Flow field control via aeration adjustment for the enhancement of larval survival of the kelp grouper Epinephelus bruneus (Perciformes:Serranidae)
Abstract
Flow field control via aeration adjustment for the enhancement of larval survival of the kelp grouper Epinephelus bruneus was examined. Aeration rate of 300 mL min−1 was introduced during daylight (07:00–19:00 hours) and adjusted to 0, 300 and 900 mL min−1 at night (19:00–07:00 hours). Larval sinking velocity±SD increased from 0.08 ± 0.05 to 0.26 ± 0.24 cm sec−1 from 4 to 12 days after hatching (DAH), indicating their susceptibility to sink. Larvae reared in 300 mLmin−1 attained the highest survival rate at 24.9 ± 3.4%, but remained significantly smaller in growth: 4.54 ± 0.56 mm compared with 4.82 ± 0.53 mm in 900 mL min−1. The flow field in 300 and 900 mL min−1 was at 10–20 and 15–25 cm above the bottom of the tank and 8.0 and 1.0 cm beneath the water surface. A favourable rearing condition was observed in 300 mL min−1 as larvae were away from the bottom and surface areas, thus preventing them from dying due to sinking and surface tension-related death (STRD). Although sinking death was decreased with an increasing aeration rate, the stronger flow had increased larval susceptibility to STRD. Our findings suggest that aeration at 300 mL min−1 could enhance larval survival by reducing both sinking death and STRD.
Introduction
The kelp grouper, Epinephelus bruneus is a highly valued marine finfish, making them one of the targeted species for aquaculture in Japan (Kato, Ishimaru, Sawada, Matsuro, Miyashita, Murata & Kumai 2004). However, survival of artificially reared larvae has remained low owing to a high mortality rate at the early developmental stages (Hirata, Hamasaki, Teruya & Mushiake 2009). Accordingly, attempts have been made to enhance the larval survival of E. bruneus including studies of optimum rearing conditions, such as temperature, light intensity and first feeding (Teruya & Yoseda 2006).
Sinking syndrome-related mortality has been widely documented and frequently observed in the larviculture stage. It is closely associated with the phenomenon of larval sinking tendency at night (Masuma, Miyashita, Yamamoto & Kumai 2008; Tanaka, Kumon, Nishi, Eba, Nikaido & Shiozwa 2009). In fact, most fish larvae are negatively buoyant particularly at night (Hare, Walsh & Wuenshel 2006), presumably because they do not feed at night and became inactive and gradually sink to greater depths. This has been reported in the greater amberjack Seriola dumerili (Shiozawa, Takeuchi & Hirokawa 2003), barfin flounder Verasper moseri (Kayaba, Sugimoto & Matsuda 2003), bluefin tuna Thunnus orientalis (Miyashita 2006) and the red sea bream (Kitajima, Yamane, Matsui, Kihara & Furuichi 1993). This marks the need for immediate action to introduce an applicable approach to the hatchery operation to resolve larval sinking syndrome-related mortality for successful mass larval production.
However, little is known about the existence of sinking death in groupers. Hirata et al. (2009) justified the existence of negative buoyancy in E. bruneus at night and considered sinking death as one of the causes of early mortality in larval production. Sundby (1983) suggested that larvae follow the movement of water currents at night owing to a cessation of swimming activity. We hypothesized that a hydrodynamic approach controlling flow rates at night would be useful for regulating larval distribution in the rearing tank by transporting larvae away from the bottom and surface of the tank, to avoid both sinking death and surface tension-related death (STRD). A similar approach has been used in bluefin tuna, Thunnus orientalis (Nakagawa, Kurata, Sawada, Sakamoto & Miyashita 2011 in press) while Sakakura, Shiotani, Chuda and Hagiwara (2007) used aeration without adjustment at night in the sevenband grouper, E. septemfasciatus.
Using a 500-L tank as an experimental case model, we visualized the flow field generated in the larviculture tank of E. bruneus, measured larval sinking incidence and velocity at night, and observed the onset of larval spine formation. We determined the effect of aeration rate adjustment at night on survival, growth and feeding intake. When this information was combined, a favourable flow field in the larviculture tank at night in relation to reduce larval sinking syndrome-related mortality could be obtained and applied in various volumes and shapes of culturing tanks.
Materials and methods
Egg collection
Fertilized eggs of E. bruneus were obtained through artificial insemination, following by human chorionic gonadotropin (HCG) hormone injection into female brood stock at the Ohshima Experiment Station, Fisheries Laboratory of Kinki University (FLKU) on 11 May 2010. The eggs were transported to Shirahama Fish Nursery Center of Kinki University on 12 May 2010 and gently transferred into each experimental tank at a density of 3000 eggs. Hatching occurred the next day (hatching rate 93.0% at 21.8°C).
Larval rearing
Rearing experiment consisted of ten 500-L cylindrical polycarbonate tanks with flat bottom (diameter 100 cm, height 62 cm), nine tanks were prepared as triplicates for three different aeration rates (0, 300 and 900 mL min−1) and one tank served as a sampling tank for measurement of larval sinking velocity. A single piece of rounded ceramic air stone (diameter 50 mm) was placed on the centre of tank bottom. Aeration rate was uniformly fixed in all tanks at 300 mL min−1 during daytime (07:00–19:00 hours) and was only adjusted to 0, 300 and 900 mL min−1 at night (19:00–07:00 hours).
Fluorescent light was used to introduce approximately 500-lx luminance from the open water surface and the photoperiod was fixed at 12L:12D (07:00–19:00 hours and 19:00–07:00 hours). The water exchange rate was introduced from 4 days after hatching (DAH) at a volume calculated by DAH multiplied by 10% of tank volume. First feeding occurred at 3 DAH and an S-type rotifer (Brachionus plicatilis sp. complex) and commercialized Super Chlorella V12 (Chlorella Industry, Fukuoka, Japan) were introduced into each rearing tank to maintain a density of 25 individuals mL−1 and 0.75 ×106 million cells mL−1. Feed oil at 0.1 mL (NMT Himaku Oil, Nissin Marinetech, Yokohama, Japan) was introduced daily to prevent STRD at approximately 07:00 hours after the light was switched on and aeration was adjusted. The oil film on the water surface was removed daily using a paper sheet three times daily (08:30, 13:00 and 16:00 hours) to encourage larval swim bladder inflation. The number of STRD larvae were recorded and removed daily. Water temperature, dissolved oxygen (DO) and pH were recorded twice daily at 08:00 and 16:00 hours using multiple water quality sensors (556 MPS, YSI Incorporation, Ohio, USA). The rearing protocols are similar in both sampling and experimental tanks and summarized in Table 1.
| Parameters | Readings |
|---|---|
| Number of eggs per tank | 3,000 |
| Density of Brachionus plicatilis sp. complex (ind. mL−1) | 25 |
| Density of Nannochloropsis oculata (million cells. mL−1) | 0.75 |
| Photoperiod | 07:00–19:00 |
| Illuminance (lx) | 500 |
|
Water exchange rate (% day−1) |
day number after hatching × 10% of tank volume |
| Water temperature (°C) | 24.0 ± 1.1 |
| Dissolved oxygen (mg L−1) | 6.5 ± 0.9 |
| pH | 7.9 ± 0.2 |
| Salinity | 33.4 ± 0.3 |
Larvae (n = 5) were collected from the rearing tank using 5-L beakers from 4 to 12 DAH at 2 days intervals. The growth in total length (TL) was measured under a stereomicroscope (SMZ1500, Nikon, Nikon Corporation, Japan). The larva was shifted to a light microscope (ECLIPSE 80i, Nikon, Nikon Corporation, Japan) to count the number of rotifers in the digestive tract. The average number of rotifers (ANR) was determined using the following formula: ANR= ∑Li/N, where Li represents the number of rotifers in the digestive tract of the ith larval individual (i = 1,.n), and N is the number of sampled larvae. On the final day of the experiment (14 DAH), surviving larvae from each tank were counted. The larval survival rate (SR) on the final day of the experiment was determined by dividing the number of surviving larvae by the initial number of hatched larvae.
Larval sinking velocity
Larval sinking velocity is defined as sinking speed in known distance over time. The measurement of larval sinking velocity was performed at night (20:00–22:00 hours) from 4 to 12 DAH. To measure larval sinking velocity Vl (cm s−1), random sampling was performed to collect larvae (n = 20) from the sampling tank using a 5-L beaker. TL of the sampled larvae were measured and observed individually under a stereomicroscope and a photo was taken for each observation. Number of larvae with spine was also recorded. Afterwards, larvae were anesthetized using 200-ppm eugenol (FA-100, Mitsubishi Tanabe Pharma, Osaka, Japan). Anesthetized larvae were gently transferred using a glass pipette and released at the top of a 2-L cylindrical glass filled with filtered rearing water taken from the sampling tank. A 10-cm measurement zone was marked on the cylindrical glass and the time required by larvae to sink 10 cm was recorded using a stopwatch. Larval Vl was calculated using Vl = 10 cm/dt, where 10 cm is the known distance and dt is the time required by larvae to sink 10 cm. This sinking velocity measurement was conducted in an air-conditioned room (24.0°C), which was equal to the rearing temperature at 5 DAH.
Flow field measurement
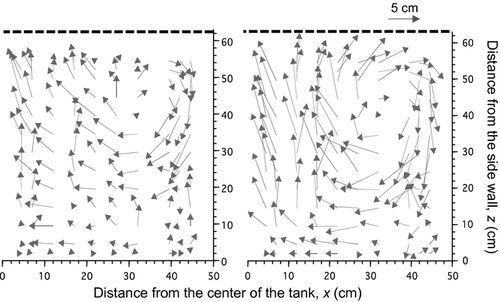
Flow field in the larviculture tank with an aeration rate supplied at 300 and 900 mL min−1 was quantified using an Acoustic Doppler velocimeter (MicroADV, SonTek/YSI, CA, USA) in three directions, horizontal rectangular (x, y) and vertical z axis (from 16 to 28 November 2010), while the water temperature was maintained at 24.0°C. The velocimeter was installed on a portable trestle to allow the sensor to take both vertical and horizontal measurements. For the improvement of sonic reflection, a shell fossil powder (Fish Green, Green Culture, Toyama, Japan) was added to the rearing tank. The velocimeter and sensor were kept at 5 cm due to the difficulty of obtaining velocity data at less than 5 cm below water surface. Measurements were taken in 130 points with 2.5, 4.5 and 5.0 cm intervals. Flow rate in each point was measured at 25 Hz for 3 min and mean data from 1 min was collected after the flow rate was stabilized. All measurements were taken above 5 cm below the water surface and 5 cm away from the tank wall. The mean velocities of all three-dimensional components in the rearing tank were obtained from sampling data and Delta graph 5.0 (Red Rock Software Incorporation, Utah, USA) was used to illustrate the flow velocity distribution in the tank.
Statistical analysis
Statistical analysis was performed using spss 15.0 software (SPSS Incorporation, Chicago, IL, USA) and a significance level of P < 0.05 was applied. One-way anova was performed to compare larval SR, TL, ANR, STRD and sinking velocity. When a significant difference was found, a post hoc test using Tukey's HSD was performed to ascertain any significant differences between treatment means.
Results
Larval survival and growth
This study revealed that E. bruneus SR±SE and TL±SE were greatly affected by the flow field control via aeration adjustment at night (Table 2). The highest SR was observed in 300 mL min−1, 24.87 ± 3.53% and remained significantly higher (P < 0.05) compared with those reared in 0 mL min−1, 1.36 ± 2.21% and 900 mL min−1, 16.75 ± 0.77%. The highest TL was observed in 900 mL min−1, 4.82 ± 0.53 mm and remained significantly larger (P < 0.05) compared with those reared in 300 mL min−1, 4.54 ± 0.56 mm and 0 mL min−1, 4.35 ± 0.48 mm.
| Aeration rate (mL min−1) | |||
|---|---|---|---|
| 0 | 300 | 900 | |
| Survival rate (%) | 1.36 ± 2.21a | 24.87 ± 3.53b | 16.75 ± 0.77c |
| Total length (mm) | 4.34 ± 0.44a | 4.55 ± 0.56a | 4.82 ± 0.53b |
- a Larval survival rate and growth values with different superscripts are significantly different.
Surface tension-related death (STRD)
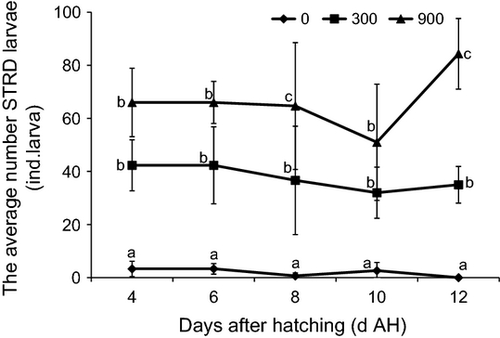
Larval STRD±SE of E. bruneus in 900 mL min−1 was found higher throughout the experimental period (Fig. 1). From 4 to 12 DAH, larval STRD in 0 mL min−1 (3.33 ± 0.58, 3.33 ± 1.53, 6.66 ±0.58, 2.67 ± 1.53 and 0 ind. day−1) was significantly lower (P < 0.05) compared with 300 mL min−1 (42.33 ± 11.13, 36.33 ± 1.53, 36.66 ± 12.90, 32.00 ± 16.52 and 35.00 ±10.00 ind. day−1) and 900 mL min−1 (66.00 ±19.32, 66.00 ± 11.13, 64.67 ± 18.03, 51.00 ± 8.00 and 84.33 ± 7.37 ind. day−1) respectively. However, no significant difference was detected (P > 0.05) between 300 and 900 mL min−1 on 4, 6 and 10 DAH.
Average number of rotifers (ANR)
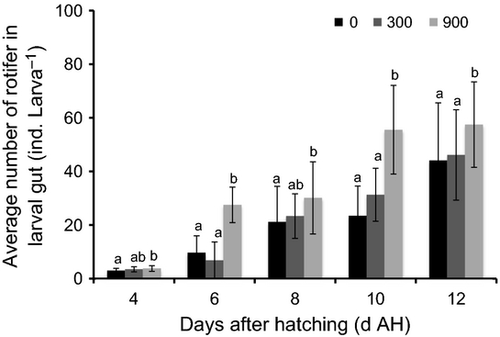
The larval ANR±SE during the daytime was greatly affected by the aeration rates at night-time in the kelp grouper (Fig. 2). From 4 to 12 DAH, larval ANR in 0 mL min−1 (3.00 ± 0.84, 9.73 ± 3.71, 21.20 ± 13.24, 23.45 ± 8.35 and 44.10 ± 16.52 ind. larva−1) was insignificant (P > 0.05) with those observed in 300 mL min−1 (3.53 ± 0.82, 6.81 ± 4.33, 23.36 ± 7.85, 31.30 ± 7.81 and 46.18 ± 14.02 ind. larva−1) respectively. However, it remained significantly lower (P < 0.05) compared with 900 mL min−1 (3.80 ± 1.04, 27.53 ± 6.62, 30.13 ± 13.47, 55.53 ± 18.35 and 57.46 ± 15.93 ind. larva−1). However, no significant difference was detected (P > 0.05) between 300 and 900 mL min−1 on 4 and 8 DAH.
Larval sinking velocity
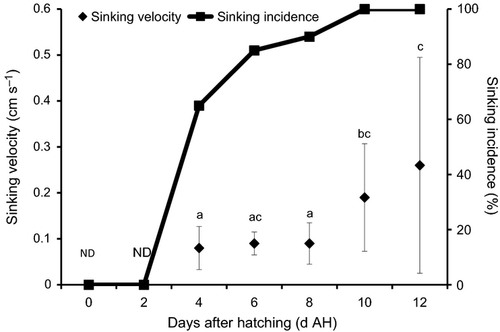
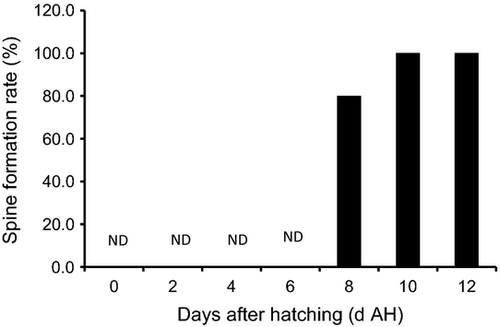
The larval sinking velocity±SE of E. bruneus was found gradually increased with their age from 4 to 12 DAH (Fig. 3). The larval Vl was 0.08 ± 0.05 cm s−1 at 4 DAH (mean 2.94 ±0.05 mm TL), followed by slightly increasing to 0.09 ± 0.02 cm s−1 at 6 DAH (mean 3.17 ±0.06 mm TL) and 8 DAH (mean 3.45 ± 0.13 mm TL). Within this period, no significant differences (P > 0.05) were detected in larval Vl from 4 to 8 DAH. A remarkable increase (P < 0.05) in larval Vl was observed at 10 DAH, as larvae rapidly sank at 0.19 ± 0.12 cm s−1 (mean 3.81 ± 0.15 mm TL). At 12 DAH, larval Vl increased to 0.26 ±0.24 cm s−1 (mean 4.28 ± 0.16 mm TL), but the difference was insignificant (P > 0.05) as compared with those at 6 and 10 DAH, owing to large variation found at 12 DAH. In terms of larval spine formation, 80% of E. bruneus had developed spine at 8 DAH and followed by 100% spine formation as they aged and grew (Fig. 4).
Flow velocity distribution
A circulation flow was observed in both larviculture tanks supplied with an aeration rate at 300 and 900 mL min−1 respectively (Fig. 5). The formation of circulation was initially generated by the vertical flows derived from air bubbles released by an aerator placed centrally at the bottom of the tank. These air bubbles moved towards the open water surface, and at this point, the direction of vertical flow gradually changed to horizontal flow approaching the sidewall. At the sidewall, horizontal flow moved towards the bottom of the tank, and upon reaching the bottom, changed direction and moved towards the centre of the tank, where the circulation flow came to completion. Circulation velocity increased and the thickness of the boundary layer between the turbulent zone and the bottom of the tank decreased at a higher air supply. The circulation flow in 300 and 900 mL min−1 was observed at 10–20 and 15–25 cm away from the bottom of the tank and 8.0 and 1.0 cm beneath the water surface respectively (Fig. 5).
Discussion
In general, the flow field in fish rearing tanks differs depending on the aeration rate (Shiotani, Hagiwara, Sakakura & Chuda 2005). E. bruneus reared in the larviculture tank with an aeration rate of 300 mL min−1 resulted in the highest larval survival. A favourable circulation flow observed in the 300-mL min−1 treatment was the main factor responsible for the higher survival. Although the circulation flow consisted of a rapid downward flow observed near the sidewall, these vertical flows were immediately transformed into horizontal flow at 2.5 cm away from the tank bottom, and prevented larvae from being distributed near this area. Meanwhile, the upward flow did not radiate out to the water surface, but was transformed into horizontal flow at 8.0 cm beneath the water surface. Accordingly, larvae were distributed away from the water surface and prevented them from experiencing STRD. In addition, circulation flow in the inner region was not as rough as that observed in 900 mL min−1. This reduces larval risk of contact with each other in the larviculture tank, which is significantly beneficial for larvae, particularly after the onset of spinal development on 8 DAH, because rough circulation flow might distribute larvae unevenly and the chances of spinal entanglement are greater. Accordingly, larvae will eventually encounter a higher incidence of mortality caused by entanglement with other larvae as reported in other groupers including the red spotted grouper, E. akaara (Kusaka, Yamaoka, Yamana, Abe & Kinoshita 2001) and the mouse grouper, Cromileptes altivelis (Sugama & Ikenoue 1999).
The dissimilarities of flow fields observed in the larviculture tanks with aeration rates of 300 and 900 mL min−1 were evidently accountable for the significant differences in E. bruneus survival. This study shows that the remarkably rapid flow observed in the 900-mL min−1 treatment was unfavourable for E. bruneus survival. A circulation flow produced by the 900-mL min−1 flow was observed at 5.0 cm above the bottom of the tank, but it went abruptly to the water surface. As we assumed that E. bruneus were transported according to the flow velocity distribution in the larviculture tanks at night, larvae might have lifted near or exceeded the water surface, eventually subjecting them to STRD. A study conducted by Sawada, Kato, Okada, Kurata, Mukai, Miyashita, Murata and Kumai (1999) observed mucous cells on the entire epidermis of E. bruneus since an early stage. Similarly to other groupers, E. bruneus are well characterized by a dense mucous cell covering on the body epidermis and tend to produce a greater number of mucous substances under stressful conditions (Yamoaka, Nanbu, Miyagawa, Isshiki & Kusaka 2000; Sarasquete, Gisbert, Ribeiro, Viera & Dinis. 2001). As we observed water conditions directly in the rearing tank, water was abruptly moved, which Sakakura, Shiotani, Chuda and Hagiwara (2006) termed as physical stress, and might have triggered larvae to secret more mucous. Larvae with glue-like mucous on the body epidermis were easily attached to the water surface and subjected to STRD. This is in agreement with the findings in our study, as E. bruneus reared in 900 mL min−1 demonstrated higher STRD compared with those in 300 mL min−1.
In contrast, E. bruneus reared in the larviculture tank with an aeration rate of 0 mL min−1 at night resulted the lowest survival rate in this study. The absence of a flow field created a region of stagnant water in the larviculture tank (Şahin & Üstündağ 2003). With the absence of a flow field in the rearing tank, we assumed that larvae might be unable to remain in the water column owing to the increasing sinking velocity at night along with their growth. Although larval sinking velocity had no significant difference from 8 to 10 DAH owing to the variance in larval sizes, we observed an increasing sinking velocity pattern. Larvae were presumed to be highly susceptible to sinking to the bottom and dying during 8–10 DAH. As larvae reared in 0 mL min−1 attained the lowest STRD, whereby only a few number of dead bodies were found floated on the water surface, while survival rate remained the lowest, larvae were probably died at the bottom of the tank. This assumption was hard to be justified due to the difficulties to allocate larval dead body at the bottom of the tank with the presence of green water (Nannochloropsis sp.) for water conditioning and feed for rotifer purposes and also the exclusion of bottom cleaning process (siphoning larval dead bodies) throughout the experimental period to minimize stress handling mortality and escapee. In addition, we directed a portable torchlight close to the rearing tank when sampling was performed, and no larvae were found near the water surface or in the water column. This enables us to further justify the existence of a sinking tendency in E. bruneus at night under no flow conditions. In practical hatchery operations, particularly using large-scale production tanks and to avoid larval sinking death, a flow field is necessary to prevent stagnant water flow in the larviculture tank.
In terms of growth performance, E. bruneus in 300 mL min−1 was found to be significantly smaller compared with those reared in 900 mL min−1. According to Kohno (1998), the negative density-dependent effect is one of the responsible factors for the poor growth in grouper larval stages, as higher larval density might lead to intra-specific food competition and exhibit lower growth as in the orange spotted grouper, E. suillus (Duray, Estudillo & Alpasan 1997) and C. altivelis (Sugama & Ikenoue 1999). In contrast, our findings show that intra-specific food competition did not exist in this study as larval feeding intake between 300 and 900 mL min−1 showed no significant difference throughout the experimental period, except 8 DAH. Therefore, we neglected the negative density-dependent effect in this study. However, we speculated that E. bruneus in 900 mL min−1 might have become stronger than larvae in 300 mL min−1 as they were able to survive under a rougher rearing environment. According to Fuiman, Cowan, Michael, Jr and O'Neal. (2005), fish larvae with higher survival skills tend to demonstrate better feeding performance as found in the orange spotted grouper, E. coioides (Toledo, Caberoy, Quintio, Choresca & Nakagawa 2002) and the grouper, E. suillus (Duray, Estudillo & Al pasan L.G. 1996). In the 900-mL min−1 treatment, smaller larvae may have died because of STRD. As a result, the proportion of strong, larger larvae in 900 mL min−1 that survived was higher compared with those in 300 mL min−1, and mean TL in 900 mL min−1 was more than in 300 mL min−1.
Apart from aeration rate, it is important to note that creating favourable flow field in fish rearing tank is species dependent, differs depending on aerator position and varies in various sizes and shapes of culturing tanks (Sakakura et al. 2007). However, the principle of flow field works in a similar way and it enables us to illustrate larval distribution in the larviculture tank. Aeration rate is easy for fish culturists to control and ensure larvae are distributed away from the bottom and water surface to avoid sinking death and STRD. Other than aerations, equipment such as commercialized propellers, customized wave makers (Sakakura et al. 2007), horizontal paddles and vertical plunges (Howell, Day, Ellis & Baynes 1998) have a similar function to produce water currents in hatchery operations. To economize cost production in hatcheries, the usage of aeration is recommended, as it provides the most immediate, easy and cheap method to produce water currents to resolve larval sinking death mortality.
In the case of a 500-L cylindrical tank, aeration at 300 mL min−1 at night is recommended to culture E. bruneus by taking into account the flow field that is favourable to reduce sinking death and STRD, and enhance larval survival. This recommendation is made possible by the quantification of flow field in the larviculture tank, with measurements of larval sinking and larval STRD. Rearing experiments including a hydrodynamics approach are not only useful for discovering the most favourable rearing conditions to resolve both sinking death and STRD, but are also helpful to estimate the flow field in different scales of tanks.
Acknowledgments
This study was supported by the global COE Program (International Education and Research Center for Aquaculture Science of Bluefin Tuna and Other Cultivated Fish) of the Ministry of Education, Culture, Science, Sports and Technology, Japan, sanctioned to Kinki University. The authors thank the staff of Shirahama and Uragami Fish Nursery Center and Shirahama Experimental Station of the Fisheries Laboratory of Kinki University, Japan, for their unconditional assistance throughout the experimental period.




