mitigation of different stresses by dietary incorporation of tryptophan in several fish species. However, to the best of our knowledge, literature is not available regarding the protective effect of dietary tryptophan against nitrite toxicity in fish. Hence, the present investigation was designed to explore the effect of dietary supplementation of L-tryptophan on metabolic and haemato-immunological responses of nitrite exposed L. rohita juveniles, the most preferred candidate species of tropical aquaculture.
Materials and methods
Experimental diet
Three iso-nitrogenous purified diets with graded levels of dietary supplemental tryptophan were formulated keeping the optimum tryptophan requirement of L. rohita as 0.36–0.38% of the diet (Abidi & Khan 2010). The three experimental diets were TRP0 (0% supplemental tryptophan in the diet but suffices normal tryptophan requirement for L. rohita), TRP0.75 (0.75% supplemental tryptophan in the diet) and TRP1.5 (1.5% supplemental tryptophan in the diet). All dietary ingredients were well mixed and pelletized through a hand pelletizer (Ace Exports, Mumbai, India). Percentage incorporation of various dietary ingredients and proximate composition of the diets is presented in Table 1. The proximate composition of the experimental diets was determined following the standard methods of AOAC (1995). All the experimental diets were analysed for tryptophan by the spectrophotometric method described by Sastry and Tammuru (1985).
| Ingredients | % Inclusion in the diets | ||
|---|---|---|---|
| TRP0 | TRP0.75 | TRP1.5 | |
| Casein (vitamin free)a | 30.00 | 30.00 | 30.00 |
| Gelatina | 10.00 | 10.00 | 10.00 |
| Dextrina | 10.00 | 10.00 | 10.00 |
| Starch solublea | 30.00 | 30.00 | 30.00 |
| Cellulose powdera | 7.99 | 7.24 | 6.49 |
| Cod liver oilb | 4.00 | 4.00 | 4.00 |
| Sunflower oile | 4.00 | 4.00 | 4.00 |
| Vit.Min mixc | 1.96 | 1.96 | 1.96 |
| Vitamin Cd | 0.01 | 0.01 | 0.01 |
| Vitamin E | 0.01 | 0.01 | 0.01 |
| Carboxymethylcellulosea | 2.00 | 2.00 | 2.00 |
| Betaine Hydrochloridea | 0.02 | 0.02 | 0.02 |
| Butylated Hydroxy Toluenea | 0.01 | 0.01 | 0.01 |
| Tryptophan (TRP)a | 0.00 | 0.75 | 1.5 |
| Total | 100.00 | 100.00 | 100.00 |
| Proximate analyses [% dry matter (DM) basis] | |||
| Moisture | 8.32 ± 0.12 | 8.37 ± 0.02 | 8.61 ± 0.14 |
| Crude Protein | 35.35 ± 0.22 | 35.39 ± 0.21 | 35.17 ± 0.31 |
| Ether Extract | 8.04 ± 0.22 | 8.52 ± 0.11 | 8.42 ± 0.53 |
| Ash | 9.26 ± 0.40 | 8.18 ± 0.26 | 9.39 ± 0.58 |
| Total carbohydrate | 47.07 ± 0.37 | 46.99 ± 0.9 | 46.84 ± 1.6 |
| Tryptophan (g kg−1 diet) | 3.5 ± 0.04 | 10.8 ± 0.01 | 18.2 ± 0.05 |
- a Himedia Laboratories, India.
- b Procured from local market.
- c Prepared manually and all components from Himedia Ltd, India, except Vitamin A and D3.
- d Sd Fine Chemicals Ltd., India.
- e Ruchi Soya Industries Ltd., Raigad, India.
- Data expressed as Mean ± SE, n = 3. Composition of vitamin mineral premix (quantity/250 g starch powder) prepared manually: ++Vitamin A 550 000 IU; ++Vitamin D3 110 000 IU; Vitamin B2 200 mg; Vitamin K 100 mg; +Vitamin B12 30 μg; Calcium Pantothenate 250 mg; Nicotinamide 1000 mg; Choline Chloride 15 g; Mn (Mnso4) 2700 mg; I (KI) 100 mg; Fe (Ferric citrate) 75 mg; Zn (Znso4) 500 mg; Cu (Cuso4) 200 mg; Co (CoCl2) 45 mg; Ca (CaCo3 and dibasic calcium phosphate) 50 g; P (dibasic calcium phosphate) 30 g; Selenium (Sodium selenite) 50 ppm. ++Glaxo Pharmaceuticals, Mumbai, India.
Experimental animal and design
Juveniles of Labeo rohita (weight 66.5 ± 0.5 g and length 14.56 ± 1.42 cm) were procured from Prem Fisheries Consultancy, Gujarat, India and brought to the wet laboratory of Central Institute of Fisheries Education, Mumbai, India. Fish were acclimatized for 15 days and then transferred to the experimental rearing units consisting of 12 uniform size plastic tanks (300 L capacity) following completely randomized design. The three treatment groups were: TRP0 (with 6 replicates), TRP0.75 (with 3 replicates) and TRP1.5 (with 3 replicates). The fish were fed their respective diets for 60 days. After 60 days of feeding, the TRP0 treatment group was split into two sub-treatment groups with triplicates. The remaining two treatments (TRP0.75 & TRP1.5) and one of the sub-treatment groups of TRP0 were exposed to sub-lethal concentration of nitrite-nitrogen for another 45 days with the same feeding regime. That is, there were all together four treatment groups as follows: TRP0-N (0% supplemental tryptophan in the diet and unexposed to nitrite), TRP0+N (0% supplemental tryptophan in the diet and exposed to nitrite), TRP0.75+N (0.75% supplemental tryptophan in the diet and exposed to nitrite) and TRP1.5+N (1.5% supplemental tryptophan in the diet and exposed to nitrite). Except TRP0-N all other groups were exposed to sub-lethal concentrations (2.0 mg L−1 i.e. 1/5th of 96 h LC50 (11.28 mg L−1) of NO2-N) of nitrite for L. rohita. Nitrite concentrations (mg NO2-N L−1) were obtained by using sodium nitrite (Himedia Laboratories, Mumbai, India). Stock solution of nitrite (1000 mg/L) was prepared from sodium nitrite (Himedia Laboratories, Mumbai, India). An appropriate volume of the freshly prepared stock solution was added to water of the experimental rearing units to obtain the final desired concentration. The total volume of the water in each tank was maintained at 250 L throughout the experimental period. Continuous aeration was provided to ensure the optimum level of dissolved oxygen. Feeding was done ad libitum twice a day. Every morning, the faecal matters were removed by siphoning and about 75% water of the tanks was exchanged with nitrite treated water in order to maintain the desired level of nitrite. Moreover, every day before and after water exchanges, the nitrite content in the water of the units was measured spectrophotometrically following the method of Shechter, Gruener and Shuval (1972). The differences between nominal and real nitrite concentrations in the water of the units were not significant (Table 2). The physicochemical parameters of water were within the optimum range (dissolved oxygen: 5.7–7.5 mg L−1; temperature: 26.1–27.4°C; pH: 7.4–8.6; ammonia nitrogen: 0.013–0.026 mg L−1; nitrate nitrogen: 0.01–0.08 mg L−1; chloride: 10.2 mg L−1) throughout the experimental period.
| Treatments | TRP0-N | TRP0+N | TRP0.75+N | TRP1.5+N |
|---|---|---|---|---|
| NO2-N before water renewal | 0.06 ± 0.01 | 1.98 ± 0.08 | 1.96 ± 0.07 | 1.98 ± 0.03 |
| NO2-N after water renewal | 0.02 ± 0.01 | 2.02 ± 0.04 | 2.04 ± 0.02 | 2.09 ± 0.05 |
- Data expressed as mean ± SE.
Growth study
The weight gain% of the fish after 60 days of feeding is presented in Table 3. During the exposure period of 45 days the fish were batch weighed at every 2 weeks, after 1 day feed deprivation. No mortality was observed during the experimental trial. At the end of the exposure period, growth parameters of fish for each treatment were measured in terms of weight gain (%), specific growth rate (SGR) and feed conversion ratio (FCR) using the following equations.
| Treatments | Weight gain% |
|---|---|
| TRP0 | 61.67a ± 0.93 |
| TRP0.75 | 67.59b ± 0.86 |
| TRP1.5 | 75.31c ± 1.24 |
- Different superscripts in the same column signify statistical differences (P < 0.05) (mean ± SE) (n = 6).
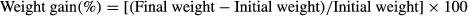
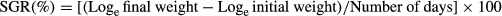
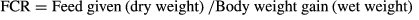
Sample preparation
Six fish from each treatment were anaesthetized with CIFECALM at 50 μL L−1. CIFECALM is an herbal anaesthetic formulation containing natural alcoholic extracts of Eugenia caryophyllata and Mentha arvensis developed by Central Institute of Fisheries Education, Mumbai, India. For enzyme assays, tissues (muscle, liver and brain) were homogenized with chilled 0.25M sucrose solution. The homogenized samples were centrifuged (7000 g, 4°C for 10 min) and supernatants were collected and stored at −20°C for subsequent enzyme assays (acetylcholine esterase, catalase, SOD, lactate dehydrogenase, aspartate aminotransferase and alanine aminotransferase).
Enzyme assays
Acetylcholine esterase (AchE) was assayed from brain of L. rohita by the method of Hestrin (1949) modified by Augustinsson (1957). Catalase activity was estimated according to the method of Takahara, Hamilton, Nell, Kobra, Ogura and Nishimura (1960). The SOD activity was estimated by the method of Misra and Fridovich (1972). Aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) activities were measured by the method described by Wooten (1964). Lactate dehydrogenase (LDH) was assayed following the method of Wrobleuiski and Ladue (1955). Protein estimation in different tissues was carried out according to the method of Lowry, Ronebrough, Farr and Randall (1951).
Blood collection and serum preparation
After 45 days of exposure to sub-lethal concentration of nitrite, fish were anaesthetized with CIFECALM (50 μL L−1) and the body surfaces were cleaned with blotting paper. Blood was collected by puncturing the caudal vein using a syringe (No. 23), which was previously rinsed with 2.7% EDTA solution (as anticoagulant) and shaken gently in order to prevent haemolysis of blood. The collected blood was used for the estimation of whole blood glucose and total leucocyte count. For serum preparation, blood was collected without anticoagulants and allowed to clot for sometime till the straw colour serum separated out. This serum was then stored at −80°C for further analysis.
Haemato-immunological studies

Whole blood glucose was estimated by the method of Nelson and Somogyi (1945). Hundred microlitre of blood was taken in a test tube and deproteinized with zinc sulphate and barium hydroxide. The mixture was then filtered and the supernatant was collected. The supernatant was taken in a test tube followed by adding alkaline copper sulphate and placed in a boiling water bath for 20 min. The test tubes were then cooled, arsenomolybdate reagent was added and absorbance was read at 540 nm against a blank in a spectrophotometer.
Serum protein and albumin were assessed using biuret (Reinhold 1953) and BCG dye binding method (Doumas, Watson & Biggs 1971) respectively using total protein and albumin kit (Qualigens pharmaceutical Limited). Globulin was calculated by subtracting albumin values from total serum protein. A/G ratio was calculated by dividing albumin values by globulin values.
The activity of serum lysozyme was measured using a kit (Bangalore Genei, Bangalore, India) following the manufacturer's protocols. Briefly, serum samples were diluted with phosphate buffer (pH 7.4) to a final concentration of 0.33 mg protein mL−1. Three millilitres of Micrococcus luteus suspension in phosphate buffer (A450 = 0.5–0.7) was taken in a cuvette and 50 μL of diluted serum was added followed by mixing for 15 s. Reading was taken in a spectrophotometer at 450 nm after 60 s of addition of serum sample. This absorbance was compared with standard lysozyme of known activity following the same procedure as above. The activity was expressed as U min−1 mg−1 protein.
Statistical analysis
The treatment effects of all parameters were determined by subjecting the data to one-way anova. Duncan's Multiple Range Test was used to determine the significant differences between any two means at 5% probability level. All the data were analysed using statistical package spss (Version 16).
Results
Growth and body indices
Nitrite exposure significantly (P < 0.05) affected the weight gain percentage, SGR and FCR (Table 4). The highest weight gain percentage was observed in control (TRP0-N) and lowest in TRP0+N group. Dietary supplementation of higher levels of tryptophan significantly augmented weight gain percentage of nitrite exposed fish, even though it was less than TRP0-N. A similar trend was found in SGR. In this study, highest FCR and HSI were observed in TRP0+N and lowest in TRP0-N (Table 4). Nitrite exposure significantly increased FCR and supplementation of additional levels of tryptophan significantly reduced FCR and HSI compared with TRP0+N. The RSI values were significantly affected (P < 0.05) by nitrite exposure (Table 4). The lowest RSI was observed in the control (TRP0-N) and all nitrite exposed groups showed similar RSI values.
| Treatments | Wt gain% | SGRw | FCRx | HSIy | RSIz |
|---|---|---|---|---|---|
| TRP0-N | 33.80c ± 0.46 | 0.49c ± 0.01 | 1.90a ± 0.03 | 1.20a ± 0.04 | 0.34a ± 0.01 |
| TRP0+N | 25.95a ± 0.09 | 0.38a ± 0.01 | 2.36c ± 0.01 | 1.36b ± 0.13 | 0.38b ± 0.01 |
| TRP0.75+N | 28.26b ± 0.23 | 0.41b ± 0.01 | 2.22b ± 0.01 | 1.21a ± 0.09 | 0.38b ± 0.01 |
| TRP1.5+N | 28.81b ± 0.50 | 0.42b ± 0.01 | 2.18b ± 0.04 | 1.19a ± 0.11 | 0.38b ± 0.01 |
- Different superscripts in the same column signify statistical differences (P < 0.05) (mean ± SE) (n = 6).
- SGR, Specific growth rate; FCR, Feed Conversion Ratio; HSI, Hepato Somatic Index; RSI, Reno-Somatic Index.
Haematological parameters
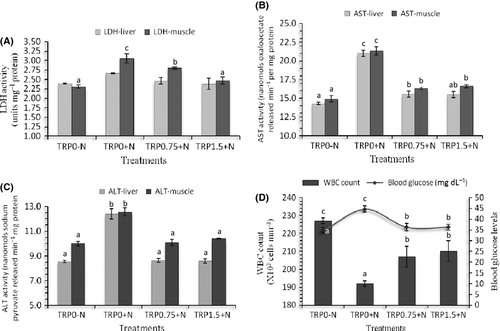
In this study, lowest blood glucose was noticed in control (TRP0-N) and nitrite exposure significantly increased blood glucose levels (Fig. 1). Dietary supplementation of higher levels of tryptophan (0.75% and 1.5%) reduced the elevation of blood glucose levels of nitrite exposed groups compared with those fed normal requirement of tryptophan (TRP0+N). The highest and lowest WBC count was observed in TRP0-N and TRP0+N groups respectively (Fig. 1). Supplementation of higher levels of tryptophan in the diet significantly reduced the reduction in WBC count in nitrite exposed groups.
Metabolic enzymes
Significant effect of nitrite exposure (P < 0.05) on metabolic enzymes likes LDH, AST and ALT (Fig. 1) was seen at the end of 45 days of exposure. Nitrite exposed groups recorded higher LDH, AST and ALT activities compared with the control. However, liver LDH activity was not significantly different among the different treatment groups. In liver and muscle, the highest activities of AST and ALT were observed in TRP0+N group. Dietary supplementation with additional levels of tryptophan found to significantly reduce the activities of these enzymes in nitrite exposed groups.
Antioxidative enzymes
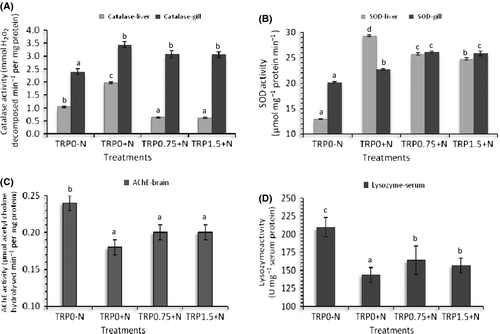
Nitrite exposure significantly (P < 0.05) increased catalase and SOD activities both in the liver and in the gill (Fig. 2). In the liver, highest activities of both catalase and SOD were observed in TRP0+N. Supplementation of elevated amounts of tryptophan in the diet (0.75% and 1.5%) significantly lowered the activities of these enzymes in the liver. However, gill catalase activities of all nitrite exposed groups were higher than that of the control (TRP0-N) irrespective of tryptophan levels in the diet. Gill SOD activity was higher in groups exposed to nitrite and fed elevated amount of tryptophan (Fig. 2).
Enzyme of neurotransmission
The highest activity of acetylcholine esterase in the brain was evidenced in the control group (TRP0-N). Nitrite exposure significantly reduced the activity of this enzyme and all nitrite exposed groups irrespective of tryptophan levels in the diet showed similar levels of activity (Fig. 2).
Serum immunological parameters
In this study, nitrite exposure significantly reduced the activity of lysozyme and feeding with additional amounts of tryptophan found to reduce the decline in lysozyme activity (Fig. 2). The highest lysozyme activity was observed in TRP0-N and the lowest in TRP0+N.
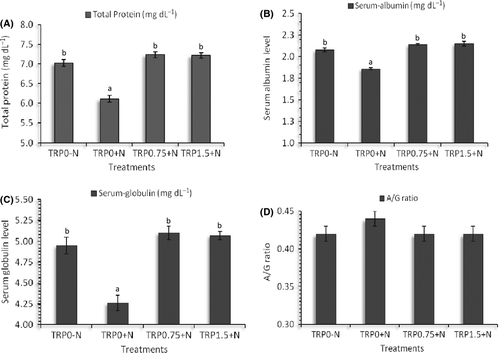
Serum total protein, albumin and globulin values were significantly affected (P < 0.05) by nitrite exposure in L. rohita juveniles (Fig. 3). It was found that nitrite exposure significantly reduced total protein, albumin and globulin levels compared with the unexposed group and the groups which were fed additional amounts of tryptophan maintained total protein, albumin and globulin values similar to that of the nitrite-unexposed groups (TRP0-N). However, the A/G ratio was found to be not significantly different (P > 0.05) among the different treatment groups (Fig. 3).
Discussion
In this study, weight gain percentage and SGR were significantly reduced in the group fed normal requirement of tryptophan when exposed to sub-lethal dose of nitrite (TRP0+N). The increased energy demand for the maintenance and repair of tissues under stress (Kumaraguru & Beamish 1986) might have resulted in the decrease in growth rate of these groups. Nitrite exposure has been found to manifest reduced growth in channel catfish (Colt, Ludwig, Tchobanoglous & Cech 1981), Atlantic cod (Siikavuopio & Saether 2006) and silver perch (Frances, Allan & Nowak 1998). However, in groups supplemented with higher levels of dietary tryptophan, a moderate decrease in percentage weight gain and SGR was observed. This augmentation of growth in nitrite exposed groups due to tryptophan supplementation might be owing to the stress relieving functions of tryptophan as reported by various authors (Lepage et al. 2002; Hseu et al. 2003; Tejpal et al. 2009; Hoseini & Hosseini 2010). However, dietary tryptophan did not reduce the muscle nitrite accumulation. The significantly increased HSI observed in groups fed control diet upon exposure to nitrite could be due to malfunctioning of liver under nitrite stress. The increased reno-somatic index upon nitrite exposure observed in this study is in agreement with Deane and Woo (2007), who documented that nitrite exposure leads to reduced expression of water channel proteins and resulting in increased water accumulation. Similarly, exposure to nitrite leads to increased tissue water retention in Kuruma shrimp (Cheng & Chen 1998).
In this study, reduced activities of antioxidative enzymes in tryptophan supplemented groups upon nitrite exposure might be due to antioxidant properties of tryptophan and its various metabolites, as reported earlier (Keithahn & Lerchl 2005; Reyes-Gonzales, Fuentes-Broto, Martı′nez-Balları′n, Miana-Mena, Berzosa, Garcı′a-Gil, Aranda & Garcı′a 2009). The reactive oxygen species formed during exposure to various pollutants can react with nitric oxide and form highly reactive and potent peroxynitrite capable of inducing oxidative damage (Jensen & Hansen 2011). As oxidative stress has been reported as a result of nitrite exposure, antioxidant property of tryptophan might mitigate nitrite toxicity. This topic could be pursued in the future research.
In this study, the increased LDH activity observed in groups exposed to nitrite compared to control group indicate hypoxic state resulting in change of metabolism from aerobic to anaerobic. During stress LDH activity usually increases (Das et al. 2004b; Tejpal et al. 2009; Akhtar et al. 2010). It has been demonstrated that nitrite exposure induces gill damage (Margiocco et al. 1983), injury to the respiratory system, methaemoglobinemia (Jensen 2003) and reduces the oxygen carrying capacity of blood consequently leading to a hypoxic condition. This might have led to increased LDH activity as suggested by Das et al. (2004a). Fish fed high levels of tryptophan were able to maintain their LDH activity as par with control group that exhibits the positive role of tryptophan supplementation in combating the nitrite stress.
Increase in ALT and AST activity after nitrite exposure in this investigation is in agreement with the previous studies (Das et al. 2004b). Huang and Chen (2002) also observed elevated AST activities upon exposure to nitrite in Anguilla anguilla. ALT and AST are metabolic enzymes (Gharaei, Ghaffari, Keyvanshokooh & Akrami 2011) present in cytoplasm. Higher levels of these two enzymes in blood might occur as a result of leakages from cells due to high concentrations as well as cell damage leading to their release into the bloodstream (Gharaei et al. 2011). Higher enzyme activity after nitrite exposure in the control group compared with tryptophan supplemented group might be due to cell damage. On the other hand, these enzymes are involved in gluconeogenesis and their activity enhances during stress to increase the availability of amino acid substrates for gluconeogenesis in order to meet the higher energy demand to cope up with stress (Masola, Chibi, Kandare, Naik & Zaranyika 2008; Tejpal et al. 2009).
In this study, nitrite exposure caused a decline in the AChE activity, which indicates nitrite induced stress in L. rohita. Our results are in agreement with Das et al. (2004a) who reported a reduction in AChE activity in Indian major carps on nitrite exposure. Several authors have reported reduction in AChE activity on exposure to stressors in different fish species (Das et al. 2004b; Akhtar et al. 2010). This reduction in AChE activity might be attributed to accumulation of cholinesters. The inhibition of AChE activity due to nitrite exposure is found to be reduced with dietary supplementation of additional levels of tryptophan. This topic could be pursued in the future research.
The haematological parameters particularly blood glucose and WBC count has been used as indicators of health status of aquatic organisms including fish (Maheswaran, Devapaul, Muralidharan, Velmurugan & Ignacimuthu 2008; Akhtar et al. 2010). In order to meet the increased energy demand during stress, a surge in glucose level usually occurs through induction of glycolytic and gluconeogenetic pathways (Barton 2002). In this study, the increase in blood glucose after nitrite exposure is low in the tryptophan supplemented group compared with the control. Earlier reports also documented a decrease in induction of glucose production in stressed fish fed tryptophan (Tejpal et al. 2009; Hoseini & Hosseini 2010). In this study, a significant decrease in total leucocyte count was observed in nitrite exposed groups. A reduction in WBC count has been reported in Indian major carps upon nitrite exposure (Das et al. 2004b; Ciji, Sahu, Pal, Dasgupta & Akhtar 2012). Dietary supplementation of higher levels of tryptophan might have helped in counteracting the nitrite induced stress as evidenced by increase in WBC count in these groups.
Serum proteins, particularly albumin and globulins, are known to play key role in immunity and wellbeing of fish (Kumar, Sahu, Pal & Kumar 2007). Aquatic organisms including fish with higher levels of total serum protein, albumin and globulin are believed to manifest stronger innate immune response (Wiegertjes, Stet, Parmentier & Van Muiswinkel 1996). In this study, a decrease in total protein, albumin and globulin levels occurred in nitrite exposed groups fed diets containing normal requirement of tryptophan. Kumar et al. (2007) reported decrease in serum protein levels in Aeromonas hydrophila challenged L. rohita. Das et al. (2004b) documented reduction in serum proteins on nitrite exposed mrigal. This reduction may be attributed to leaking of serum proteins into the peripheral fluids(Ellis, Hastings & Munro 1981) along with their proteolysis to meet the increased energetic demand during stress (Ellis et al. 1981; Das et al. 2004b). It is evident that nitrite oxidizes haemoglobin to methaemoglobin and lowers its capacity to bind with oxygen (Tomasso 1986; Urrutia & Tomasso 1987; Huang & Chen 2002) which may induce hypoxia at the tissue level (Das et al. 2004b) and eventually affect the normal respiratory metabolism leading to increased energy demand. Moreover, nitrite exposure has also been reported to increase plasma corticosteroid concentrations in channel catfish (Tomasso, Davis & Simco 1981) causing elevated energetic demand. The haemolysis and shrinkage of RBC could be the other factors leading to reduction in serum proteins as reported in mrigal (Das et al. 2004b). In this study, elevated level of tryptophan supplementation had augmented the serum proteins which envisage the immuno-stimulating effect of tryptophan. This may be due to the ability of tryptophan to reduce corticosteroid production (Lepage et al. 2002).
Lysozyme stimulates the phagocytosis of bacteria by lysing their cell wall and imparts innate immunity (Yousif, Albright & Evelyn 1994). It has been reported that lysozyme activity increases in parallel with leucocyte numbers (Fletcher & White 1973). In this study, the groups that had the maximum lysozyme activity also had the maximum leucocyte count.
From the results obtained in this study, it may be concluded that nitrite exposure significantly affected growth, metabolic activity and immunity of L. rohita juveniles. The normal requirement of tryptophan (0.35% of the diet) was unable to combat with the nitrite induced stress. Dietary fortification with higher levels of tryptophan (a minimum of 0.75% of the diet in addition to normal requirement) found to be effective in combating the nitrite induced stress and triggering the immunity. Hence, dietary supplementation of higher levels of tryptophan can be useful for reducing nitrite induced stress. Although dietary supplementation of L-tryptophan in purified form may not be economically feasible to the farmers, the inclusion of higher amounts of tryptophan rich feed ingredients like soy protein, seaweeds, sesame meal, rapeseed meal and cotton seed meal can be a possible option to tackle nitrite issues in culture systems.
Acknowledgments
The authors are grateful to Dr. W. S. Lakra, Director and Vice Chancellor, Central Institute of Fisheries Education (CIFE), Mumbai, for providing facilities and financial assistance for carrying out the study. The first author is grateful to CIFE for awarding the Institutional Fellowship. The authors have no any conflict of interest. We would also like to thank the anonymous reviewers for improving the manuscript.




