Effects of starvation on δ15N and δ13C in Atlantic bonito, Sarda sarda (Bloch, 1793)
The Atlantic bonito, Sarda sarda (Bloch, 1793), is a scombrid that occurs throughout the Atlantic Ocean and Mediterranean Sea. In spite of the commercial importance of this species, very few studies have been conducted recently to improve the knowledge and understanding of its trophic biology (Campo, Mostarda, Castriota, Scarabello & Andaloro 2006). Stable isotope analysis is widely used to reconstruct diets and elucidate migration patterns in fish (Olson, Popp, Graham, López-Ibarra, Galván-Magaña, Lennert-Cody, Bocanegra-Castillo, Wallsgrove, Gier, Alatorre-Ramírez, Balance & Fry 2010; Logan, Rodríguez-Marín, Goñi, Barreiro, Arrizabalaga, Golet & Lutcavage 2011; McMahon, Hamady & Thorrold 2013). Nitrogen stable isotope ratios (15N/14N, δ15N) provide useful information about the trophic level of organisms in food webs, while carbon stable isotope values (13C/12C, δ13C) are indicative of energy sources (Fry 1988; Vander Zanden & Rasmussen 1999; Post 2002). Food shortage has been suggested to alter the isotopic values in muscle and liver tissues of fish (Doucett, Booth, Power & McKinley 1999; Estrada, Lutcavage & Thorrold 2005; Varela, Rodríguez-Marín & Medina 2013), and consequently may complicate the estimations of relative contributions of food sources to the diet using stable isotopes. The effect of starvation on stable isotope compositions may be particularly relevant in tunas, as some species often fast for long periods (Rivas 1954, 1955; Rodríguez-Roda 1964; Medina, Abascal, Megina & García 2002), which can bias dietary reconstructions based on isotopic models. Therefore, laboratory experiments are needed to quantify and understand isotopic changes under food-deprivation conditions. The aim of this study is to assess the effects of starvation on δ15N and δ13C values in muscle and liver in the Atlantic bonito.
Immature Atlantic bonito from a single batch (body mass mean ± SD = 445 ± 107 g, fork length mean ± SD = 30.40 ± 2.19 cm, n = 17) were provided by the ‘Planta Experimental de Cultivos Marinos’ (Instituto Español de Oceanografía, C. O. de Murcia, Mazarrón, Spain). Throughout the experimental period (November 11, 2011–December 26, 2011), the fish were maintained under fasting conditions and natural photoperiod, temperature (17–20°C) and salinity (37 ppt). Samples of liver and muscle were collected from three individuals at the start of the experiment (day 0) and subsequently on days 7, 14, 25 and 45. Body mass and length were recorded during samplings, and the Fulton's condition index (K) calculated as K (g cm−3) = 100 weight length−3.
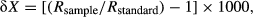
Trends of δ15N, δ13C and Fulton's condition index (K) in the course of the experiment, as well as the relationship between δ15N and K, were assessed by simple linear regression analysis with a significance level of α = 0.05. The statistical analyses were performed using Statgraphics Centurion XV.II.
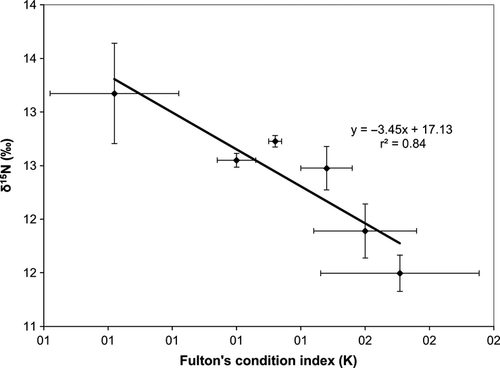
The Fulton's index showed a significant decrease with time (K = −0.01 time (days) + 1.57; r2 = 0.94, P < 0.001) (Fig 1a), indicating that starvation strongly affected the condition of the fish. Otherwise, liver δ15N was the only isotopic variable that increased significantly under nutritional stress (δ15N = 0.03 time (days) + 11.72, r2 = 0.84, P < 0.05) (Fig 1b), whereas no significant trends were observed for δ13C in muscle and liver nor for 15N in muscle (P > 0.05) (Fig 1b, c). Consequently, similar to what was observed in wild Atlantic salmon, Salmo salar (Doucett et al. 1999), there was a significant negative linear relationship between liver δ15N and fish condition of starved bonito (δ15N = −3.45K + 17.13; r2 = 0.84, P < 0.01) (Fig 2).
The estimated average rate of liver δ15N increase (Δ15N = 0.03‰/day) in Atlantic bonito was somewhat lower than that previously reported in the gobiid Pomatochistus minutus (0.05‰ day−1) (Guelinckx, Maes, Van Den Driessche, Geysen, Dehairs & Ollevier 2007). The rate of δ15N increase caused by starvation appears to be highly variable among different animals. Adams and Sterner (2000) found Δ15N to be relatively high (ca. 0.90‰ day−1) in the freshwater cladoceran, Daphnia magna, following 5 days of fasting, while Oelbermann and Scheu (2002) estimated that Δ15N was ca. 0.11‰/day in Pardosa lugrubis spiders starved for 12 days. Unlike these arthropods, the terrestrial flatworm, Arthurdendyus triangulates, showed a low δ15N increment (ca. 0.01‰/day) after a 243-day fast (Boag, Neilson & Scrimgeour 2006). Such results suggest large differences in the effect of starvation on shifts in δ15N levels among taxa. Isotopic changes under fasting conditions can also be influenced by a variety of factors such as the duration of food deprivation, the previous nutritional status of the individuals (Gaye-Siessegger, Focken, Abel & Becker 2007) and/or the species' metabolic rate.
The significant increase in δ15N found in liver tissue can be caused by preferential excretion of molecules bearing the light isotope relative to those bearing the heavy isotope, which would not be replaced by dietary proteins (Gannes, O'Brien & Martínez del Rio 1997). Another plausible explanation for δ15N increase could be related with changes in amino acid composition caused by fasting (Hobson & Clark 1992). The lack of significant δ15N variations in muscle tissue is probably due to the short duration of the experiment, as the muscle shows a relatively low turnover rate (ca. 4–5 months) in tunas (Graham 2008; Varela, Larrañaga & Medina 2011), and hence its response to food deprivation is slower than that of other organs, such as the liver, which presents fairly faster turnover rates (ca. 3–4 weeks) (Graham 2008).
δ13C values remained similar throughout the experiment in both muscle and liver tissues (Fig. 1b, c), though δ13C was more variable in liver. This suggests that either fasting does not affect δ13C values in Atlantic bonito or δ13C changes do not become apparent in less than 45 days of starvation. Similarly, in P. minutus, starved for 20 days, δ13C did not change in muscle and liver, but did significantly decrease in heart (Guelinckx et al. 2007). In contrast, δ13C increased in red and white muscle and liver of Atlantic salmon (Salmo salar) during the natural fasting period that occurs in the reproductive migration (Doucett et al. 1999). δ13C also increased in muscle of Japanese flounders starved for 21 days (Tominaga, Uno & Seikai 2003). The δ13C increase reported in these studies can be explained by the preferential loss of 12C during oxidation of acetyl groups derived from catabolism of lipids, proteins and carbohydrates (Hobson, Alisauskas & Clark 1993).
In conclusion, the results derived from this study suggest that food deprivation induces increased δ15N and higher variability in δ13C values in liver of Atlantic bonito, whereas the isotopic values of muscle do not significantly change throughout a fasting period of 45 days. Therefore, we believe that muscle rather than liver may be a suitable tissue to be used in studies aimed to estimate diet composition or trophic position involving stable isotope analysis in poor–condition fish or fish in which condition is unknown.
Acknowledgements
We thank María Lema (Unidad de Técnicas Instrumentales de Análisis, Universade da Coruña) for isotopic analysis. This study was supported by the Spanish Ministry of Science and Innovation (projects CTM2011-27505 and CTM2011-29525-C04). This is CEI-MAR contribution #33.




