Dietary microbial levan ameliorates stress and augments immunity in Cyprinus carpio fry (Linnaeus, 1758) exposed to sublethal toxicity of fipronil
Abstract
A 45-day feeding trial was conducted to study the stress ameliorating and immunomodulatory role of microbial levan in Cyprinus carpio fry exposed to sublethal dose (1/10th LC50) of fipronil [(±)-5-amino-1-(2,6-dichloro-α,α,α-trifluoro-p-tolyl)-4-trifluoromethylsulfinylpyrazole-3-carbonitrile]. Two hundred and twenty-five fry were randomly distributed in five treatments in triplicates. Four purified diets were prepared with graded levels of microbial levan. Five different treatment groups were levan control L0P0 (basalfeed + 0% levan without exposure to pesticide); pesticide control L0P1 (basalfeed + 0% levan with exposure to pesticide); L0.25P1 (basalfeed + 0.25% levan with exposure to pesticide); L0.50P1 (basalfeed + 0.50% levan with exposure to pesticide) and L0.75P1 (basalfeed + 0.75% levan with exposure to pesticide). Lactate dehydrogenase (LDH), malate dehydrogenase (MDH) and fructose-1,6-diphosphatase (FDPase) activites were significantly (P < 0.05) increased, whereas alkaline phosphatase (ALP), adenosine triphosphatase (ATPase) and acetyl choline esterase (AchE) activities were significantly (P < 0.05) reduced in higher levan-fed groups. RBC, haemoglobin and WBC counts were significantly (P < 0.05) increased in the levan-fed groups. Similar trends were also observed for the total serum protein, globulin, NBT and lysozyme activities. Blood glucose and serum cortisol exhibited a third order polynomial relationship with increasing level of dietary levan. Overall result showed stress ameliorating, immunostimulating and protective role of microbial levan against fipronil-induced stress in C. carpio fry at 0.75% level of dietary levan supplementation.
Introduction
Aquatic pollution has become major global concern nowadays. Indiscriminate and widespread use of pesticides primarily in the agricultural sector ultimately leads to pollution of aquatic environment and thus it becomes hazardous to the aquatic life. Increased use of pesticide results in the excess inflow of toxic chemicals into the aquatic ecosystem (Baskaran, Palanichamy, Visalakshi & Balasubramanian 1989; Kalavathy, Sivakumar & Chandran 2001). Kilgore and Mingyuli (1975) reported that concentration of pesticide residues were found to be more in aquatic ecosystem rather than in terrestrial ecosystem. Aquatic organisms are highly vulnerable to the residues of these toxic substances. Fish being one of the important aquatic organisms are no exception to this. Quite a few species of fish have been reported to have deleterious effects when exposed to pesticides (Khangarot, Ray & Singh 1988; Areechon & Plump 1990).
Fipronil is a new broad-spectrum phenylpyrazole insecticide, which is commercially sold in the Indian market under various trade names like regent, termidor. Because of impending bans on application of dieldrin, lindane and DDT, use of fipronil is gaining considerable attention in recent years. Even minute concentration of fipronil is highly effective against various insects and pests of crops, notably rice insects, thrips and termites (Mulrooney, Wolfenbarger, Howard, Goli & Goli 1998) owing to its lipophilicity and persistency properties. Fipronil is used to protect paddy crop from the attack of various pests in paddy-cum-fish integrated farming system and also to control bund-destroying crabs in rice field. Thus, there is potential threat on non-target species such as fish. Moreover, fipronil is reported to be highly toxic to rainbow trout and very highly toxic to bluegill sunfish with 96 h LC50 of 0.246 mg L−1 and 0.083 mg L−1 respectively. It is also highly toxic to Japanese carp with 96 h LC50 value of 0.34 mg L−1 (Colliot, Kukorowski, Hawkins & Roberts 1992) and Sheephead minnows (EC50 = 0.13 mg L−1) on acute basis [USEPA (U.S. Environmental protection Agency) 1996].
Recent studies in fish have indicated that immunostimulants can activate various immune functions, even in stressful situations, and therefore reverse the deleterious effects mediated by stress (Ortuno, Esteban & Meseguer 2003; Sarma, Pal, Sahu, Ayyappan & Baruah 2009). Madhuban and Anilava (2003) reported that dietary supplementation of ascorbic acid at higher doses (1 g kg−1) can counter the stress of the pesticide deltamethrin in Clarias gariepinus. A number of synthetic compounds viz. vitamin C (Manush, Pal, Das & Mukherjee 2005), alpha-tocopherol (Belo, Schalch, Moraes, Soares, Otoboni & Moraes 2005), tryptophan (Tejpal, Pal, Sahu, Jha, Muthappa, Sagar & Rajan 2008) and pyridoxine (Akhtar, Pal, Sahu, Ciji, Gupta, Choudhary, Jha & Rajan 2010) have been reported to ameliorate stress in fish. In recent years, levan has received considerable attention due to its multi-functional features (Rairakhwada, Pal, Bhathena, Sahu, Jha & Mukherjee 2007; Gupta, Pal, Sahu, Dalvi, Kumar & Mukherjee 2008). Levan, a type of fructan is a non-digestible food fibre, composed of monomers of d-fructose attached by ß (2→6) linkages. Although levan is a diversely distributed component, particularly in plants, yeasts, fungi and bacteria (Jang, Kang, Cho, Kim, Lee, Hong, Seong, Kim, Kim, Rhee, Ha & Choue 2003), many microbial species such as Zymomonas mobilis, Bacillus subtilis, Bacillus polymyxa and Acetobacter xylinum also produce extracellular levan of high molecular weight when grown on sucrose, which is serologically active (Clarke, Roberts & Garegg 1997).
Previous studies have shown levan to have anti-tumour (Stark & Leibovici 1986), immunostimulating (Xu, Yajima, Li, Saito, Ohshima, and Yoshikai 2006) and prebiotic activities (Bello, Walter, Hertel & Hammes 2001) in humans. In our earlier studies, we observed that dietary microbial levan increases the immunological responses of Cyprinus carpio (Rairakhwada et al. 2007) and L. rohita (Gupta et al. 2008) and protects the animals against Aeromonas hydrophila challenge. Furthermore, dietary microbial levan (1.25%) could be an effective means to enhance tolerance to thermal stress and improve the immune status of L. rohita juveniles (Gupta, Pal, Sahu, Dalvi, Akhtar, Jha & Baruah 2010). Besides these properties, the role of microbial levan to combat pesticide stress has never been studied. Hence, this study was aimed to delineate the role of dietary levan in ameliorating fipronil-induced stress and possible immuno-augmentation in C. carpio fry.
Materials and methods
Experimental animals
Cyprinus carpio fry with an average weight of 3.26 ± 0.43 g were procured from Palghar fish farm, Maharashtra during the month of February, 2011. They were transported, stocked in cement tank and left undisturbed during the whole night. The next day, fish were given a mild salt treatment to ameliorate the handling stress. The stock was acclimatized under aerated condition for 15 days. During acclimation, fish were fed with pelleted diet prepared in the laboratory (30% CP).
Chemicals
Technical grade fipronil (C12H4Cl2F6N4OS) (99.1% pure) manufactured by Bio Quest International Private Limited, Mumbai, India, consisting of alpha and beta isomers at the ratio of 50:50, was procured from Malti enterprises, Mumbai. It was kept in an airtight container in the refrigerated condition. A stock solution of fipronil was prepared using analytical grade acetone (solubility of fipronil in acetone is 545.9 g L−1 at water pH 9.0). Required quantity of fipronil was drawn from this stock solution for the experimental use. Range finding test was conducted using methodology as suggested by APHA–AWWA– WPCF (1975). Bioassay experiment was carried out to determine the median lethal concentration (LC50) of fipronil in fish for 96 h using probit analysis method (Finney 1971). The 96 h LC50 value for fipronil was found to be 0.428 mg L−1. Based on this value, the sublethal fipronil dose of 0.0428 mg L−1 (1/10th LC50 for 96 h) was selected for this study to simulate the fipronil-contaminated water bodies of India. All other chemicals used in the experiment were of analytical grade.
Experimental site, design and feeding
The laboratory analysis was carried out at the Fish Nutrition, Biochemistry and Physiology Laboratory of Central Institute of Fisheries Education (CIFE), Mumbai and the experimental setup was maintained in the wet laboratory of the Aquaculture division. Two hundred and twenty-five fry of C. carpio were randomly distributed in five treatment groups, each with three replicates following a completely randomized design. The experimental rearing system consisted of 15 uniform size circular fibre reinforced plastic tanks (150-L capacity). The total volume of the water in each tank was maintained at 75 L throughout the experimental period.
Four experimental diets were prepared (Table 1). The fish were divided into five different treatment groups as levan control L0P0 (basalfeed + 0% levan); pesticide control L0P1 (basalfeed + 0% levan); L0.25P1 (basalfeed + 0.25% levan); L0.50P1 (basalfeed + 0.50% levan) and L0.75P1 (basalfeed + 0.75% levan). Purified microbial levan were produced and characterized as described by Rairakhwada et al. (2007) and provided by Dr Zarine P. Bhathena, Department of Microbiology, Bhavans College, Mumbai, Maharashtra, India. The dietary ingredients along with the necessary levan were mixed with gelatin, water and oil to make dough, followed by cooking in an autoclave at 15 psi for 20 min. After cooling, vitamins and minerals were added. Finally, the dough was pressed through a hand pelletizer to obtain uniform size pellets and sun dried for 4 h. The pellets were then kept in a hot air oven (50–60°C) overnight for complete drying, packed in polythene bags and stored at 4°C throughout the experimental period. All the groups except L0P0 were exposed to sublethal dose of fipronil. Continuous aeration was provided. Feed was given to satiation level for 45 days twice daily. Daily ration was divided into two parts: about 2/3rd of total ration was given at 09:00 hours, and the rest 1/3rd at 18:00 hours. The uneaten feed and faecal matters were removed by siphoning out about 50% of the tank water on alternate days with care to avoid stress of the test organisms. Test solution was renewed with firponil-treated water prepared from the stock solution to provide constant sublethal effect of the fipronil. Similar methodology was followed up for sublethal toxicity studies as previously described by (Das & Mukherjee 2003) and (Prusty, Kohli, Sahu, Pal, Saharan, Mohapatra & Gupta 2011). The physico-chemical parameters of the rearing water were within the optimum range (dissolved oxygen: 6.56–7.1 mg L−1; pH: 7.25–7.8; temperature: 26.6–28.2°C; alkalinity 46–58 mg L−1 and hardness 48–64 mg L−1) throughout the experimental period (Prusty et al. 2011).
| Ingredients | Experimental diets (% inclusion) | |||
|---|---|---|---|---|
| L0P0 | L0.25P1 | L0.50P1 | L0.75P1 | |
| Casein fat freea | 35 | 35 | 35 | 35 |
| Gelatina | 9.5 | 9.5 | 9.5 | 9.5 |
| Dextrina | 10 | 10 | 10 | 10 |
| Starch solublea | 25 | 25 | 25 | 25 |
| Cellulose powdera | 9.0 | 8.75 | 8.5 | 8.25 |
| Cod liver oilb | 4 | 4 | 4 | 4 |
| Sun flower oilc | 4 | 4 | 4 | 4 |
| Vitamin mineral mixd | 2.88 | 2.88 | 2.88 | 2.88 |
| Vit-Ce | 0.10 | 0.10 | 0.10 | 0.10 |
| Microbial levan | – | 0.25 | 0.5 | 0.75 |
| CMCa | 0.48 | 0.48 | 0.48 | 0.48 |
| Betain Hydrochloridea | 0.02 | 0.02 | 0.02 | 0.02 |
| BHTa | 0.02 | 0.02 | 0.02 | 0.02 |
| Total | 100 | 100 | 100 | 100 |
- a Himedia Laboratories, Mumbai, India.
- b Densa Pharma, Mumbai, India.
- c Ruchi Soya Industries, Raigad, India.
- d Composition of vitamin mineral mix (EMIX PLUS) (quantity 2.5 kg−1). Vitamin A 55 00 000 IU; Vitamin D3 11 00 000 IU; Vitamin B2 2000 mg; Vitamin E 750 mg; Vitamin K 1000 mg; Vitamin B6 1000 mg; Vitamin B12 6 μg; Calcium Pantothenate 2500 mg; Nicotinamide 10 g; Choline Chloride 150 g; Mn 27 000 mg; I 1000 mg; Fe 7500 mg; Zn 5000 mg; Cu 2000 mg; Co 450 mg; Ca 500 g; P 300 g; l-lysine 10 g; dl-Methionine 10 g; Selenium 50 mg L−1; Selenium 50 mg L−1; Satwari 250 mg L−1 (Lactobacillus 120 million units and Yeast Culture 3000 crore units).
- e Stay C (Hoffman La Roche, Nutley, NJ, USA) 15% ascorbic acid activity.
Tissue homogenate preparation
At the end of the feeding trial, six fish per treatment were sampled and anaesthetized with CIFECALM (50 μL L−1). CIFECALM is an herbal anaesthetic formulation containing natural alcoholic extracts of Eugenia caryophyllata and Mentha arvensis (developed by CIFE, Mumbai). The muscle, liver, gill and brain tissues were dissected and weighed carefully. A 5% homogenate was prepared with chilled sucrose solution (0.25 M) in a glass tube using teflon-coated mechanical tissue homogenizer. The tube was continuously kept in ice to avoid heating. The homogenate was centrifuged at 5000 g for 10 min at 4°C in a cooling centrifuge. The supernatant was collected and stored at −20°C for enzyme studies.
Enzyme assays
Lactate dehydrogenase (LDH) and Malate dehydrogenase (MDH)
Lactate dehydrogenase (l-lactate NAD+ oxidoreductase; E.C.1.1.1.27) was assayed using 0.1 M phosphate buffer (pH 7.5), 0.2 mM NADH solution in 0.1 M phosphate buffer. The reaction was initiated by adding 0.2 mM sodium pyruvate as the substrate and OD was recorded at 340 nm as described by (Wroblewski & Ladue 1955). A similar reaction mixture was used for the estimation of malate dehydrogenase (l-malate: NAD+ oxidoreductase; E.C.1.1.1.37) except for the substrate (1 mg oxaloacetate/ml of chilled triple distilled water) (Ochoa 1995).
Fructose-1, 6-diphosphatase (FDPase)
Fructose-1, 6-diphosphatase (d-FDP-1-Phosphohydrolase; E.C. 3.1.3.11) was estimated using 50 mM borate buffer (pH 9.5), 0.05 M fructose-1, 6-diphosphate (pH 7–7.3) and 0.5 M MgSO4 (Freeland & Harper 1959). The enzyme (E.C.3.1.1.7) was assayed using the method of Hestrin (1949) modified by Augustinsson (1957). Phosphate liberated was estimated at OD of 660 nm (Fiske & Subbarow 1925).
Alkaline phosphatase (ALP)
The ALP (E.C. 3.1.3.1) activity was determined using the method of Garen and Levinthal (1960). The assay mixture comprised of 0.2 mL bicarbonate buffer (0.2 M), 0.1 mL of 0.1 M MgCl2, 0.1 mL tissue homogenate, 0.5 mL of distilled water and 0.1 mL of freshly prepared 0.1 M para-nitrophenyl phosphate. The reaction mixture was incubated in water bath at 37°C for 15 min and the reaction was arrested by 1.0 mL of 0.1 N NaOH and OD was taken at 410 nm.
Adenosine triphosphatase (ATPase)
Adenosine triphosphatase activity was determined (adenosine triphosphate phosphohydrolase, E.C. 3.6.1.3) using a reaction mixture of 0.1 M Tris-HCl buffer (pH 7.8), 100 mM NaCl, 20 mM KCl, 3 mM MgCl2, 5 mM ATP. The mixture was incubated for 15 min and the reaction was terminated by means of 10% trichloroacetic acid (McKim 1977). Phosphate liberated was estimated at OD of 660 nm (Fiske and Subbarow 1925).
Acetylcholine esterase (AchE)
The enzyme (E.C.3.1.1.7) was assayed using the method of Hestrin (1949) modified by Augustinsson (1957). Acetylcholine esterase assay system comprised of 1.0 mL of M/15 phosphate buffer (pH 7.2), 1 mL acetylcholine (0.004 M, pH 4.0) substrate buffer mixture (1:9 dilution) and 0.2 mL of homogenate, and incubated for 30 min at 37°C. Alkaline hydroxylamine (2.0 mL) was added to terminate the reaction. The solution was mixed thoroughly and 1 mL of HCl (2:1) was added followed by thorough mixing. Enzyme solution was then added to the control tubes. The colour was developed by the addition of 1 mL of FeCl3 (10%) and OD was recorded at 540 nm after thorough mixing. In this assay, mixing the solution in every step is very essential to avoid trapping of air bubbles.
Protein estimation
Protein estimation in different tissues was carried out using alkaline CuSo4 and freshly prepared 1 N Folin- Ciocalteau reagents (Lowry, Ronebrough, Farr, and Randall 1951). A 10% tissue homogenate was prepared with chilled sucrose solution (0.25 M) in a glass tube using teflon-coated mechanical tissue homogenizer. 2 mL of 5% TCA solution was added with 0.1 mL of homogenate in a tube and centrifuged at 5000 g for 10 min at 4°C. The supernatant was discarded and residue was dissolved by adding 0.5 mL of 0.1 N NaOH. Freshly prepared bovine serum albumin was used as the protein standard.
Collection of blood
Blood was collected by puncturing the caudal vein using a medical syringe (No. 23), which was previously rinsed with 2.7% EDTA solution (as an anticoagulant) and shaken gently to prevent haemolysis of blood. The blood samples were used for determination of haemoglobin content, total erythrocyte counts, total leucocyte counts and Nitroblue Tetrazolium (NBT) assay. For serum, again three fish from each replicate and a total of 15 from each treatment were anesthetized; the blood was collected without anticoagulant and allowed to clot for 2 h, centrifuged (3000 g for 5 min) and then kept at −80°C until use.
Haemato-immunological studies
The haemoglobin percentage was determined by estimating cyanmethemoglobin using Drabkin's Fluid (Qualigens, Division of Glaxo SmithKline Pharmaceutical Ltd., Mumbai, India). Five milliter of Drabkin's working solution was taken in a clean and dry test tube and 20 μL of blood was added to it. The absorbance was measured using a spectrophotometer (MERCK, Nicolet, evolution 100, Darmstadt, Germany) at a wavelength of 540 nm. The final concentration was calculated by comparing with standard cyanmethemoglobin (Qualigens). Total erythrocytes and leucocytes were counted in a haemocytometer using erythrocyte and leucocyte diluting fluids (Qualigens) respectively. Twenty microlitre of blood was mixed with 3980 μL of diluting fluid in a clean glass test tube. The mixture was shaken well to suspend the cells uniformly in the solution. Then, the cells were counted using a haemocytometer.

Serum protein, albumin, globulin and A/G ratio
Serum protein was estimated using Biuret and the bromocresol green (BCG) dye binding method (Reinhold 1953) using a total protein and albumin kit (Qualigens Diagnostics). Albumin was estimated using the bromocresol green binding method (Doumas, Watson & Biggs 1971). The absorbance of standards and tests was measured against the blank in a spectrophotometer at 630 nm. Globulin was calculated by subtracting albumin values from total serum protein. The albumin/globulin (A/G) ratio was calculated by dividing albumin values by globulin values.
Serum lysozyme activity
Serum lysozyme activity was measured using an ion exchange chromatography kit (Bangalore Genei, Bangalore, India). Serum samples were diluted with phosphate buffer (pH 7.4) to a final concentration of 0.33 mg mL−1. Three millilitre of Micrococcus luteus suspension in phosphate buffer (A450 = 0.5–0.7) and 50 μL of diluted serum sample were mixed well in a cuvette for 15 s and reading was taken in a spectrophotometer at 450 nm, 60 s after addition of the serum sample. This absorbance was compared with standard lysozyme of known activity, following the same procedure. The activity was expressed as Units min/mg protein.
Respiratory burst activity (NBT)
Respiratory burst activity of phagocytes was measured using the NBT assay following the method of Secombes (1990), as modified by Stasiack and Bauman (1996). Fifty microlitre of blood was placed into the wells of ‘U’ bottom microtitre plates and incubated at 37°C for 1 h to allow adhesion of cells. The supernatant was then removed and the wells were washed three times in phosphate-buffered saline (PBS). After washing, 50 μL of 0.2% NBT was added and incubated for a further 1 h. The cells were then fixed with 100% methanol for 2–3 min and washed three times with 30% methanol. The plates were then air-dried and 60 μL 2 N potassium hydroxide and 70 μL dimethyl sulphoxide were added to each well. The OD of the solution was then recorded in an ELISA reader at 540 nm.
Blood glucose and serum cortisol
Glucose was estimated using the method of Nelson and Somogii (1944,1945). Serum cortisol levels were determined using a commercially available cortisol-specific competitive binding enzyme linked immunosorbent assay (ELISA) kit (Neogen Corporation, Lexington, KY, USA) from triplicate samples. These cortisol ELISA kit and methodologies have been successfully validated and utilized in many fish species (Afonso, Basu, Nakano, Devlin & Iwama 2003; Stefansson, Nilsen, Ebbesson, Wargelius, Madsen, Bjornsson & McCormick 2007; Fast, Hosoya, Johnson & Afonso 2008).
Challenge study with Aeromonas hydrophila
After 45 days of feeding, 12 fish from each group were challenged with virulent A. hydrophila strain 018 (obtained from Aquatic Animal Health Management Division, CIFE, Mumbai, USA). The pathogenic isolates of A. hydrophila were grown on nutrient broth for 24 h at 30°C in a BOD incubator and harvested by centrifuging the culture broth at 10 000 g for 10 min at 4°C. The cells were then washed thrice in sterile PBS (pH 7.2) and final concentration was maintained at 1.8 × 108 CFU mL−1 by serial dilution. The fish in each experimental group were intraperitoneally injected with 0.2 mL of bacterial suspension. Mortality was observed for all groups for 10 days. Tissues were taken from dead fish for bacteriological culture and to confirm A. hydrophila as the cause of disease.
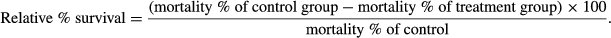
Statistical analysis
Mean values of all parameters were subjected to one-way anova to study the treatment effect and Duncan's Multiple Range Tests (DMRT) were performed to determine the significant differences between the mean values. Comparisons were made at 5% probability level. Polynomial relationship with dietary levan was carried out to fit the trend line of blood glucose and serum cortisol parameter. All the data were analysed using statistical package spss (Version 16) (SPSS Inc., Chicago, IL, USA).
Results
Enzyme assays
Higher value of LDH activity in muscle was observed compare with liver. Significantly (P < 0.05) highest LDH activity was found in L0P1 group in both liver and muscle, and the activity significantly (P < 0.05) reduced in higher levan-fed groups (L0.50P1 and L0.75P1) (Table 2). Similar trend was observed for MDH activity in both liver and muscle.
| Enzyme | Tissues | Treatments | ||||
|---|---|---|---|---|---|---|
| L0P0 | L0P1 | L0.25P1 | L0.50P1 | L0.75P1 | ||
| LDH | Liver | 3.30b ± 0.45 | 4.28c ± 0.57 | 3.01a,b ± 0.10 | 2.34a,b ± 0.36 | 2.15a ± 0.09 |
| Muscle | 10.91b,c ± 0.87 | 15.22d ± 2.11 | 11.67c ± 1.67 | 7.51b ± 1.43 | 7.10a ± 0.70 | |
| MDH | Liver | 12.44a ± 2.60 | 20.16b ± 1.38 | 14.52a ± 0.47 | 13.37a ± 1.83 | 11.15a ± 0.59 |
| Muscle | 14.21b ± 0.63 | 22.99c ± 2.14 | 12.30a,b ± 0.89 | 9.50a,b ±1.30 | 11.21a,b ± 0.94 | |
| FDPase | Liver | 2.83a ± 0.23 | 3.91b ± 0.02 | 3.36a ± 0.15 | 3.22a ± 0.24 | 2.88a ± 0.019 |
| ALP | Liver | 34.05b ± 3.41 | 18.74a ± 5.91 | 35.64b ± 2.47 | 36.97b ± 7.78 | 50.43c ± 2.53 |
| ATPase | Gill | 1.91c ± 0.03 | 1.12a ± 0.05 | 1.56b ± 0.17 | 1.61b ± 0.06 | 1.93c± 0.07 |
| AchE | Brain | 5.23c ± 0.56 | 3.01a ± 0.39 | 3.92b ± 0.11 | 4.74b,c ± 0.28 | 4.30b,c ± 0.29 |
- Mean values bearing different superscript (a, b, c, d) in a row are significantly different (P < 0.05).
- LDH: U min−1 mg−1 protein at 37°C.
- MDH: U min−1 mg−1 protein at 37°C.
- FDPase: μg phosphorus released mg−1 protein min−1 at 37°C.
- ALP: nmol p-nitrophenol released mg−1 protein min−1 at 37°C.
- ATPase: μg phosphorus released mg−1 protein min−1 at 37°C.
- AchE: μmol acetylcholine hydrolysed mg−1 protein min−1 at 37°C. Data are expressed as mean ± SE, n = 6.
- AchE, acetyl choline esterase; ALP, alkaline phosphatase; ATPase, adenosine triphosphatase; LDH, lactate dehydrogenase; FDPase, fructose-1,6-diphosphatase; MDH, malate dehydrogenase.
Highest (P < 0.05) FDPase activity in the liver was recorded in group exposed to fipronil and fed without levan supplemented diet (L0P1). Lowest FDPase activity among levan-fed group was observed with 0.75% supplementation (L0.75P1), which did not vary significantly (P > 0.05) with other levan-fed groups (Table 2).
Alkaline phosphatase activity in liver increased with increasing concentration of levan in the diet and highest (P < 0.05) value was observed in the group fed with 0.75% levan supplementation. Fipronil treatment was found to inhibit ALP activity in C. carpio fry and lowest value (P < 0.05) was observed in the L0P1 group (Table 2).
Dietary levan supplementation had significant (P < 0.05) impact on ATPase activity in the gill of treated groups (Table 2). Highest ATPase activity was observed in the L0.75P1 group fed with highest level of dietary levan that varied significantly from all other groups, except L0P0. Lowest value was observed in the L0P1 group fed without levan and exposed to fipronil.
Highest AchE activity was observed in the L0P0 group, which was reared without exposure to fipronil and it was similar to the groups fed with 0.50% and 0.75% levan diet. Treatment with fipronil led to significant (P < 0.05) decline in the AchE activity in the L0P1 group (Table 2).
Haemato-immunological studies
Maximum and minimum WBC count, RBC count and haemoglobin content were observed in L0.75P1 and L0P1 group respectively. Total serum protein and globulin contents were recorded highest (P < 05) in the L0.50P1 group, which was similar to L0.75P1 group. Lower value of A/G ratios was found in L0.50P1 and L0.75P1, which did not detect significant (P > 05) changes between groups. No significant (P > 05) changes were observed in the value of albumin content (Table 3).
| Treatments | Total Leucocyte count (×103 cells mm−3) | Total Erythrocyte count (×106 cells) mm−3) | Haemoglobin content (g dL−1) | Total Serum protein (g dL−1) | Albumin (g dL−1) | Globulin (g dL−1) | A:G Ratio | |
|---|---|---|---|---|---|---|---|---|
| L0P0 | 238.17b ± 0.39 | 1.04b ± 0.03 | 7.10b,c ± 0.15 | 1.44a ± 0.03 | 0.48 ± 0.02 | 0.96a ± 0.06 | 0.50b ± 0.06 | |
| L0P1 | 212.34a ± 1.61 | 0.96a,b ± 0.02 | 5.46a ± 0.38 | 1.31a ± 0.02 | 0.45 ± 0.03 | 0.86a ± 0.04 | 0.52b ± 0.06 | |
| L0.25P1 | 239.27b ± 2.26 | 1.01b ± 0.02 | 6.40a,b ± 0.47 | 1.37a ± 0.09 | 0.39 ± 0.02 | 0.98a ± 0.10 | 0.41a,b ± 0.06 | |
| L0.50P1 | 233.67b ± 2.41 | 0.97a,b ± 0.02 | 7.10b,c ± 0.17 | 1.93b ± 0.08 | 0.44 ± 0.02 | 1.49b ± 0.07 | 0.30a ± 0.02 | |
| L0.75P1 | 253.70c ± 4.93 | 1.22c ± 0.04 | 7.86c ± 0.46 | 1.78b ± 0.03 | 0.48 ± 0.03 | 1.30b ± 0.06 | 0.37a,b ± 0.04 | |
- Values in the same column with different superscript (a,b,c) differ significantly (p<0.05). Data are expressed as mean ± SE, n=6.
Serum lysozyme activity and nitroblue tetrazolium (NBT)
Higher value of serum lysozyme activity was noticed in the L0.75P1 group fed diet with the highest level of levan supplementation. Highest (P < 0.05) NBT reduction value after challenge with A. hydrophila was recorded in the L0.75P1 group, whereas lowest value was observed in L0P1 and L0.25P1 group (Table 4).
| Treatments | Serum lysozyme (A/620 nm) | NBT (OD/540 nm) |
|---|---|---|
| L0P0 | 833.23a,b ± 23.95 | 0.25b ± 0.01 |
| L0P1 | 760.65a ± 42.15 | 0.14a ± 0.01 |
| L0.25P1 | 804.37a ± 38.10 | 0.17a ± 0.01 |
| L0.50P1 | 826.83a,b ± 28.08 | 0.26b ± 0.02 |
| L0.75P1 | 929.92b ± 15.75 | 0.30c ± 0.03 |
- Values in the same column with different superscript (a, b, c) differ significantly (P < 0.05).
- Data are expressed as mean ± SE, n = 6.
- NBT, nitro blue tetrazolium.
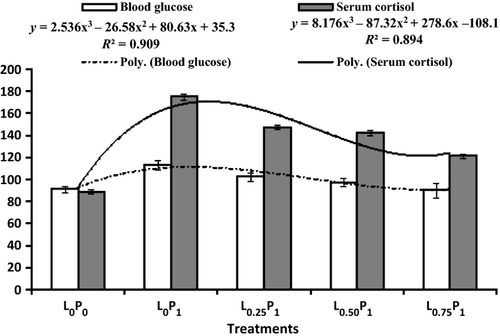
Blood glucose and serum cortisol
A gradual decline in the blood glucose level was noticed with the increasing level of dietary levan. Similar trend was also noticed for the serum cortisol level. Blood glucose and serum cotisol level were found to be highest in the L0P1 group exposed to fipronil and fed without levan diet (Fig. 1).
Challenge study with Aeromonas hydrophila
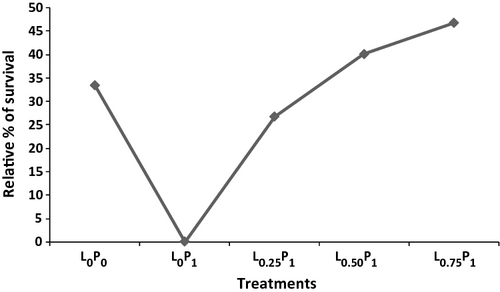
Lower mortalities were observed in fish fed with levan supplemented diets after challenge with A. hydrophila. Among the levan-fed groups, maximum relative % of survival was found in L0.75P1 (46.67%) and L0.50P1 (40.0%) while minimum was recorded in L0.25P1 group (26.67%) (Fig. 2).
Discussion
The present experiment aimed to elucidate the stress ameliorating and immunomodulatory role of microbial levan in C. carpio fry exposed to sublethal fipronil toxicity. Pesticides have been shown to inhibit the activity of several metabolic enzymes in fish (Mishra & Shukla 1997; Tripathi & Verma 2004). Like many toxic chemicals, fipronil has been well known to affect metabolic enzyme profile and thus can alter the physiological and biochemical responses of aquatic organisms [USEPA (U.S. Environmental protection Agency) 1996; Cary, Chandler, Volz, Walse & Ferry 2004].
In general, LDH activity increases in stress (Vijayaraghavan & Rao 1986). In both, liver and muscle, the LDH activity was maximum in the L0P1 group, suggesting that the animals were under stress induced by fipronil. LDH activity decreased significantly (P < 0.05) in the groups fed with higher microbial levan supplemented diets indicating the stress ameliorating efficacy of levan. Significantly (P < 0.05) higher activity of MDH, a TCA cycle intermediate, in L0P1 group indicates greater activity of TCA cycle due to increased energy demands during stress. The dietary supplementation of microbial levan significantly (P < 0.05) decreased the MDH activity both in the liver and muscle indicating the stress mitigating effect of levan. Tejpal et al. (2008) and Akhtar et al. (2010) also reported decreased MDH activity in Cirrhinus mrigala and Labeo rohita fingerlings under crowding and endosulfan stress when fed with pyridoxine and l-tryptophan respectively.
The highest value of FDPase in the liver of L0P1 indicates that gluconeogenic pathway get activated in fish as they prefer to utilize the protein and lipids rather than carbohydrate for energy requirements (Demeal 1978). However, in fish under stress, glucose is mainly derived by gluconeogenesis (Vijayan, Foster & Moon 1993). Shaffi (1982) reported rise in FDPase activity in the hepatic tissues of Labeo rohita, Clarias batrachus and Channa punctatus due to sublethal exposure of DDT. An increase in transaminase activity might have provided valuable precursor for gluconeogenesis. Lowest activity in the L0.75P1 group indicates reduced glucose production by gluconeogenesis due to stress amelioration efficacy of dietary levan.
Alkaline phosphatase, a zinc-containing metallo-enzyme, plays an important role in phosphorus metabolism in the body. Lowest value of ALP in the liver of L0P1 group fed diet without levan and exposed to fipronil is in agreement with the previous results of (Sharma 1990) in Channa gachua and (Verma, Pal, Manush, Das, Dalvi, Chandrachoodan & Apte 2007) in C. carpio exposed to endosulfan toxicity. Significant increase in ALP activity in the L0.75P1 group might be attributed to hydrolysis of high-energy phosphate bonds to liberate phosphate ions to combat stressful condition or higher metabolic rate. Our results are supported with the findings of Sarma et al. (2009) in C. punctatus who fed with high protein and high vitamin C diet and exposed to endosulfan stress.
ATPase activity had been used as a sensitive biomarker for assessment of pollutant-induced damage to the osmoregulatory and acid-base regulatory system in the gills (Stagg, Rusin & Brown 1992). Inhibitions of the gill ATPase activity upon exposure to pesticides are well documented for other fish (Lionetto, Giordano, Vilella & Schettino 2000; Waring and Mooreb 2004). Similarly in this study, lowest gill ATPase activity was found in the L0P1 group fed without levan and exposed to fipronil. Supplementation of levan led to significant increase in ATPase activity in high levan-fed groups, suggesting their anti-stress effect. Similar findings were observed by (Sarma et al. 2009) in C. punctatus fed with high protein and high vitamin C diet and exposed to endosulfan stress.
AchE is one of the most commonly used enzymes as a biomarker for the pollution studies. Acetylcholine is synthesized in nervous tissue by enzyme choline acetylase and rapidly destroyed by cholinesterase, a group of related enzymes, which are hydrolytic in action (Stowe 1969). Cholinesterase hydrolyses the acetylcholine and hence its production decreases at higher activity of cholinesterase. The lowest activity observed in the L0P1 group indicates highest stress in animals induced by accumulation of acetylcholine. Murthy, Reddy, Reddy, Bhaskar and Govindappa (1984) observed the inhibition of acetylcholine esterase in tilapia on exposure to acidic media. Similarly, Tejpal et al. (2008) observed inhibition of acetylcholine esterase in brain of C.mrigala fingerlings under crowding stress due to accumulation of acetylcholine. The best result obtained with group fed either 0.50% or 0.75% levan diet indicates the stress ameliorating effect of levan in C. carpio fry.
Highest leucocyte numbers (WBC) were observed in the group fed diet with 0.75% levan supplementation, which is in agreement with the results of earlier work on glucan (Jeney & Anderson 1993a; Siwicki, Anderson & Rumsey 1994). Selvaraj, Sampath and Sekar (2005) also reported significant increase in total leucocyte count with increasing dose of glucan in C. carpio. As neutrophils form the major fraction of the phagocytic leucocytes, thus it indicates that levan might be responsible for the increased phagocytic activity. Augmentation of erythrocytes and haemoglobin contents were also observed with increased supplementation of levan in the diet, indicating an improvement in the health status of the fish. Our findings are corroborated with the previous studies performed by and Rairakhwada et al. (2007) in C. carpio and Gupta et al. (2008) in L. rohita after feeding graded level of levan diet.
Increase in the serum protein and globulin levels is thought to be associated with a stronger innate response in fish (Wiegertjes, Stet, Parmentier & Muiswinkel 1996). The maximum total serum protein and globulin content was found in the group fed diet supplemented with either 0.50% or 0.75% levan. This suggests that levan might be responsible for the bactericidal activity of serum, as the complement fraction constitutes a large part of the globulin. The 1.7-fold reduction in the A/G ratio was observed in fish fed diet containing 0.5% levan. The A/G ratio is a measurable humoral component of non-specific defence. The decline of A/G ratio and increase in globulin levels in levan-fed groups might play an important part in the immune-protective mechanisms of fish, as indicated by Sahoo and Mukherjee (2001) and Misra, Das, Mukherje, and Pattnaik (2006). Our results are in agreement with Rairakhwada et al. (2007) and Gupta et al. (2008), who also found an elevation of total serum protein and globulin contents as well as reduction in A/G ratio after feeding levan.
There were 1.2-fold increments in serum lysozyme activity in the group supplemented with 0.75% levan in the diet. Enhancement of lysozyme could be correlated with enhanced phagocytic activity. This observation was supported by Rairakhwada et al. (2007) in C. carpio and Gupta et al. (2008) in L. rohita who observed higher lysozyme activity in levan-fed groups. This suggests immunoboosting properties of levan in C. carpio fry.
A variety of immunostimulants, including levamisole (Jeney & Anderson 1993b; Secombes 1996), glucans (Sakai, Taniguchi, Mamoto, Ogawa & Tabata 2001) and yeast RNA (Choudhury, Pal, Sahu, Kumar, Das & Mukherjee 2005) are known to increase respiratory burst activity. Increased respiratory burst activity can be correlated with increased bacterial pathogen killing activity of phagocytes (Sharp & Secombes 1993), and hence a better immunity status of fish. In this study, the respiratory burst activity of phagocytes was measured by reduction in NBT by intracellular superoxide radicals produced by leucocytes. After challenged with A. hydrophila, the respiratory burst activity was significantly (P < 0.05) higher in the L0.75P1 group, which was fed with diet supplemented with 0.75% of levan. Our finding is similar to previous studies on L. rohita juveniles (Choudhury et al. 2005; Gupta et al. 2008), suggesting that levan at 0.75% maintains the immunity status of C. carpio fry.
Cortisol level has been used as an indicator of primary stress response, whereas blood glucose test is the most common of the secondary stress response parameter (Chatterjee, Pal, Das, Manush, Sarma, Venkateshwarlu & Mukherjee 2006). Elevated cortisol levels upon different environmental stressors were reported by many workers (Brown, Eales, Evans & Hara 1984) in rainbow trout upon acid water (Bleau, Daniel, Chevalier, Van-Rra & Hontela 1996), upon mercury chloride and methyl mercury, and Akhtar et al. (2010) upon endosulfan exposure. Similarly, rise in the blood glucose level on application of various stressors have been accounted by several workers, like handling (Wedemeyer 1972; Carey & Mc Cormick 1998), transportation (Barton & Schreck 1987), claw ablation of prawn (Manush et al. 2005), crowding (Tejpal et al. 2008) and pesticide stress (Prusty et al. 2011). Cortisol and glucose levels were highest in the L0P1 group fed diet without levan and exposed to fipronil stress than the levan supplemented groups. Both blood glucose and serum cortisol exhibited a third order polynomial relationship with inclusion of dietary levan (Y = 2.036x3 – 21.15x2 + 64.56x + 46.1, R2 = 0.957) and (Y = 8.176x3 – 87.32x2 + 278.6x–108.1, R2 = 0.894). Among the levan supplemented groups, maximum reduction in cortisol level was noticed with 0.75% level of levan incorporation in the diet. Therefore, it can be inferred that levan can reduce the fipronil-induced stress by lowering the cortisol level in the serum. Parallel trend was also found in blood glucose. Significant decrease in glucose level in the higher levan supplemented groups may be attributed to efficient utilization of glucose from the blood. Our results are in agreement with the findings of Tejpal et al. (2008) and Akhtar et al. (2010) in C. mrigala and L. rohita fingerlings respectively.
Aeromonas hydrophila, one of the most commonly encountered bacterial pathogen in carps in India, which causes various diseases in fish such as haemorrhagic septicaemia, infectious dropsy, tropical ulcerative disease and fin rot leading to heavy mortality in aquaculture farms (Kumar & Dey 1988; Rath 1993; Karunasagar, Ali, Otta & Karunasagar 1997). Dietary supplementation of levan registered an improved relative percentage of survival of C. carpio against an intraperitoneal challenge with A. hydrophila. Highest relative percentage of survival recorded in 0.75% levan-fed group. Selvaraj et al. (2005) reported similar results with dietary glucan in the common carp, indicating immunomodulating properties of levan in C. carpio fry.
To summarize the above findings, biochemical parameters were in favour of L0.75P1 group fed with 0.75% levan supplemented diet. The heamatological parameters, serum lysozyme and NBT support the ability of C. carpio fry to withstand the sublethal fipronil exposure. It is concluded that dietary supplementation of levan at 0.75% level ameliorates fipronil-induced stress and helps to augment immunity in C. carpio fry. This amelioration method can be potentially suggested especially for commercial culture of common carp, where fish are exposed to sublethal contamination of fipronil, and thus it provides appropriate management option to overcome fipronil-induced stress.
Acknowledgments
The first author is grateful to the Director, Central Institute of Fisheries Education, Mumbai for providing the necessary facilities for this study and the Department of Microbiology, Bhavans College, Andheri, Mumbai for providing microbial levan. The financial support provided by ICAR, New Delhi in the form of institutional fellowship to S.K. Gupta is duly acknowledged.




