The use of Kluyveromyces marxianus in the diet of Red-Stirling tilapia (Oreochromis niloticus, Linnaeus) exposed to natural climatic variation: effects on growth performance, fatty acids, and protein deposition
Abstract
The purpose of this study was to evaluate the use of single cell proteins (Kluyveromyces marxianus) as fishmeal substitutes for Oreochromis niloticus during long-term feeding under natural climatic conditions. Juveniles (150) were divided into two groups: the control group, fed with conventional fish diet (T1); and the experimental group, fed with the alternative diet (T2). T1 animals had a higher final weight and per cent weight gain than T2 animals, which we mainly observed in winter and autumn. The lipid content ranged from 1.9% to 3.8% in liver and from 0.2% to 1% in white muscle for both groups. T2 animals had a higher protein concentration in the fillet and the lipid content in this tissue was not altered by diet. Liver and plasma protein content in both groups and the muscle content in T1 decreased during growth. Specific fatty acids (FAs) were selectively retained in fillet phospholipids (PLs), primarily as C22:6n3, which was found at high levels in the PLs of both groups during all seasons. Despite the differences observed, the percentage of polyunsaturated FAs in the fillet was similar throughout the experiment for both diets, reaching approximately 50% of the total FA in the PLs.
Introduction
The rapid expansion of fish culture in recent years has created a demand for alternative supply resources that reduce the cost of nutrition in aquaculture, which can be 50–80% of total fish production costs (El-Sayed, Mansour & Ezzat 2003). The majority of such costs are due to high prices and shortages of fishmeal, the main ingredient used in the diets (Hansen, Roselund, Karlsen, Koppe & Hemre 2007), and no strategies for increasing fishmeal production currently exist. Forecasts to 2020 indicate that the worldwide demand for fishmeal might exceed its production (New & Wijkstrøm 2002), making its future use unfeasible. Consequently, alternative protein and oil sources that are suitable for aquafeed are urgently needed to ensure the sustainability of both aquacultural and fishery industries (Hardy & Barrows 1988). Plant proteins appear to be the most suitable fishmeal alternatives (Bhosale, Bhilave & Nadaf 2010; Hardy 2010). The efficiency of the various alternative protein sources, including sunflower meal (Olvera-Novoa, Olivera- Castillo & Martínez-Palacios 2002), soybean meal (Wang, Jiang, Ji & Xie 2010), linseed meal (Gaber 2003) and canola meal (Zhou & Yue 2009) as either partial or complete replacements for fishmeal has been individually evaluated in fish diets. Besides the protein sources, vegetables are also used worldwide as alternative lipid sources in fish feed (Tacon & Metian 2008; Nasopoulou & Zabetakis 2012).
The need to develop aquaculture that does not damage the environment (i.e. environmentally friendly aquaculture), and uses microorganisms (e.g. single cell proteins), either with or without probiotic activity, have been widely promoted (Wang & He 2008). Single cell proteins (SCPs), including microalgae, bacteria and yeast, which are alternative non-conventional protein sources, are frequently used as ingredients in fishmeal due to their high levels of nutrients, such as proteins, B-vitamins, pigments and complex carbohydrates (Oliva-Teles & Gonçalves 2001). These SCPs have been used as supplemental animal feed to compensate for amino acid and vitamin deficiencies in cereals, and they are recommended as substitutes for soybean oil in the diets of fowl (Göhl 1981). Oliva-Teles and Gonçalves (2001) succeeded in replacing 50% of the diet of Dicentrarchus labrax with yeast (Saccharomyces cerevisiae) base that had no negative effects on fish growth. Studies that evaluated the effects of Candida utilis as a substitute for fishmeal in the diets of Oreochromis mossambicus larvae have shown that these animals accepted the substitution of 30% yeast in their diet and had high growth parameters without adverse effects on fish performance and culture profit (Olvera-Novoa et al. 2002).
Single cell proteins are less expensive dietary supplements when compared with conventional supplements because they are easily produced on an industrial level from a number of carbon-rich substrate by-products (Lee & Kim 2001). Agro industrial residues are considered rich raw material for the production of SCPs. However, such residues are often discarded directly into the environment, where they can have significant negative impact. Biotechnological use of these residues leads to the production of diets with excellent nutritional profiles and a reduction in the level of pollutants, thereby increasing residue value (Vendruscolo, Ribeiro, Esposito & Ninow 2009).
According to a review by Glencross, Booth and Allan (2007), a number of strategies for the inclusion and substitution of ingredients in aquaculture can be used to test them in fish diets, including tests of limited inclusion on one or two levels that maintain equivalent values of protein and energy. These studies provide the standards needed to use different inclusion levels and ensure that substitutions are made based on equivalent digestible levels.
In addition to nutritional status, aquatic organisms are exposed to fluctuations in environmental conditions throughout the year, both in their natural habitat and on fish farms, which can ultimately cause stress to animals. Temperature is one of the most important biotic factors affecting growth, food consumption and feeding efficiency in fish (Martinez, Cristina & Ros 1996; Azaza & Dhraïef 2007) because it regulates protein turnover and growth efficiency (Jobling 1994), among other functions. Behavioural and physiological acclimations are effective responses to potential stressors (Arts & Kohler 2009); however, in culture conditions, it is important to prevent and mitigate these stressors. Changes in food availability during these periods are therefore important (Fountoulaki, Vasilaki, Hurtaro, Grigorakis, Karacostas, Nengas, Rigos, Kotzamanis, Venou & Alexis 2009). Traditionally, fish biologists use indices based on weight and length standards (Anderson & Neumann 1996) to evaluate the general health condition of fish. Considering the inter- and intra-specific variation in these indices, the results can be overestimated in aquacultural studies. Metabolic indicators, such as proteins and lipids (including fatty acids profiles), are complimentary ways to assess the health and condition of fish that use a more specific method compared with zootechnical indices (Arts & Kohler 2009). A number of studies have shown environmental and nutritional effects on fat and the composition of fat content (Polvi & Ackman 1992; Kiessling, Pickova, Johansson, Åsgård, Storebakken & Kiessling 2001).
Fatty acids (FAs) are carboxylic acids that have important functions in a number of metabolic pathways (mainly energetic and structural) (Ratnayake & Ackman 1979; Linko, Kaitaranta & Vuorela 1985). The FA composition of fish tissue is variable even within species, depending on different abiotic and biotic factors, such as season, type and amount of food availability, water temperature, pH, salinity and reproductive stage (Bayir, Sirkecioğlu, Aras, Aksakal, Haliloğlu & Bayir 2010). Temperature variations alter the melting point of esterified FAs in the PLs of biological membranes, thereby altering membrane fluidity (Hazel 1989). In human diets, fish are the major source of polyunsaturated fatty acids (PUFAs) via the omega 3 series (n3), and, in western countries, a minimum consumption level of fish has been recommended with the goal to either prevent or reduce premature heart disease, inflammatory disorders and many other health problems (Turner, Else & Hulbert 2003).
The purpose of this study was to evaluate the use of single cells (live Kluyveromyces marxianus) as substitutes for fishmeal through long-term feeding under natural ambient conditions during the growth of Oreochromis niloticus (Red-Stirling) and assess biochemical and zootechnical indices. Tilapia have fast growth, high-quality flesh, disease resistance and adaptability to a wide range of environmental conditions, all of which make this species an excellent choice in nutritional studies, especially for tropical and subtropical environments (Beveridge & McAndrew 2000; Nguyen, Davis & Saoud 2009). Kluyveromyces marxianus is a common yeast in the microbiota of whey cheese and has been well documented as a producer of inulinase and β-galactosidase, thereby allowing lactose hydrolysis (Belem & Lee 1998). This is the first study to use of this microorganism as a food source for fish and evaluate its effect on fish growth and biochemical parameters of the liver, fillet and plasma, including FA content, during a long-term feeding trial under natural ambient conditions.
Material and methods
Experimental diet
This experiment was performed for 8 months (February to October) at the Ponte Nova Fish Farm (23°35′33.8″S and 45°58′09.1″W). Fermentation occurred and the fish feed was manufactured in the Laboratory of Biological Chemistry and Biotechnology at Mogi das Cruzes University.
The yeast used for fermentation (K. marxianus CBS 6565) was provided by the Laboratory of Fermentation of Technological Food Institute, which is located at the Federal University of Rio Grande do Sul (RS-Brazil). This strain was maintained in 2% agar-cheese whey at pH 6 and 5°C. To produce biomass, discontinuous fermentation was performed for 14 h to obtain a whey cheese volume of 100 L−1, at pH = 6.0, 30°C, and using 4.5 vvm of aeration and 10% of the inoculum. After this period, the fermentation product was centrifuged at 223.5 × g for 10 min and added to the fish diets in a proportion of 15% (w/w), without any treatment (Ribeiro 2008). We chose this level of substitution to maintain both isonitrogenous and isoenergetic diets.
The following two experimental diets were formulated: T1, which was similar to a conventional fish diet, with fish meal as the main protein source; and T2, which was the test diet in which the fishmeal was completely replaced by the submerged culture of K. marxianus in cheese whey. The compositions of the ingredients, and the biochemical analyses of the diets, are presented in Table 1. Both diets were formulated with equal amounts of total protein (28%) and lipids (7–8%). Dietary lipids were also extracted for fatty acid profiling (Table 2), and the amino acid profiles for the three protein sources used in the diets are presented in Table 3. The feed was pelleted by compression, using a California Pellet Mill pelletizer with a 3-mm diameter. The animals were manually fed twice daily at 6% of fish biomass/day, except 24-h before weighing and tissue collection. During the acclimation period, the animals were fed with commercial feed (PURINA: crude protein = 32%, crude lipids = 6.5%, moisture = 8%, crude fibre = 7%, mineral mixture = 10%, calcium = 1.2%, phosphorus = 0.6% and vitamin C = 0.32%).
| T1 (%) | T2 (%) | |
|---|---|---|
| Ingredients (%) | ||
| Yeasta | 0.0 | 15.0 |
| Fish meal | 10.0 | 0.0 |
| Soybean meal | 43.7 | 38.7 |
| Corn meal | 20.0 | 20.0 |
| Wheat meal | 25.0 | 25.0 |
| Lysine | 0.3 | 0.3 |
| Methionine | 1.0 | 1.0 |
| Vitamin premixb | 0.002 | 0.002 |
| Phosphates | 0.0035 | 0.0035 |
| Biochemical analysis (%) | ||
| Crude protein | 28.0 | 28.0 |
| Crude fat | 7.0 | 8.0 |
- a Kluyveromyces marxianus: 42.7% of crude protein, 1.3% of crude fat and 87.6% of water content.
- b Vitamin premix (each 1.0 kg contain): folic acid 250 mg; panthenoneic 5000 mg; Vit. A 1,000,000 UI; Vit. B1 250 mg; Vit. B12 2500 mg; Vit. B2 1750 mg; Vit. B6 875 mg; Vit. C 12,500 mg; Vit. D3 600,000 UI; Vit. E 12,500 UI; Vit. K 315 mg; niacin 3750 mg; cobalt 24,999 mg; copper 11,249 mg; iron 106 mg; manganese 3749 mg; selenium 75 mg; zinc 17,499 mg; antioxidant 0.25 g; vehicle QSP.
| Fatty acid (%) | T1 | T2 |
|---|---|---|
| C14:0 | 0.2 | 1.7 |
| C16:0 | 15.0 | 12.2 |
| C18:0 | 7.1 | 4.4 |
| C22:0 | 3.4 | 1.1 |
| Σ SFA | 25.9 | 19.4 |
| C16:1 | 3.0 | ND |
| C18:1 | 24.0 | 26.8 |
| C20:1 | 2.5 | 1.0 |
| Σ MUFA | 29.5 | 28.5 |
| C18:2n6 | 18.0 | 45.0 |
| C18:3n3 | 1.3 | 3.6 |
| C20:4n6 | 1.3 | ND |
| C20:5n3 | 4.6 | ND |
| C22:5n3 | 1.4 | ND |
| C22:6n3 | 10.8 | 1.1 |
| Σ PUFA | 38.0 | 50.7 |
| Σ n6 | 20.0 | 45.5 |
| Σ n3 | 18.0 | 5.3 |
| n3/n6 | 0.9 | 0.1 |
| <1.00% | 5.5 | 5.1 |
- Fatty acids with values below 1.00% were summed and showed together. Σ SFA, Σ MUFA, Σ PUFA, Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3 respectively. ND, not detected.
| Amino acids (%) | Protein ingredients | ||
|---|---|---|---|
| Kluyveromyces Marxianus a | Soybeanb | Fishmealb | |
| Threonine | 3.1 | 2.5 | 3.0 |
| Valine | 3.3 | 2.1 | 5.1 |
| Methionine | 1.5 | 0.6 | 2.3 |
| Leucine | 6.6 | 3.6 | 6.2 |
| Phenylalanine | 2.3 | 2.4 | 3.1 |
| Lysine | 3.1 | 2.9 | 4.6 |
| Histidine | 0.9 | 1.4 | 2.2 |
| Arginine | 1.9 | 3.5 | 5.1 |
| Isoleucine | 2.9 | 2.1 | 3.4 |
| Tryptophan | 1.4 | 0.7 | 0.6 |
- a Analysis performed by ITAL – Instituto de Tecnologia de Alimentos.
- b Data from the producer.
This experiment is the first using K. marxianus in O. niloticus nutrition; therefore, we measured the apparent digestibility coefficients of the experimental diets prior to the beginning of the experiment to verify the digestibility of this new ingredient. To calculate the apparent digestibility coefficients, we used the indirect method described by Furukawa and Tsukahara (1966), which uses 1% chromic oxide, as a dietary marker, to analyse the diets and the faeces. A total of 50 animals from the same lot were used for the digestibility analysis, and the faeces were collected using the Guelph system described by Cho, Cowey and Watanabe (1985). The tests indicated that the digestibility of the T2 diet did not differ (82.40 ± 6.93%) from the digestibility of the T1 diet (82.97 ± 9.95%); hence, the substitution percentages used in the diet supplemented with the SCPs were maintained.
Experimental design
A total of 150 Red-Stirling tilapia were obtained from the Empresa Brasileira do Peixe Ltda (Jundiaí, SP, Brazil). All the animals were males, which were created by sex reversal through the addition of the androgen steroid, 17 α-methyltestosterone (MT), into their diet at 60 mg MT kg−1 for 28 days. At the Ponte Nova Fish Farm, the animals had an initial body weight of 86.8 ± 11.37 g and an initial body length of 17.0 ± 0.85 cm. The animals were randomly distributed into two experimental groups (T1, fed with control diet; and T2, fed with unicellular protein diet) for an acclimation period of 30 days. The animals were then redistributed among six 10-m3 ponds to create three replicates (ponds) for each diet. Each pond had continuous water flow (1800-L h−1), and storage density was 5 fish m−3. The fish were maintained under natural photoperiod and environmental conditions. Dissolved oxygen and water temperature were monitored daily using an oximeter (Model 55; YSI, Yellow Springs, OH, USA), and pH was monitored using a commercial kit (Merck Chemicals, Darmstadt, Germany).
Growth parameters
To evaluate fish growth and performance from experimental diets, biometrical parameters were collected in every season at 2-month intervals. All the animals from each pond were measured at these intervals, and the results were used to calculate: Weight Gain (%) = 100[(final weight−initial weight)/initial weight]; and the Seasonal Mass Increase = Mass in Season X−Mass in Season Y.
Fish sampling and metabolic analyses
Eighteen animals from each experimental group were sampled each season (six animals from each replicate). Captured fish were anaesthetized with a benzocaine solution to a concentration of 70 mg L−1. Blood samples were collected using heparinized syringes (Liquemine; Roche®, Rio de Janeiro, RJ, Brazil), and needles were used to puncture the caudal vasculature. The blood samples were centrifuged at 655.1 × g for 10 minutes, and the plasma was separated into aliquots that were immediately frozen at −80°C until processing. Each animal was subsequently sacrificed by decapitation, and the whole liver and portions of the white muscle were removed and stored at −80°C.
Plasma total protein was measured according to the method of Lowry, Rosenbrough, Farr and Randal (1951). Precipitation and solubilization were performed as suggested by Milligan and Girard (1993). The total protein was measured in a spectrophotometer at 660 nm based on a standard curve generated from bovine serum albumin (Sigma Diagnostics INS, St. Louis, MO, USA).
The total lipids of the tissues and diet were extracted using the method of Folch, Less and Stanley (1957), which was adapted by Parrish (1999) for aquatic organisms. The lipid extracts (as described above) were separated into polar lipids (phospholipids) and neutral lipids (triglycerides) using an activated silica column (Yang 1995). Methylation of each fraction was performed with acetyl chloride (5% HCl in methanol) (Christie 2003), and the FA composition was determined with methyl esters, using a Varian Model 3900 Gas Chromatograph (Walnut Creek, CA, USA) coupled to a flame ionization detector. The FAs were identified by comparing their retention times to known standard retention times for fatty acid methyl esters (Supelco, 37 components, Sigma-Aldrich, Chicago, IL, USA; Larodan, Mixture Me93, and Qualmix PUFA fish M; and Menhaden Oil, Larodan, Malmö, Sweden). The fatty acid methyl esters were analysed in a capillary column (CP Wax 52 CB, 0.25 mm thickness, 0.25-μm diameter, and 30-m length). Hydrogen was used as a carrier gas at a linear velocity of 22 cm s−1. The column was programmed at 170°C for 1 min, followed by a 2.5°C min−1 ramp to 240°C and a final hold time of 5 min. The injector and flame ionization detector temperatures were 250 and 260°C respectively.
Statistical analysis
Resulting growth parameter percentages (initial weights, final weights, WGs, concentrations of proteins and lipids and fatty acid profiles) were each expressed as a mean ± standard error of the mean. The growth parameters were compared using t-tests, and the physiological parameters, between diets and seasons, were compared using a two-way analysis of variance, followed by a Tukey's test. For all analyses, differences were considered significant when P < 0.05. These analyses were performed using the statistical software, BioStat 3.0 (AnalysSoft Inc., Vancouver, BC, Canada).
Results
Food and environmental parameters
The biochemical analyses, FA profiles, and amino acid compositions of both diets are presented in Tables 1–3 respectively. The analyses revealed the same percentage of proteins and lipids in both diets. The FA analysis of the dietary lipids revealed the presence of 25.93% saturated fatty acids (SFAs), in T1, and 19.38%, in T2; monounsaturated fatty acids (MUFAs) ranged from 28.48%, in T2, to 29.51%, in T1; and PUFAs were 37.96%, in T1 and 50.75%, in T2. In T2, C18:2n6 contributed to 44.99% of the total PUFA, whereas the T1 diet had a major diversity of PUFAs. The essential amino acid analysis showed a higher percentage of leucine and tryptophan in the yeast, and tilapia nutritional requirements were supplied in the diets offered (Santiago & Lovell 1988).
During the experiment, the mean values of water temperature, dissolved oxygen and pH during the experiment for both treatments were: 20.4 ± 3.8°C, 4.6 ± 0.09 mg L−1 and 7.0 ± 0.3 respectively. These parameters demonstrated no significant differences between the treatments; however, water temperatures showed high variation during the year. In the early months of the experiment (summer to February), mean water temperature was 28.0 ± 1.21°C. In autumn (April), mean water temperature was around 23.3 ± 1.50°C, whereas in winter (August), mean temperature was 18.3 ± 2.30°C. At the end of the experiment (spring–October), mean temperature was 24.3 ± 3.57°C (Fig. 1). During the entire experimental period, therefore, mean water temperature was 19.57 ± 4.48°C.
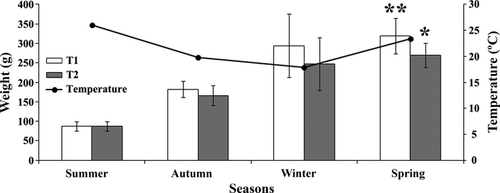
Biometrical parameters
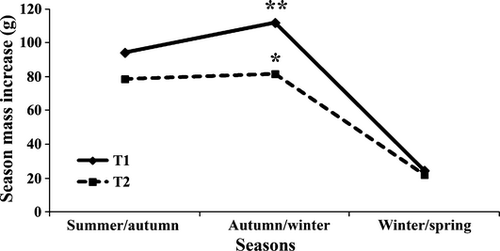
At the end of the experimental period, no mortality occurred in either group. The animals fed with the conventional diet (T1) had a higher final weight and percentage weight gain (%WG) than the group fed with the alternative diet (T2) (Table 4 and Fig. 1). When analysing the mass increase during the experimental period, the difference between both groups was evident in autumn/winter, with the lower value in T2, whereas in other periods, no statistical differences occurred in both groups (Fig. 2).
| Growth parameters | T1 | T2 |
|---|---|---|
| Initial weight (g) | 89.7 ± 18.22 | 88.6 ± 12.65 |
| Final weight (g) | 318.7 ± 45.00* | 270.0 ± 31.00** |
| WG (%)a | 263.81 ± 21.33* | 208.21 ± 46.89** |
- a Per cent weight gain WG (%).
- Different symbols indicate statistical differences (P < 0.05) between diets.
Fish body composition
The protein and lipid contents in the white muscle, liver and plasma of animals are shown in Table 5. In T1, we found a decrease in the protein content of the white muscle in the autumn, and the lower concentrations were maintained during winter and spring. In T2, the decrease in muscle protein content, when compared with the initial sampling, was observed only in the spring. During the autumn and winter, the animals from T2 had higher protein content in the white muscle compared to the animals from T1. When the temperature started to increase (e.g. in spring), no differences were found.
| Parameters | Summer | Autumn | Winter | Spring | S | D | S × D | |||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |||||
| Crude protein | ||||||||||
| Muscle | 17.0 ± 0.42a | 8.9 ± 1.37b* | 14.0 ± 1.47ab** | 8.2 ± 1.05b* | 13.0 ± 1.09ab** | 10.1 ± 0.69b | 9.9 ± 1.11b | P < 0.05 | P < 0.05 | NS |
| Liver | 16.0 ± 0.40a | 6.9 ± 0.73b | 8.3 ± 2.71b | 7.1 ± 0.90b | 7.7 ± 0.25b | 11.3 ± 0.55c* | 8.3 ± 0.63b** | P < 0.05 | P < 0.05 | NS |
| Plasma | 9.2 ± 0.34a | 4.7 ± 0.33b | 4.1 ± 0.74b | 4.4 ± 0.39b | 4.6 ± 0.21b | 6.1 ± 0.35b* | 3.3 ± 0.31b** | P < 0.05 | P < 0.05 | NS |
| Crude lipid | ||||||||||
| Muscle | 0.2 ± 0.0a | 0.3 ± 0.03a | 0.3 ± 0.06ab | 1.0 ± 0.15b | 0.9 ± 0.04b | 0.8 ± 0.05b | 0.9 ± 0.05b | P < 0.05 | NS | NS |
| Liver | 1.9 ± 0.29a | 3.8 ± 0.43b | 3.6 ± 0.45b | 2.3 ± 0.42ac | 3.4 ± 0.47b | 2.8 ± 0.12c* | 3.3 ± 0.13b** | P < 0.05 | P < 0.05 | NS |
| Plasma | 2.4 ± 1.1a | 5.5 ± 1.86a | 9.1 ± 3.24a | 8.8 ± 1.36b | 10.1 ± 1.60b | 8.2 ± 2.76b | 7.5 ± 0.37b | P < 0.05 | NS | NS |
- Different symbols indicate statistical differences (P < 0.05) between diets; Different letters indicate statistical differences among seasons, S – statistical difference during the seasons within each group; D – statistical difference between diets; S × D – two-way ANOVA considering season and diet as variables; NS – not significant.
In the liver and the plasma, the proteins showed the same pattern of concentration change over the experimental period. Over the period from summer to autumn, the concentration of the proteins decreased by approximately 50%, and these low values were sustained in the winter for both experimental groups. In the spring, however, a significant increase in hepatic protein was observed in animals from T1, but not in animals from T2. When temperature increased, the animals from T1 demonstrated a higher hepatic content compared to T2 animals.
During growth, the tissue lipid content showed a different pattern than the protein (Table 5). Both groups showed a general pattern of increasing concentration for total lipids. In the winter and spring, lipid content of white muscle and plasma increased in animals from both groups, whereas hepatic lipids increased for both groups in the autumn. During the winter, only T1 decreased in hepatic lipids. We also observed that the diet had no influence on lipid concentration in the plasma and the muscle. At the end of the experiment in the spring, however, the animals fed the experimental diet (T2) presented a higher hepatic lipid content when compared to the animals from T1.
Liver and white muscle FAs were analysed separately in the fractions that corresponded to triglycerides (TGs = neutral lipids) or phospholipids (PLs = polar lipids), whereas the plasma FAs were analysed for total lipids (Tables 6-10). The FA profile in all the tissues of both groups consisted predominantly of palmitic acid (C16:0), oleic acid (C18:1n9), linoleic acid (C18:2n6), arachidonic acid (C20:4n6) and docosahexaenoic acid (C22:6n3).
| Fatty acid (%) | Summer | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| C14:0 | 1.4 ± 0.15a | 1.8 ± 0.52 | 2.8 ± 0.17ab | 2.4 ± 0.55 | 2.5 ± 0.96ab | 2.8 ± 0.27 | 4.0 ± 0.16b |
| C16:0 | 18.0 ± 0.34 | 18.9 ± 1.67 | 20.6 ± 0.77 | 18.0 ± 2.03 | 18.6 ± 1.34 | 17.0 ± 1.16 | 22.1 ± 0.31 |
| C17:0 | 2.2 ± 1.81a | 0.5 ± 0.10ab | 1.1 ± 0.84 | 0.5 ± 0.13ab | 1.1 ± 0.18 | 0.6 ± 0.05b | 0.6 ± 0.09 |
| C18:0 | 15.5 ± 9.20a | 8.8 ± 1.49 | 7.8 ± 0.49 | 8.1 ± 2.33 | 9.6 ± 1.38 | 7.4 ± 0.29b | 7.1 ± 0.42 |
| C21:0 | 0.9 ± 0.09 | 0.5 ± 0.12 | 0.4 ± 0.14 | 0.8 ± 0.08 | 1.1 ± 0.31 | 0.8 ± 0.22 | 0.9 ± 0.36 |
| Σ SFA | 38.4 ± 8.97a | 31.1 ± 1.40ab | 33.3 ± 0.55 | 29.2 ± 2.18ab | 33.4 ± 2.24 | 28.0 ± 0.48b | 35.4 ± 1.02 |
| C16:1 | 3.1 ± 0.49 | 3.2 ± 0.82 | 7.8 ± 3.37 | 3.4 ± 0.43 | 4.2 ± 1.29 | 3.9 ± 0.26 | 4.7 ± 0.39 |
| C17:1 | 2.4 ± 2.04 | 0.5 ± 0.13 | ND | 0.3 ± 0.11 | 0.8 ± 0.27 | 0.4 ± 0.10 | ND |
| C18:1n9 | 27.3 ± 1.97 | 23.6 ± 4.08 | 30.5 ± 0.28 | 28.4 ± 3.61 | 22.3 ± 4.48 | 25.5 ± 1.13 | 26.9 ± 2.14 |
| C20:1n9 | 1.0 ± 0.49 | 1.6 ± 0.66 | 0.9 ± 0.07 | 0.6 ± 0.44 | 1.1 ± 0.42 | 1.1 ± 0.43 | 1.0 ± 0.15 |
| Σ MUFA | 33.9 ± 1.57 | 27.4 ± 5.73 | 39.2 ± 3.19 | 35.5 ± 1.87 | 28.8 ± 4.72 | 30.4 ± 1.21 | 33.6 ± 1.51 |
| C18:2n6 | 18.7 ± 8.89 | 20.9 ± 3.08 | 19.9 ± 2.73 | 22.8 ± 3.88 | 19.9 ± 2.24 | 21.5 ± 0.78 | 20.9 ± 1.06 |
| C18:3n6 | 1.5 ± 0.04 | 2.5 ± 1.41** | 0.8 ± 0.18* | 0.9 ± 0.16 | 0.8 ± 0.19 | 7.2 ± 3.70* | 1.0 ± 0.44** |
| C18:3n3 | 1.3 ± 0.57 | 1.9 ± 0.44 | 1.7 ± 0.45 | 1.7 ± 0.32 | 3.5 ± 1.43 | 3.7 ± 0.92 | 3.2 ± 0.50 |
| C20:2n6 | 1.3 ± 0.31 | 1.5 ± 0.20 | 0.9 ± 0.16 | 1.7 ± 0.35 | 1.5 ± 0.30 | 1.1 ± 0.38 | 1.0 ± 0.21 |
| C20:4n6 | 0.6 ± 0.17a | 3.3 ± 2.10b** | 0.8 ± 0.24* | 2.7 ± 1.81 | 2.8 ± 1.72 | 1.2 ± 0.16* | 0.7 ± 0.03** |
| C22:4n6 | 0.5 ± 0.02 | 1.4 ± 0.58 | 0.4 ± 0.03 | 1.0 ± 0.48 | 1.2 ± 0.71 | 0.7 ± 0.14 | 0.6 ± 0.22 |
| C22:5n6 | 0.7 ± 0.04 | 2.1 ± 1.76 | 0.4 ± 0.12 | 1.8 ± 1.27 | 1.6 ± 1.15 | 0.6 ± 0.09 | 0.4 ± 0.11 |
| C22:5n3 | 0.5 ± 0.01 | 1.4 ± 0.84 | 0.9 ± 0.12 | 0.8 ± 0.36 | 1.1 ± 0.46 | 1.0 ± 0.38 | 1.0 ± 0.32 |
| C22:6n3 | 1.2 ± 0.20 | 4.2 ± 2.70 | 0.7 ± 0.07 | 3.2 ± 2.22 | 4.1 ± 2.50 | 1.6 ± 0.52 | 1.4 ± 0.50 |
| Σ PUFA | 27.7 ± 9.73a | 41.5 ± 6.51b** | 27.5 ± 3.27* | 35.2 ± 1.95 | 37.8 ± 3.78 | 41.6 ± 1.68** | 31.1 ± 0.59* |
| Σ n6 | 24.3 ± 9.24 | 32.5 ± 3.12 | 24.0 ± 2.85 | 31.0 ± 1.54 | 28.4 ± 2.10 | 34.7 ± 3.33** | 25.1 ± 0.52* |
| Σ n3 | 3.4 ± 0.55 | 8.9 ± 4.02 | 3.5 ± 0.65 | 4.2 ± 0.82 | 9.4 ± 2.56 | 7.0 ± 1.78 | 5.9 ± 1.05 |
| n3/n6 | 0.2 ± 0.10 | 0.3 ± 0.10 | 0.1 ± 0.02 | 0.1 ± 0.03 | 0.3 ± 0.09 | 0.2 ± 0.08 | 0.2 ± 0.05 |
| Σ <1.00% | 1.8 ± 0.90 | 1.8 ± 0.61 | 1.9 ± 0.53 | 1.2 ± 0.43 | 2.3 ± 0.79 | 2.1 ± 0.59 | 2.3 ± 0.44 |
- Fatty acids with values below 1.00% were sum and showed together. Σ SFA. Σ MUFA. Σ PUFA. Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3. Different symbols indicate statistical differences (P < 0.05) between diets; Different letters indicate statistical differences among seasons. ND, not detected.
| Fatty acid (%) | Summer | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| C14:0 | 0.7 ± 0.06 | 2.2 ± 0.66 | 1.0 ± 0.20 | 1.6 ± 0.58 | 2.1 ± 0.63 | 0.9 ± 0.21 | 0.9 ± 0.23 |
| C16:0 | 10.2 ± 1.61a | 17.4 ± 3.44b | 13.9 ± 1.44ab | 15.3 ± 2.26ab | 19.6 ± 2.77b | 10.8 ± 0.96a | 15.0 ± 0.48a |
| C17:0 | 1.4 ± 0.77 | 0.8 ± 0.09 | 1.4 ± 0.13 | 0.6 ± 0.07 | 0.6 ± 0.07 | 0.5 ± 0.04 | 0.6 ± 0.07 |
| C18:0 | 15.9 ± 1.02 | 12.0 ± 3.47 | 16.2 ± 0.71 | 11.7 ± 2.04 | 11.0 ± 2.47 | 14.6 ± 1.23 | 12.0 ± 0.48 |
| C21:0 | 0.7 ± 0.42 | 0.9 ± 0.29 | 1.2 ± 0.11 | 0.7 ± 0.24 | 1.2 ± 0.35 | 0.8 ± 0.20 | 1.2 ± 0.31 |
| Σ SFA | 29.1 ± 1.38a | 33.5 ± 1.19b | 34.1 ± 1.85ab | 30.1 ± 1.34ab | 34.8 ± 1.93b | 27.9 ± 0.80a | 29.9 ± 1.05a |
| C16:1 | 1.1 ± 0.21 | 2.6 ± 1.00 | 1.6 ± 0.29 | 2.6 ± 0.55 | 3.1 ± 0.84 | 1.4 ± 0.12 | 1.8 ± 0.31 |
| C18:1n9 | 13.0 ± 2.01 | 18.8 ± 3.37 | 12.3 ± 4.78 | 25.3 ± 3.94a | 21.0 ± 5.09 | 11.5 ± 0.79a | 13.9 ± 2.08 |
| C20:1n9 | 1.1 ± 0.75 | 0.8 ± 0.22 | 1.1 ± 0.06 | 1.3 ± 0.21 | 0.6 ± 0.29 | 0.7 ± 0.10 | 0.9 ± 0.28 |
| Σ MUFA | 15.2 ± 1.56a | 22.4 ± 4.50ab | 15.0 ± 4.49 | 29.3 ± 4.44b | 25.0 ± 5.98 | 14.0 ± 1.05a | 16.6 ± 2.61 |
| C18:2n6 | 12.2 ± 1.16 | 16.1 ± 3.43 | 12.8 ± 0.71 | 19.6 ± 2.02 | 19.5 ± 2.65 | 12.2 ± 0.54 | 13.2 ± 0.97 |
| C18:3n6 | 1.0 ± 0.30 | 1.8 ± 0.82 | 0.7 ± 0.11 | 0.7 ± 0.12 | 1.0 ± 0.21 | 1.8 ± 0.16 | 1.8 ± 0.29 |
| C18:3n3 | 0.6 ± 0.06 | 0.6 ± 0.31 | 0.7 ± 0.16 | 0.7 ± 0.35 | 1.6 ± 0.68 | 1.0 ± 0.67 | 1.2 ± 0.24 |
| C18:4n6 | 0.9 ± 0.18 | 1.5 ± 0.81 | 0.6 ± 0.06 | 1.3 ± 0.52 | 1.0 ± 0.46 | 1.1 ± 0.03 | ND |
| C20:2n6 | 1.6 ± 0.34 | 1.4 ± 0.34 | 1.0 ± 0.43 | 1.4 ± 0.39 | 1.5 ± 0.23 | 1.8 ± 0.41 | 0.8 ± 0.23 |
| C20:4n6 | 10.2 ± 1.52 | 5.9 ± 2.62 | 9.0 ± 1.01 | 5.0 ± 1.70 | 4.4 ± 2.63 | 10.6 ± 1.23 | 8.1 ± 0.81 |
| C20:3n3 | 1.6 ± 1.16 | 0.3 ± 0.12 | 0.4 ± 0.12 | 0.6 ± 0.18 | 0.5 ± 0.11 | 0.6 ± 0.16 | 0.6 ± 0.13 |
| C22:2n6 | 1.1 ± 0.48 | 0.2 ± 0.09 | 1.4 ± 0.81 | 0.1 ± 0.03 | ND | 0.5 ± 0.14 | 1.4 ± 0.08 |
| C22:4n6 | 2.8 ± 1.37 | 1.7 ± 0.50 | 1.9 ± 0.14 | 1.4 ± 0.41 | 1.4 ± 0.63 | 3.1 ± 0.56 | 3.2 ± 0.55 |
| C22:5n6 | 5.9 ± 1.97 | 3.6 ± 1.46ab | 6.1 ± 0.15 | 2.9 ± 0.98a | 2.5 ± 1.47 | 6.1 ± 0.38b | 5.1 ± 1.45 |
| C22:5n3 | 2.5 ± 0.21 | 1.6 ± 0.49 | 2.9 ± 0.62 | 1.3 ± 0.45 | 1.6 ± 0.61 | 3.9 ± 0.13 | 4.5 ± 0.70 |
| C22:6n3 | 15.8 ± 1.21b | 9.6 ± 4.06ab | 13.8 ± 2.03ab | 5.7 ± 2.42a | 5.7 ± 3.09a | 16.1 ± 2.09b | 14.7 ± 1.72b |
| Σ PUFA | 55.7 ± 2.38b | 44.1 ± 5.21ab | 50.9 ± 3.02ab | 40.5 ± 5.08a | 40.2 ± 6.53a | 58.1 ± 1.15b | 53.5 ± 2.85ab |
| Σ n6 | 33.7 ± 2.04 | 31.2 ± 1.78 | 33.0 ± 1.40 | 32.3 ± 2.21 | 31.3 ± 3.04 | 36.5 ± 3.01 | 32.4 ± 1.36 |
| Σ n3 | 22.1 ± 2.61b | 12.9 ± 4.40ab | 17.9 ± 2.58ab | 8.2 ± 3.14a | 8.9 ± 3.52a | 21.7 ± 2.38b | 21.0 ± 2.30b |
| n3/n6 | 0.7 ± 0.10a | 0.4 ± 0.13ab | 0.5 ± 0.08ab | 0.2 ± 0.08b | 0.3 ± 0.09b | 0.6 ± 0.11a | 0.7 ± 0.07a |
| Σ <1.00% | 0.2 ± 0.03 | 0.5 ± 0.16 | 0.4 ± 0.21 | 0.5 ± 0.15 | 0.8 ± 0.25 | 1.1 ± 0.49 | 0.2 ± 0.03 |
- Fatty acids with values below 1.00% were sum and showed together. Σ SFA, Σ MUFA, Σ PUFA, Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3. Different symbols indicate statistical differences (P < 0.05) between diets; Different letters indicate statistical differences among seasons. ND, not detected.
| Fatty acid (%) | Summer | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| C14:0 | 2.1 ± 0.20 | 4.2 ± 0.54 | 3.4 ± 0.68 | 1.5 ± 0.16 | 2.0 ± 0.07 | 1.9 ± 0.17 | 1.7 ± 0.12 |
| C16:0 | 22.1 ± 1.60ac | 28.5 ± 2.21c | 24.5 ± 4.36 | 14.2 ± 0.68ab | 15.9 ± 0.68 | 14.1 ± 0.40b | 15.1 ± 1.38b |
| C17:0 | 0.6 ± 0.12 | 0.4 ± 0.08 | 0.5 ± 0.10 | 0.7 ± 0.12 | 1.6 ± 0.99 | 0.7 ± 0.06 | 0.4 ± 0.05 |
| C18:0 | 12.6 ± 1.02 | 10.3 ± 2.14 | 11.0 ± 3.01 | 13.6 ± 1.09 | 11.2 ± 0.35 | 10.8 ± 0.99 | 10.8 ± 0.50 |
| C21:0 | 0.4 ± 0.13 | 0.3 ± 0.01 | 0.4 ± 0.05 | 1.5 ± 0.16 | 0.7 ± 0.09 | 0.5 ± 0.20 | 0.9 ± 0.23 |
| Σ SFA | 38.1 ± 2.56ac | 43.9 ± 0.72c | 39.9 ± 2.97abc | 31.4 ± 1.44ab | 31.6 ± 1.83abc | 28.3 ± 1.03b | 29.1 ± 1.11b |
| C16:1 | 3.3 ± 0.25 | 5.7 ± 0.10 | 4.4 ± 0.83 | 2.3 ± 0.29 | 3.8 ± 0.46 | 2.9 ± 0.10 | 3.1 ± 0.39 |
| C18:1n9 | 35.0 ± 0.84 | 35.2 ± 2.67a | 36.4 ± 5.72 | 20.3 ± 3.03b* | 32.0 ± 0.73** | 28.2 ± 2.02ab | 32.7 ± 2.00 |
| C20:1n9 | 2.1 ± 0.18 | 1.6 ± 0.11 | 2.0 ± 0.67 | 2.9 ± 1.45 | 1.9 ± 0.17 | 2.0 ± 0.42 | 2.1 ± 0.25 |
| Σ MUFA | 41.0 ± 0.95ab | 42.9 ± 2.38a | 43.3 ± 4.50 | 26.6 ± 3.91b* | 38.8 ± 1.19** | 33.3 ± 2.54ab | 38.7 ± 1.91 |
| C18:2n6 | 15.2 ± 2.03 | 9.5 ± 1.49 | 11.1 ± 1.31 | 11.3 ± 2.12 | 16.1 ± 1.97 | 20.0 ± 1.48 | 15.7 ± 1.29 |
| C18:3n3 | 0.4 ± 0.05 | ND | 1.0 ± 0.20 | 1.2 ± 0.98 | 1.1 ± 0.24 | 3.0 ± 0.48 | 1.4 ± 0.18 |
| C18:4n6 | 1.1 ± 0.26 | 0.5 ± 0.10 | 0.6 ± 0.10 | 1.5 ± 0.44 | 0.8 ± 0.50 | 0.2 ± 0.07 | 0.2 ± 0.02 |
| C20:2n6 | 1.3 ± 0.12 | 0.8 ± 0.07a | 0.6 ± 0.40 | 2.6 ± 0.36b** | 0.9 ± 0.48* | 0.7 ± 0.13ab | 0.5 ± 0.07 |
| C20:4n6 | 0.8 ± 0.19 | 0.8 ± 0.07 | 0.9 ± 0.14 | 6.0 ± 1.45** | 2.4 ± 0.27* | 3.5 ± 0.93 | 3.7 ± 0.67 |
| C20:3n3 | 0.2 ± 0.06 | ND | 0.2 ± 0.04 | 0.6 ± 0.11 | 0.6 ± 0.03 | 1.1 ± 0.16 | 0.6 ± 0.13 |
| C22:2n6 | ND | ND | 0.8 ± 0.53 | 1.1 ± 0.92 | 0.4 ± 0.16 | 0.5 ± 0.21 | 0.2 ± 0.07 |
| C22:4n6 | 0.3 ± 0.09 | 0.2 ± 0.00 | 0.3 ± 0.07 | 1.8 ± 0.42 | 1.5 ± 0.62 | 1.2 ± 0.10 | 1.5 ± 0.39 |
| C22:5n6 | 0.5 ± 0.10 | 0.3 ± 0.01 | 0.5 ± 0.25 | 6.0 ± 1.44 | 1.6 ± 0.27 | 1.8 ± 0.23 | 1.5 ± 0.47 |
| C22:5n3 | 0.2 ± 0.07 | 0.1 ± 0.01 | 0.2 ± 0.08 | 1.7 ± 0.44 | 0.9 ± 0.12 | 2.0 ± 0.23 | 1.5 ± 0.51 |
| C22:6n3 | 0.8 ± 0.06a | 0.4 ± 0.09a | 0.3 ± 0.12 | 7.8 ± 2.33b* | 3.3 ± 0.75** | 4.5 ± 1.21b | 5.0 ± 1.76 |
| Σ PUFA | 20.9 ± 2.95a | 13.0 ± 1.53c | 16.8 ± 1.57ab | 42.0 ± 2.67b* | 29.6 ± 2.18b** | 38.2 ± 2.45b | 32.3 ± 2.52b |
| Σ n6 | 19.7 ± 2.76ab | 12.5 ± 1.64a | 15.1 ± 1.61a | 31.0 ± 0.50b* | 23.7 ± 2.31b** | 27.6 ± 1.60ab | 23.7 ± 0.72ab |
| Σ n3 | 1.1 ± 0.19a | 0.5 ± 0.10a | 1.8 ± 0.44ab | 11.0 ± 2.35b* | 5.9 ± 1.00ab** | 10.6 ± 1.68b | 8.5 ± 2.29b |
| Σ <1.00% | 1.7 ± 0.17 | 1.1 ± 0.22 | 1.2 ± 0.28 | 2.4 ± 0.92 | 1.7 ± 0.36 | 2.0 ± 0.44 | 1.4 ± 0.36 |
- Fatty acids with values below 1.00% were sum and showed together. Σ SFA, Σ MUFA, Σ PUFA, Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3. Different symbols indicate statistical differences (P < 0.05) between diets. Different letters indicate statistical differences among seasons. ND, not detected.
| Fatty acid (%) | Summer | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| C14:0 | 0.5 ± 0.08 | 1.8 ± 0.18 | 2.1 ± 0.46 | 1.8 ± 0.55 | 1.9 ± 0.65 | 0.9 ± 0.16 | 1.7 ± 0.17 |
| C16:0 | 9.6 ± 3.91 | 14.5 ± 2.23 | 12.9 ± 3.26 | 19.1 ± 0.97 | 16.3 ± 1.54 | 11.0 ± 1.50* | 18.8 ± 2.96** |
| C17:0 | 1.1 ± 0.15 | 0.4 ± 0.01 | 0.5 ± 0.03 | 0.7 ± 0.05 | 0.3 ± 0.07 | 0.7 ± 0.20 | 0.5 ± 0.11 |
| C18:0 | 18.7 ± 0.19 | 9.3 ± 1.11 | 8.3 ± 2.94 | 9.9 ± 0.88 | 11.9 ± 2.79 | 10.2 ± 0.62 | 14.7 ± 2.72 |
| C21:0 | 1.1 ± 0.08 | 0.8 ± 0.18 | 0.5 ± 0.15 | 0.4 ± 0.11 | 1.0 ± 0.40 | 1.0 ± 0.18 | 1.1 ± 0.21 |
| Σ SFA | 31.2 ± 3.80 | 27.0 ± 3.07 | 24.3 ± 6.45 | 32.3 ± 1.09 | 31.6 ± 3.65 | 24.0 ± 1.93* | 37.0 ± 5.72** |
| C16:1 | 4.8 ± 2.66 | 4.1 ± 1.47 | 5.1 ± 1.39 | 3.2 ± 0.75 | 3.1 ± 0.77 | 2.3 ± 0.37 | 2.7 ± 0.61 |
| C18:1n9 | 15.0 ± 0.90 | 30.7 ± 7.45 | 36.0 ± 7.27 | 26.2 ± 7.10 | 23.4 ± 4.90 | 20.8 ± 2.00 | 24.8 ± 1.79 |
| C20:1n9 | 0.4 ± 0.11 | 1.2 ± 0.12 | 2.1 ± 1.61 | 1.4 ± 0.47 | 1.9 ± 0.38 | 1.6 ± 0.40 | 1.2 ± 0.44 |
| Σ MUFA | 20.7 ± 3.25 | 36.5 ± 8.51 | 43.6 ± 6.95 | 31.1 ± 8.15 | 29.0 ± 5.41 | 25.3 ± 1.67 | 29.7 ± 2.25 |
| C18:2n6 | 11.0 ± 0.39 | 12.9 ± 0.89 | 11.0 ± 2.28 | 15.2 ± 3.69 | 13.4 ± 2.15 | 13.4 ± 1.46 | 11.3 ± 1.57 |
| C18:3n6 | 0.7 ± 0.06 | 1.6 ± 0.91 | ND | 0.5 ± 0.11 | 0.4 ± 0.05 | 0.5 ± 0.13 | 0.8 ± 0.18 |
| C22:4n6 | 1.6 ± 0.18 | 1.7 ± 0.44 | 5.2 ± 4.85 | 1.1 ± 0.43 | 2.0 ± 0.82 | 1.9 ± 0.20 | 1.6 ± 0.28 |
| C22:5n6 | 6.0 ± 0.55 | 5.0 ± 1.52 | 1.1 ± 0.50 | 2.7 ± 1.68 | 4.4 ± 1.86 | 5.7 ± 0.49 | 3.7 ± 0.54 |
| C22:5n3 | 1.5 ± 0.33 | 0.8 ± 0.19 | 1.3 ± 0.76 | 1.9 ± 1.29 | 1.9 ± 0.91 | 3.4 ± 0.84 | 1.0 ± 0.27 |
| C22:6n3 | 14.0 ± 0.82 | 7.4 ± 2.72 | 5.6 ± 4.56 | 6.0 ± 4.00 | 9.7 ± 4.51 | 14.5 ± 1.77* | 8.2 ± 2.51** |
| Σ PUFA | 48.0 ± 1.61 | 36.4 ± 6.49 | 32.1 ± 12.1 | 36.0 ± 7.17 | 39.2 ± 9.16 | 50.7 ± 0.95* | 33.3 ± 5.37** |
| Σ n6 | 31.7 ± 0.54 | 27.4 ± 3.40 | 23.4 ± 5.96 | 25.9 ± 2.77 | 26.6 ± 4.51 | 30.0 ± 1.64 | 22.8 ± 2.80 |
| Σ n3 | 16.3 ± 1.12 | 9.0 ± 3.23 | 8.7 ± 6.50 | 10.1 ± 5.98 | 12.6 ± 5.22 | 20.7 ± 2.32* | 10.5 ± 2.82** |
| Σ <1.00% | 2.2 ± 0.39 | 1.7 ± 0.97 | 1.5 ± 0.48 | 2.5 ± 1.03 | 3.2 ± 0.89 | 3.0 ± 0.87 | 2.2 ± 0.60 |
- Fatty acids with values below 1.00% were sum and showed together. Σ SFA, Σ MUFA, Σ PUFA, Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3. Different symbols indicate statistical differences (P <0.05) between diets; ND, not detected.
| Fatty acid (%) | Autumn | Winter | Spring | |||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| C14:0 | 1.6 ± 0.29 | 2.2 ± 0.09 | 1.7 ± 0.14 | 1.5 ± 0.17 | 1.5 ± 0.38 | 1.6 ± 0.28 |
| C16:0 | 16.9 ± 1.31 | 18.4 ± 0.85 | 17.0 ± 0.81 | 16.4 ± 1.17 | 13.1 ± 1.88 | 14.8 ± 1.65 |
| C18:0 | 9.4 ± 0.25 | 9.9 ± 0.37 | 9.7 ± 0.69 | 6.8 ± 0.56 | 9.1 ± 3.30 | 8.0 ± 0.93 |
| C21:0 | 1.7 ± 0.09 | 1.7 ± 0.12 | 1.4 ± 0.02 | 1.1 ± 0.09 | 0.8 ± 0.16 | 1.6 ± 0.14 |
| Σ SFA | 30.2 ± 1.68 | 27.2 ± 4.67 | 30.1 ± 1.83 | 26.3 ± 1.96 | 25.6 ± 5.05 | 26.4 ± 2.83 |
| C14:1 | 0.5 ± 0.35 | 0.2 ± 0.04 | 0.1 ± 0.05 | 0.5 ± 0.08 | 1.0 ± 1.04 | 0.2 ± 0.07 |
| C16:1 | 2.0 ± 0.21 | 2.7 ± 0.20 | 3.2 ± 0.64 | 2.6 ± 0.27 | 2.1 ± 0.12 | 2.4 ± 0.33 |
| C18:1n9 | 20.8 ± 2.64 | 20.1 ± 2.23 | 21.9 ± 1.09* | 15.7 ± 0.72** | 13.0 ± 4.83 | 23.2 ± 3.19 |
| C20:1n9 | 1.8 ± 0.28 | 1.7 ± 0.12 | 1.3 ± 0.07 | 2.0 ± 0.26 | 2.0 ± 0.31 | 2.5 ± 0.13 |
| Σ MUFA | 25.8 ± 3.06 | 25.6 ± 1.91 | 27.0 ± 1.84* | 21.5 ± 0.79** | 18.9 ± 4.45 | 29.1 ± 3.61 |
| C18:2n6 | 17.0 ± 2.67 | 13.2 ± 1.19 | 12.2 ± 1.35 | 15.7 ± 1.55 | 13.5 ± 3.29 | 15.2 ± 0.47 |
| C18:3n3 | 0.8 ± 0.18 | 1.1 ± 0.21 | 1.9 ± 0.34 | 1.8 ± 0.25 | 2.4 ± 0.43 | 1.8 ± 0.39 |
| C20:2n6 | 1.6 ± 0.48 | 2.4 ± 1.17 | 1.4 ± 0.70 | 1.1 ± 0.30 | 2.8 ± 1.50 | 1.5 ± 0.36 |
| C20:4n6 | 5.1 ± 0.76 | 5.5 ± 0.59 | 4.7 ± 0.47 | 4.2 ± 0.11 | 4.5 ± 0.63 | 3.7 ± 0.63 |
| C22:2n6 | 1.3 ± 1.09 | 0.4 ± 0.18 | 2.9 ± 2.23 | 0.3 ± 0.09 | 7.9 ± 6.14 | 0.4 ± 0.12 |
| C22:4n6 | 1.9 ± 0.47 | 2.8 ± 0.49 | 2.3 ± 0.22 | 5.1 ± 0.67 | 3.4 ± 1.16 | 2.3 ± 0.68 |
| C22:5n6 | 4.7 ± 1.03 | 4.3 ± 0.99 | 2.3 ± 0.08 | 3.4 ± 0.51 | 3.8 ± 0.67 | 3.4 ± 0.68 |
| C22:5n3 | 1.1 ± 0.48 | 2.8 ± 0.42 | 4.9 ± 0.09 | 5.5 ± 0.97 | 5.2 ± 1.56 | 3.1 ± 0.81 |
| C22:6n3 | 9.3 ± 3.35 | 7.5 ± 1.00 | 8.8 ± 0.49* | 13.6 ± 1.71** | 13.0 ± 1.19 | 11.2 ± 3.04 |
| Σ PUFA | 44.0 ± 4.62 | 41.5 ± 2.12 | 42.9 ± 3.67* | 52.2 ± 2.48** | 55.5 ± 7.50 | 44.5 ± 6.44 |
| Σ n6 | 32.4 ± 1.46 | 29.6 ± 1.02 | 26.7 ± 3.86 | 30.4 ± 1.47 | 33.9 ± 4.69 | 27.6 ± 2.25 |
| Σ n3 | 11.6 ± 3.51 | 11.9 ± 1.30 | 16.1 ± 0.18* | 21.9 ± 2.42** | 21.6 ± 3.09 | 16.9 ± 4.21 |
| <1.00% | 2.9 ± 0.80 | 3.2 ± 0.92 | 2.3 ± 0.52 | 2.8 ± 0.41 | 3.9 ± 1.14 | 3.5 ± 1.02 |
- Fatty acids with values below 1.00% were sum and showed together. Σ SFA, Σ MUFA, Σ PUFA, Σ n6 and Σ n3 are the sum of saturated, monounsaturated, polyunsaturated, polyunsaturated n6 and polyunsaturated n3. Different symbols indicate statistical differences (P < 0.05) between diets. ND, not detected.
In the white muscle, SFAs (mainly C17:0 and C18:0) decreased in TG, with increases in body mass in the T1 group and differences between animals sampled in the summer versus the spring. In spring, the T2 C14:0 was higher in white muscle TGs when compared with the same tissue and diet group from the beginning of the experiment (summer; Table 6). On the other hand, the PUFA percentage (mainly C18:3n6 and C20:4n6) in TG was higher in animals from T1 when compared to T2 in autumn and spring; and the initial group had a lower percentage of PUFA when compared to T1 animals in autumn.
In the muscle PL (Table 7), the percentage of SFA (mainly C16:0) in T1 increased in the autumn and winter, when compared to the percentage of SFA in T2 in the summer. In T1, however, we found a subsequent decrease in these percentages, with increasing temperatures in the spring. In the T1 group, MUFAs (mainly C18:1n9) showed the same pattern as SFAs (i.e. an increase in the winter and a decrease in the spring). Conversely, in both groups, PUFA decreased in the muscle PL during the winter and increased in the spring, with only T1 showing differences from winter to spring. The difference was a consequence of the variations in PUFAn3, primarily C22:6n3. Although the T2 group showed no increase in total PUFA levels in the spring (when compared with animals sampled in the winter), the T2 group had the same increase in PUFAn3 due to variations in the percentage of C22:6n3. The n3/n6 ratio in muscle PL was not affected by diet; however, when compared with the summer, its value was reduced in the winter and increased again in the spring (for both groups).
In both experimental groups throughout the experimental period, the liver TG had an FA profile with a similar pattern as above (Table 8): The percentage of SFA was maintained until winter and decreased in spring, mainly due to differences in C16:0. When compared with the previous sampling period (autumn), the MUFA levels of animals in the T1 group (mainly C18:1n9) decreased in the winter, resulting in a higher percentage of the FAs in T2 compared to T1 in the same season.
The PUFA profile varied considerably during the experimental period. The PUFA decreased in autumn for T1, followed by an increase in winter. This increase occurred in both experimental groups and these higher values were maintained until the end of the experiment. In the winter, PUFA was higher in the T1 than in the T2 group. The PUFA changes in liver TG were very similar because they both showed an n6 PUFA pattern of variation and an increase in winter due to increases in C20:2n6 and C20:4n6, which were both higher in T1 than in T2. The animals sampled in the spring, for both groups, had lower percentages of n3 (mainly C22:6n3) than animals sampled in the summer. Because of the differences in C22:6n3 in the winter, the animals from T1 also accumulated more PUFA n3 in liver TG than did the T2 animals.
The FA from the liver PL did not change with the increase in body mass; however, diet influenced the FA composition of the liver PL, but only in spring (Table 9). When compared to animals from T2, the animals from T1 had a lower percentage of SFA (mainly C16:0). Conversely, compared to the animals from T1, the animals from T2 had a lower percentage of PUFA (mainly C22:6n3) in liver PL than T1 animals.
In the plasma, the FA also did not vary with increasing body mass, but in the winter, the use of different diets changed the profile of the circulating FA. In T1, the alternative diet (T2) decreased the percentage of MUFAs, primarily because of the C18:1n9 level. Conversely, at this same body mass, the percentage of PUFA n3 (specifically C22:6n3) was higher in the plasma of T2 animals than in the T1 animals (Table 10).
Discussion
The results of this study clearly indicate that the protein produced from the fermentation of whey, by the yeast K. marxianus, can be used as a feed supplement in a tilapia diet, thereby representing a useful destination for cheese whey. Considering body composition, this diet did not significantly influence the main substrates that we analysed, and the percentages of PUFAs in the fillet were similar throughout the experiment when comparing both diets.
Throughout the experiment, dissolved oxygen and pH values did not significantly vary and were within the optimum ranges for normal growth and health of tilapia (El-Shafai, El-Gohary, Nasr & Gijzen 2004). The fish ponds did not have any artificial devices to maintain the temperature; thus, the water temperature was directly influenced by the temperature of the environment, which was intentionally included as a part of our experimental design to mimic the production of most tilapia in outdoor systems with no temperature control. Our results can therefore be used to evaluate the ingredients and diets used in real aquacultural situations (Goda, Wafa, El-Haroun & Chowdhury 2007).
El-Sayed, El-Ghobashy and Al-Amoudi (1996) investigated the effects of pond depth and water temperature on the growth of Nile tilapia and showed that a smaller increase in weight and length were recorded in winter months, demonstrating the influence of this variable on animal growth. Those descriptions corroborate the results of this study, in which the smaller increase in body mass coincided with the winter months (sampled in spring), regardless of the diet. Only during autumn (sampled in winter) did we find body mass increases affected by the experimental diets (i.e. T1 reached higher body mass than T2). Despite these differences, however, both groups of animals failed to reach commercial size (ca. 450 g) at the end of 8 months, suggesting that the possible stress factor that delayed growth for both groups was a low temperature during the autumn and winter months. Atwood, Tomasso, Webb and Catlin (2003), investigating the effects of diet on survival of tilapia in low temperatures, showed that small animals were more susceptible to low temperature than the largest ones and that diets supplemented with coconut and menhaden oil had little influence on the ability of tilapia to survive in low temperatures.
Although the fish that were fed with the alternative diet showed a reduced body weight, their apparent digestibility (82.4%) was similar to the control diet (82.7%), which confirms published data that shows excellent digestibility of the protein fraction of yeasts (Lara-Flores, Olvera-Novoa, Guzmán-Méndez & López-Madrid 2003): When Saccharomyces cerevisae was included in diets of tilapia, average digestibility ranged from 80% to 90%. These authors suggested that the proteolytic activity of yeast could increase the digestibility of supplemented aquafeeds. Beyond the zootechnical and digestibility aspects, the use of alternative sources of ingredients in fish feed, with experimental diets, should take into account animal metabolic functions, such as protein and lipid metabolism. Some studies described the mobilization of lipids between the muscle and the liver (Moreira, Venturieri & Mimura 2002; Tolussi, Hilsdorf, Caneppele & Moreira 2010; Vieira, Hilsdorf & Moreira 2012), and demonstrated that fish are able to synthesize lipids in the liver and direct the substrate to muscle and/or other tissues in several situations (Sheridan 1988).
The lipid content in white muscle is important for determining the commercial quality of a fish (Ando, Mori & Nakamura 1993), and our results revealed that muscle lipid content was not altered by diet and remained low during all seasons, supporting evidence that tilapia has a lean fillet (Bennion 1980). In addition, the protein content in muscle was higher in the T2 animals when compared to the control fish, especially in the coldest months of the year. These data suggest that differential deposition of substrates in animals that are fed with yeast can be attributed to the biological value of the protein ingredients, thereby allowing maintenance of the muscle protein content (which is not used as an energy source). This finding is important for fillet quality and subsequent marketing (Lu, Takeuchi & Ogawa 2003). Dias, Alvares, Arzel, Corraze, Diez, Bautista and Kaushik (2005) showed that the quality of dietary protein modulates both lipid synthesis and the consequential use of those synthesized lipids as energy substrata. The deposition of lipids in fish tissue is highly related to both the balance of amino acids and the energy/protein ratio ( Botaro, Furuya, Silva, Santos, Silva & Santos 2007). According to Graig and Helfrich (2002), the increase in lipids in fish diets for use as an energy source is a trend in aquaculture that strives to reserve protein for use towards animal growth. This retention of proteins is the main goal of nutritionists who seek to develop a diet that is economically effective and environmentally sustainable.
Even when we considered a similar tissue lipid content during most of the experimental period, analyses of the FA profile revealed that the inclusion of SCPs in the diet changed the dynamics of FA metabolism in tilapia. According to Skalli and Robin (2006), the difference in the FA composition of tissues is a result of the specificity and affinity for FAs in animal metabolism. In general, FAs have a distinct role in fish metabolism; PLs are important constituents of cell membranes, acting as the main precursors of prostaglandins, thromboxanes and leukotrienes, and preventing cardiac diseases (Wahlquist 1998; Nasopoulou, Tsoupras, Karantonis, Demopoulos & Zabetakis 2011); and TGs serve as storage depots for FAs, which are catabolized to produce energy (Henderson & Tocher 1987). However, the interactions among the FAs from different sources are complex, and tissues may not necessarily assimilate or catabolize these FAs similarly (Bell, Henderson, Tocher, McGheef, Dick & Porter 2002).
In summary, we found a decrease in SFA in muscle TG in spring and an increase in PUFA in autumn for FA data in the T1 group. PL in the same tissue presented a pattern of fluctuation in SFA, MUFA and PUFA, during autumn and winter, and a return to the initial values in spring, confirming that the return to warmer temperatures re-established the FA of PL. In the liver, we found a decrease in SFA and MUFA in TG, in winter, with maintenance of low SFA values in spring. In contrast, PUFA showed a pronounced decrease in autumn, with the re-establishment of values from winter and spring. Conversely, in T2, we found a reduced number of seasonal changes that were more pronounced in muscle, with an increase in SFA in winter and a PUFA decrease in PL fraction, in winter, and a concomitant decrease in PUFA in winter (i.e. a trend that was different from T1). In the liver TG, inverse behaviour occurred for SFA and PUFA: SFA decreased in spring and PUFA increased in winter and spring.
In general, the FA profile of a fish fillet reflects the dietary FA profile. In this study,, specific FAs were selectively retained in the fillet and influenced by the diet and the season. T2 diet contained lower percentages of C22:6n3 (docosahexaenoic acid [DHA]) and considerable amounts of C18:3n3. C18:3n3 is considered an essential fatty acid (EFA) for this species and an important substrate for elongation and desaturation to DHA, enabling equivalent percentages for the group fed with the fish meal diet during most seasons. EFAs are FAs that are not synthesized endogenously and therefore must be ingested in the diet (Arts & Ackman 2001). EFAs vary by species and by the ability of such species to catalyse the FA presented in a diet, with the aid of specific enzymes, elongases and desaturases (Hastings & Agaba 2001; Zheng, Tocher, Dickson, Bell & Teale 2004).
Despite the differences we observed, the percentages of PUFA in white muscle phospholipids (i.e. the fillet) were similar throughout the experimental period when we compared both diets. These findings highlight the importance of maintaining the quality of fillets. When we used both diets, PUFA percentages reached more than 50% of the total FA, represented mainly by arachidonic acid (C20:4n6; AA) and DHA. This finding suggests that these animals are efficiently elongating and desaturating the main precursor of the n6 PUFA, C18:2n6 (Olsen, Henderson & McAndrew 1990). According to Arts and Ackman (2001), C18:2n6 is an EFA for fish and humans because it is a precursor of AA, which is needed during growth, reproduction, regulation of the immune response and physiological stress response to environmental alterations (Bell, Tocher, Macdonald & Sargent 1995). During the coldest months of the year, the n3/n6 PUFA ratio in muscle PL decreased in both experimental groups, with higher values in the spring and summer, when it was close to 0.7. The n3/n6 ratios of 0.30 and 0.50 have been recommended by several authors to promote a higher conversion of alpha-linolenic acid (C18:3n3) to C22:6n3; thus, a ratio of 0.25–0.50 has greater importance for individuals who have a low intake of EFA. However, ratios close to 1.00 are not recommended because inhibition in the conversion of precursor FAs into long chain FAs occurs (Masters 1996).
The high percentage of PUFA in the TG in many tissues of the T1 group revealed the importance of this class of lipids in the animals that were fed fish meal, suggesting that liver TGs are temporary storage deposits of PUFAs, especially for those with large and highly unsaturated chains, such as DHA ( Cejas, Tejera, Jerez, Bolaños & Lorenzo 2003). In addition, animals fed the T2 diet acquired more C14:0 in the TG fraction, from muscle, thereby supporting elongation of short chain FAs from milk that we used as a substrate for the fermentation of yeast in this trial (Mansson 2008).
The increased PUFA in liver TG, in the T1 group is a common response of animals in low temperature environments (Hazel 1984), and it is observed in the spring as a response to the colder winter temperatures. In white muscle, PUFA decreased in PL during the winter months, which could be a response that allocates fatty acids between muscle and liver, particularly because liver is responsible for processing and mobilization of FAs (Sheridan 1994). In contrast, the animals in the T2 group showed increased SFA in the spring, which is what most likely modified the fluidity of the cell membranes, when compared to the T1 animals ( Trueman, Tiku, Caddick & Cossins 2000; Pereira, Leonard & Mukerji 2003; Polley, Tiku, Trueman, Caddick, Morozov & Cossins 2003).
In summary, during the experimental period, neither group reached the size that could be used in commercial fish farms. The animals that were fed with the alternative diet had a smaller increase in mass, when compared to the control group, but this result was found mainly in the winter, most likely due to a lower deposit of PUFA in the liver TG. Animals fed the alternative diet had a higher concentration of protein in their fillet, suggesting the use of this alternative protein source. In addition, the FAs of the phospholipids had similar profiles throughout the year for both diets, which is important because PLs are an essential source of PUFA in human nutrition.
Acknowledgments
This study was supported by a research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 05/55837-6) and a student fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors also thank Empresa Brasileira do Peixe Ltda for providing the experimental animals, Ponte Nova Hatchery (Departamento de Águas e Energia Elétrica/Fundação de Amparo ao Ensino e Pesquisa [DAEE/FAEP]) for providing the facilities for the trials, and the Laboratory of Fermentation of Technological Food Institute at the Federal University of Rio Grande do Sul (RS-Brazil) for providing the yeast used for fermentation.




