Faecal biomarkers for diagnosis and prediction of disease course in treatment-naïve patients with IBD
The Handling Editor for this article was Dr Sreedhar Subramanian, and it was accepted for publication after full peer-review.
Summary
Background
Faecal biomarkers can be used to assess inflammatory bowel disease (IBD).
Aim
To explore the performance of some promising biomarkers in diagnosing and predicting disease course in IBD.
Methods
We included 65 patients with treatment-naïve, new-onset Crohn's disease (CD), 90 with ulcerative colitis (UC), 67 symptomatic controls (SC) and 41 healthy controls (HC) in this prospective observational study. We analysed faecal samples for calprotectin (FC), myeloperoxidase (MPO), human neutrophil lipocalin (HNL), eosinophil cationic protein ECP and eosinophil-derived neurotoxin (EDN) and compared markers among groups. We assessed the diagnostic capability of biomarkers with receiver operating characteristic curves. Clinical disease course was determined for each patient with IBD and analysed the association with biomarkers by logistic regression.
Results
All markers were elevated at inclusion in patients with IBD compared with HC (p < 0.001) and SC (p < 0.001). FC (AUC 0.85, 95% CI: 0.79–0.89) and MPO (AUC 0.85, 95% CI: 0.80–0.89) showed the highest diagnostic accuracy in distinguishing IBD from SC. The diagnostic ability of biomarkers differed between IBD subtypes with the highest performance for FC and MPO in CD. The diagnostic accuracy was further improved by combining FC and MPO (p = 0.02). Levels of FC, MPO and HNL at inclusion were predictive of an aggressive disease course with MPO showing the strongest association (p = 0.006).
Conclusions
This study provides new insight into the diagnostic and prognostic capability of neutrophil and eosinophil biomarkers in IBD and suggests that MPO, alone or in combination with FC, may add to the diagnostic power of faecal biomarkers.
1 INTRODUCTION
Inflammatory bowel disease (IBD) is a heterogeneous disease encompassing the two main subtypes, Crohn's disease (CD) and ulcerative colitis (UC). At diagnosis, patients may present with a broad spectrum of symptoms, and although some clinical factors associated with prognosis have been identified, the future disease course is largely unpredictable. Within IBD management, as in many other medical fields, there is a shift towards a more personalised approach. This paradigm, known as precision medicine, aspires to identify the most suitable treatment for the right patient at the right time.1
In the search for biomarkers that can guide clinical decision-making, various molecular data, including proteomics, metabolomics and genomics, are under investigation.2 However, none of these markers are yet implemented in the diagnostic algorithm for IBD, and few are available in clinical practice to guide clinicians regarding future disease course.
In contrast to the slow discovery of signatures of systemic biomarkers based on non-targeted –omics analyses, faecal biomarkers are becoming increasingly established in IBD care.3 These non-invasive markers are often identified from targeted analyses of specific proteins in stool. Among identified markers, faecal calprotectin (FC), a neutrophil cytosolic protein, is the most well-established. FC concentrations correlate with the degree of intestinal inflammation, and its high stability facilitates implementation in clinical care. In some healthcare systems, FC is used as a tool in the diagnostic algorithms for IBD, whereas in other systems, its use is restricted to longitudinal monitoring of patients with IBD. However, concerns about inter-assay differences and the test's specificity4-6 and sensitivity7 have been raised.
Additional faecal proteins reflecting the influx and activation of various innate immunity cells have been identified as potential biomarkers. Similar to FC, myeloperoxidase (MPO) is mainly released from neutrophils and demonstrates a moderate correlation to endoscopic scores.8, 9 Human neutrophil lipocalin (HNL), also known as neutrophil gelatinase-associated lipocalin or lipocalin-2, exists in different molecular forms and is released from both neutrophils and epithelial cells.10 Elevated levels of faecal HNL have been reported in patients with IBD when compared with healthy controls (HC) and with patients with irritable bowel syndrome, and HNL has been shown to be moderately to strongly correlated with endoscopic IBD activity.10, 11 Eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP) represent granular proteins released from activated eosinophils, and their faecal levels have demonstrated moderate correlations with endoscopic activity in IBD.12-14
Although these results indicate a potential role for novel faecal markers in monitoring the inflammatory activity in IBD, their role in diagnostic and prognostic algorithms for IBD remains to be explored. Therefore, we examined faecal samples from treatment-naïve patients in an inception cohort and examined associations of FC, MPO, HNL pAb/765, HNL 763/764, ECP and EDN with the diagnosis and prognosis of IBD. Furthermore, we aimed to assess their diagnostic capacity and potential to predict disease course of IBD, when used alone or in combination with clinical characteristics.
2 MATERIALS AND METHODS
2.1 Study design
This was a prospective multicentre observational cohort study of adult patients with clinical suspicion of IBD. First, we examined whether faecal markers were associated with an IBD diagnosis by comparing newly diagnosed patients with IBD with symptomatic controls (SCs), that is patients with IBD-like symptoms but without any endoscopic signs of IBD-associated inflammation at inclusion or during follow-up. Next, we explored whether faecal markers were related to future disease course by comparing IBD patients with an indolent versus an aggressive disease course during the first year of follow-up. The study was a part of the Swedish multicentre programme, ‘A multi-modal national study to identify biomarkers for diagnosis, therapy response and disease progression in IBD (BIO IBD)’.
2.2 Patient cohort
Patients aged ≥18 years, referred from a primary care physician, with suspected IBD based on symptoms such as diarrhoea, abdominal pain and blood or mucus in stool for >2 weeks were consecutively included at eight Gastroenterology centres (Ersta Hospital, Karolinska University Hospital (Huddinge/Solna), Linköping University Hospital, Sahlgrenska University Hospital (Sahlgrenska/Östra), Skåne University Hospital (Lund/Malmö), Södra Älvsborg Hospital, Uppsala University Hospital, Örebro University Hospital) in Sweden between November 2011 and December 2016. Exclusion criteria were a previous IBD diagnosis or IBD treatment and inability to provide informed consent. To be eligible for inclusion in the present study, individuals also had to provide a faecal sample at inclusion. Also, only patients with a definite subtype of IBD, that is CD or UC, were included in this study; patients with IBD unclassified were excluded.15
After obtaining informed consent, blood and stool samples were collected. All patients underwent routine diagnostic work-up for IBD, and the diagnosis was based on clinical, endoscopic, histological and cross-sectional imaging according to internationally accepted diagnosis criteria.16, 17 Information about demographics (sex, age and smoking status) was collected at baseline, and the Montreal classification was used to categorise patients according to their phenotype of CD or UC. Patients diagnosed with IBD were followed prospectively with follow-up visits and data collection at 3 and 12 months. IBD treatment was initiated according to national and international guidelines at the discretion of the treating physician. Individuals with gastrointestinal symptoms but no endoscopic or histologic signs of IBD-associated inflammation were considered SC. To establish a diagnosis, these individuals were subjected to further examinations depending on their clinical presentation including coeliac serology, upper endoscopy, faecal microbiological testing and bile acid measurements.
In addition to the inclusion of cases with newly diagnosed IBD and SCs, HC without any history of current or chronic gastrointestinal symptoms were also included and served as a separate reference group. The recruitment of HC occurred via advertisements at participating centres. After obtaining informed consent from HC, blood and faecal samples were collected, and a flexible sigmoidoscopy was performed.
2.3 Treatment outcome
The clinical disease course was defined at 12 months (±2 months) using a composite endpoint, and the definition was in line with previous work on IBD disease course.18 The composite endpoint of an aggressive disease course was recorded if the patient suffered from difficult-to-control inflammation or developed a complicated disease, both conditions defined below.
Difficult-to-control inflammation included the occurrence of hospital admission for active disease, inflammatory-related resecting surgery or stoma, use of >2 courses of corticosteroid or a total cumulative corticosteroid dose of >2.5 g within the first 12 months from diagnosis, use of ≥2 biological agents within the first 12 months from diagnosis or switch to second line due to poor clinical effectiveness.
Development of complicated disease behaviour included the development of new strictures, fistula, abscesses or perianal disease in patients with CD within the first 12 months. Also, patients undergoing stricture, fistula, abscess-related or perianal surgery due to Crohn's disease during the period were categorised into this group.
2.4 Measurement of biomarkers
All collected stool samples were transported to the University Hospital, Uppsala, Sweden, on dry ice and stored at −80°C until analysis. Samples were thawed, and proteins were extracted using a CTAB-containing buffer and prepared in a single batch as described previously.13 Faecal markers were measured using ELISAs provided by Diagnostics Development, Uppsala, Sweden, www.diagnosticdevelopment.com, that is MPO (myeloperoxidase) ELISA, HNL pAb/765 and HNL 763/764 (human neutrophil lipocalin) ELISA, ECP (eosinophil cationic protein) ELISA and EDN (eosinophil-derived neurotoxin) ELISA. All samples were assessed in duplicate, and after adjustment of assay dilution and water content in faeces, levels of markers were expressed as micrograms per gram of semi-dried faeces. The intra- and inter-assay variations were less than 12% for all assays.
Correspondingly, FC was extracted and analysed with a chemiluminescent immunoassay using the LIASON XL analyzer (DiaSorin, Saluggia, Italy), Department of Clinical Chemistry, University Hospital, Uppsala, Sweden. High-sensitivity (Hs)-CRP and albumin in serum were assayed at Uppsala BioLab, Uppsala Clinical Research Center (UCR), Uppsala, Sweden.
2.5 Statistical analyses
Categorical data were expressed as frequency and quantitative data as median and interquartile range. For the comparison of biomarkers between groups, the Kruskal–Wallis test was used, followed by pairwise analysis of subgroups with post-hoc analysis according to Dunn. To estimate the clinical performance of the biomarker to separate (A) IBD from SC and (B) aggressive disease course from an indolent course, receiver operating characteristic (ROC) curves were generated. The area under the curve (AUC) with a 95% confidence interval (95% CI) was reported. The Youden index was used to derive the optimal cut-off value, which was further used to determine sensitivity, specificity, likelihood ratio for a positive result [LR(+)] and likelihood ratio for a negative result [LR(−)]. Differences between AUCs were compared according to DeLong.19 The association of biomarkers and aggressive disease course was analysed using logistic regression. Biomarkers were analysed separately and adjusted for sex, age, diagnosis (when no subgrouping) and smoking, and p-values were adjusted for multiplicity using free step-down resampling according to Westfall-Young.20 The biomarkers were fitted using splines with three knots, while age was fitted linearly. The Spearman correlation test was used and presented in a scatterplot matrix to assess the correlation between biomarkers.
To analyse the diagnostic performance of a combination of FC and one additional marker in comparison to FC alone, a model was set up. Markers were fitted using a spline with 3 knots, and models were fitted and adjusted for sex, age and smoking. The concordance index (c-index) was calculated, and the fraction of new information, that is the additional information that was gained from a model comprising two markers compared with a model with FC alone, was assessed. This was calculated as 1-variance of the predictors for the new model divided by the variance of the predictions for the original model (1-var[prednew]/var[predoriginal]). The likelihood ratio was used to test whether adding a marker improved the fit of the model, that is the diagnostic capability of the model.
A p < 0.05 was considered significant. Statistical calculations were performed in MedCalc version 20.014 (MedCalc Software Ltd, Oostende, Belgium), GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA) and R, version 3.6.1 (Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
3.1 Study population
In total, 285 individuals were screened for inclusion in the study. Of these, 22 individuals were excluded because of a diagnosis of IBD-U (n = 1), uncertainties of diagnosis (n = 7), exposure to corticosteroids prior inclusion and no sign of inflammation on index endoscopy (n = 2), lost at follow-up (n = 1) or not providing adequate volume of faeces for analysis (n = 11). The final cohort consisted of 65 patients with CD, 90 with UC, 67 SCs and 41 HC (Table 1, Figure S1). The final diagnosis for SCs is presented in Table S1. About half of these were considered functional gastrointestinal disorders (n = 32), and other diagnoses were microscopic colitis (n = 7) and coeliac disease (n = 3). For eight individuals, no diagnosis could be established despite a solid gastrointestinal assessment. Table 2 summarises the leading symptoms of the screened individuals at presentation.
| Treatment-naive Crohn's disease (n = 65) | Treatment-naive ulcerative colitis (n = 90) | Symptomatic controls (n = 67) | Healthy controls (n = 41) | |
|---|---|---|---|---|
| Demographic | ||||
| Sex, female, n (%) | 33 (51) | 43 (48) | 44 (66) | 24 (59) |
| Age median (range) | 29 (18–85) | 34 (18–81) | 37 (18–79) | 26 (19–48) |
| Smoking | ||||
| Current/former/never | 11/12/42 | 7/35/46 [2] | 10/12/42 [3] | 3/7/28 [3] |
| Location, CD | ||||
| L1/L2/L3/L4 | 25/27/13/2 | |||
| Behaviour, CD | ||||
| B1/B2/B3 | 57/7/1 | |||
| Perianal disease, p | 6 | |||
| Extent, UC | ||||
| E1/E2/E3 | 31/26/33 | |||
| Biochemical data | ||||
| CRP (mg/L) | 7.20 (1.90–26.8) [1] | 3. 30 (1.20–7.70) | 1.50 (0.70–5.20) | 0.60 (0.30–1.10) [1] |
| Albumin (g/L) | 38.0 (34.3–41.0) [1] | 40.0 (37.0–43.0) | 40.0 (38.0–44.0) | 41.5 (40.0–43.8) [1] |
- Note: [] indicates the number of missing observations.
| Symptoms, n (%) | IBD (n = 155) | Symptomatic controls (n = 67) |
|---|---|---|
| Diarrhoea | 75 (48.4) | 28 (41.8) |
| Blood in stool | 97 (62.5) | 21 (31.3) |
| Urgency | 93 (60.0) | 43 (64.2) |
| Abdominal pain | 110 (71.0) | 43 (64.2) |
| Missing data | 17 (11.0) | 10 (14.9) |
Included patients with IBD had to be naïve to IBD therapy. However, patients who had started 5-aminosalicylate suppositories or enemas less than 10 days before inclusion (n = 2) and patients with a short treatment of corticosteroid (less than 2 days if intravenous [n = 2] and less than 10 days if oral therapy [n = 2]) were accepted if the endoscopic assessment in immediate connection to inclusion indicated moderate-to-severe inflammation and/or aphthous ulcers.
There was no difference in sex or smoking habits across the groups. The HCs were younger compared with SCs (p < 0.001) and patients with UC (p < 0.001) but not with CD (p = 0.26).
In total, 38% (25/65) of patients with CD and 19% (17/90) with UC fulfilled the criteria for an aggressive clinical course in terms of inflammation after 1 year of follow-up. Twelve per cent (8/65) of patients with CD met the criteria for complicated disease behaviour. Six of these eight patients also meet the criteria for uncontrolled inflammation.
Patients who reached the outcome fulfilled one or several criteria, where the criteria of an extensive need for corticosteroids (>2.5 g prednisolone [59%] and/or >2 courses of corticosteroids [24%]), as well as hospital admission (40%), were most frequent.
Three patients with CD underwent ileocecal resection during the first year from diagnosis, whereas no patient with UC underwent colectomy during the corresponding period.
3.2 Levels of faecal markers at diagnosis
Baseline levels of FC, MPO, HNL pAb/765, HNL 763/764, ECP and EDN in faeces were analysed across groups. All faecal markers were significantly elevated in patients with IBD compared with HC. Furthermore, patients with CD and UC displayed higher levels of all faecal markers than SC (p < 0.001), except for the levels of EDN in patients with CD (ns). The neutrophil markers FC, MPO, HNL pAb/765 and HNL 763/764, but not the eosinophil markers ECP and EDN, were significantly higher in SC than in HC. In contrast, there were no statistically significant differences in levels of faecal markers between patients with CD and UC (Figure 1).
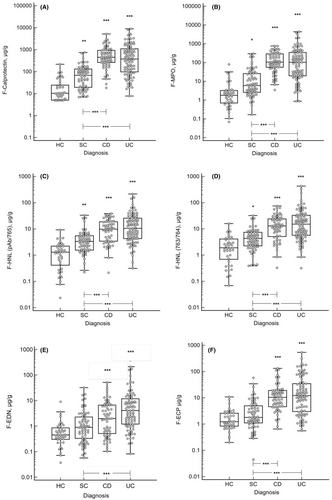
There was no difference in the levels of faecal markers at baseline when categorising patients with CD by disease location (Table S2), disease behaviour or presence of perianal disease (data not shown). In contrast, baseline levels of FC and ECP (p < 0.01), MPO, HNL pAb/765 and HNL 763/764 (p < 0.05) were higher in patients with extensive UC (E3) than in patients with ulcerative proctitis (E1). Levels of eosinophil markers (ECP and EDN) were higher in left-sided UC (E2) than in ulcerative proctitis (both p < 0.05), while the other markers did not differ between these two UC phenotypes (Table S2).
3.3 Diagnostic capability of single faecal markers to differentiate between IBD and SC
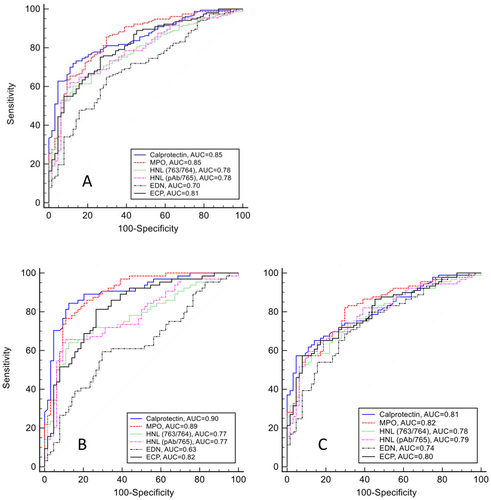
The capability of each marker to differentiate IBD from non-IBD SC was examined by constructing ROC curves (Figure 2). To reflect a diagnostic scenario where patients with gastrointestinal symptoms seek healthcare and are examined, we first explored the ability of each marker to distinguish between IBD (CD and UC combined) and SC. The neutrophil markers, FC (AUC 0.85, 95% CI: 0.79–0.89) and MPO (AUC 0.85, 95% CI: 0.80–0.89), displayed the highest AUCs followed by ECP (AUC 0.80, 95% CI: 0.74–0.85) and both variants of HNL (Table 2). The eosinophil marker EDN performed poorly in separating IBD from SC. Pairwise comparison of FC and MPO revealed no significant difference in their diagnostic capability, but both markers performed significantly better than HNL pAb/765 (FC p < 0.05, MPO p < 0.01), HNL 763/764 (FC p < 0.01, MPO p < 0.01) and EDN (FC p < 0.001, MPO p < 0.001) in differentiating IBD from SC. The diagnostic capability of MPO was better than ECP (p < 0.05), while no difference was seen between FC and ECP (ns) in this regard. The sensitivity and specificity of each marker at its optimal cut-off (Criterion) and the corresponding likelihood ratios are shown in Table 3.
| Faecal marker | n, IBD | n, SC | AUC (95% CI) | Criterion | Sens/Spec % | LR (+) | LR (−) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| FC | 154 | 64 | 0.85 (0.79–0.89) | >176 | 71.6/87.5 | 5.73 | 0.32 | 79.6 |
| MPO | 155 | 67 | 0.85 (0.80–0.89) | >52.3 | 65.8/89.6 | 6.3 | 0.38 | 77.7 |
| HNL pAb/765 | 154 | 67 | 0.78 (0.72–0.84) | >7.17 | 62.3/89.6 | 5.97 | 0.42 | 76.0 |
| HNL 763/764 | 154 | 67 | 0.78 (0.72–0.83) | >8.89 | 60.3/88.1 | 5.01 | 0.45 | 74.2 |
| ECP | 154 | 67 | 0.80 (0.74–0.85) | >4.26 | 75.3/73.1 | 2.8 | 0.34 | 74.2 |
| EDN | 154 | 67 | 0.70 (0.64–0.76) | >1.68 | 64.9/71.6 | 2.29 | 0.49 | 68.2 |
Next, the capacity of each faecal marker to discriminate between IBD subtypes and SC was examined (Figure 2B,C). For distinguishing CD and SC, FC and MPO yielded the highest AUCs (FC [AUC 0.90, 95% CI: 0.84–0.95] and MPO [AUC 0.90, 95% CI: 0.83–0.94]), which were significantly different from AUCs for both variants of HNL (p < 0.001), ECP (p < 0.01) and EDN (p < 0.001). The AUCs of FC and MPO for differentiating UC and SC were lower (AUC 0.81, 95% CI: 0.74–0.87 and AUC 0.82, 95% CI: 0.75–0.87, respectively) and not significantly different from the other markers, which had an overall lower discriminating capacity between CD and UC versus SC (Figure 2, Table S3). Among the eosinophil markers, ECP (AUC 0.82, 95% CI: 0.74–0.88) performed better than EDN (AUC 0.63, 95% CI: 0.55–0.72) in distinguishing between patients with CD and SC (p < 0.001).
The discrimination between IBD and HC according to faecal markers is presented in Figure S2.
3.4 Diagnostic capability of multiple faecal markers to differentiate between IBD and controls
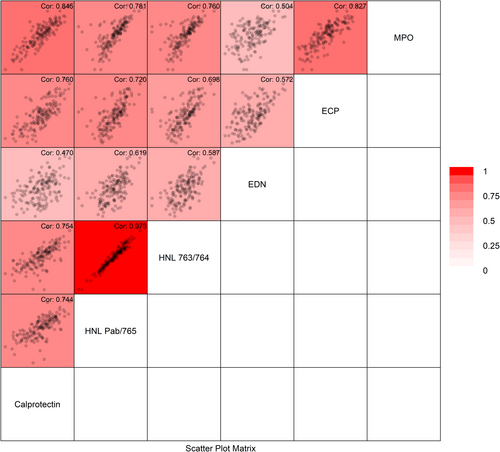
Overall, the faecal biomarkers showed strong-to-moderate internal correlations, as demonstrated in Figure 3. The strongest correlation, except for the HNL variants, was seen between FC and MPO (r = 0.85) and between MPO and ECP (r = 0.83), followed by MPO and HNL pAb/765 (r = 0.78). The diagnostic performance by a combination of markers was tested using logistic regression analysis, starting with FC and adding a second faecal marker. Adding MPO to FC yielded 9% new information and significantly improved the diagnostics (p = 0.02). On the contrary, adding HNL pAb/765, HNL 763/764, ECP or EDN to the FC model did not improve its diagnostic capability (Table 4).
| Faecal marker | C-index | 95% CI | Fraction new information | p-value |
|---|---|---|---|---|
| FC | 0.89 | 0.84–0.93 | ||
| MPO | 0.90 | 0.85–0.94 | 0.09 | 0.02 |
| HNL pAb/765 | 0.89 | 0.84–0.93 | 0.01 | 0.64 |
| HNL 763/764 | 0.89 | 0.84–0.93 | 0.01 | 0.74 |
| ECP | 0.89 | 0.84–0.93 | 0.03 | 0.33 |
| EDN | 0.89 | 0.84–0.93 | 0.00 | 0.85 |
3.5 Influence of clinical characteristics on disease course of IBD
A multivariate analysis was performed to estimate the influence of clinical characteristics on the clinical disease course of IBD. In the cohort, the risk of an aggressive disease course was lower in females (OR = 0.37, 0.17–0.80) compared with males, but higher in CD compared with UC (OR = 3.14, 1.46–6.76), and in extensive UC compared with ulcerative proctitis (OR = 7.47, 1.45–38.5). Smoking habits and age at diagnosis demonstrated no associations with the disease course.
3.6 Associations between levels of faecal markers at diagnosis and future disease course
Patients with an aggressive disease course had significantly higher levels of FC, MPO and both variants of HNL at diagnosis compared with patients with an indolent disease course (Table S4). Analyses by subtype of IBD revealed an elevation of all markers in patients with an aggressive course of UC compared with those with an indolent course (Table 5), whereas no differences were seen between patients with an aggressive and indolent course of CD (Table S5). Stratified analyses by location of CD (L1–L3) yielded similar findings (not shown).
| Aggressive course (n = 17) | Indolent course (n = 73) | p-value | |
|---|---|---|---|
| UC | |||
| Sex, female, n (%) | 4 (24) | 41 (56) | 0.03 |
| Age, median (range) | 34 (18–72) | 34 (18–81) | 0.57 |
| Smoking | [1] | [1] | >0.99/0.61/0.82 |
| Current/former/never | 1/5/10 | 6/30/36 | |
| UC, extent | |||
| E1/E2/E3 | 2/1/14 | 29/25/19 | 0.04/0.02/<0.001 |
| Faecal marker, Median (IQR; μg/g) | |||
| FC | 927 (440–2968) | 305 (86.5–967) | 0.02 |
| MPO | 340 (104–1469) | 68.6 (17.8–212) | 0.006 |
| HNL pAb/765 | 32.0 (11.0–52.0) | 8.10 (4.20–20.0) | 0.004 |
| HNL 763/765 | 38.0 (17.5–74.5) | 9.80 (5.30–22.7) | 0.002 |
| EDN | 11.0 (0.50–46.5) | 3.27 (1.17–8.61) | 0.05 |
| ECP | 34.0 (11.0–92.5) | 10.6 (2.55–26.7) | 0.02 |
- Note: [] indicates missing value.
3.7 The capability of faecal markers to differentiate patients by future disease course
To examine the capability of each marker to distinguish between a future aggressive or indolent disease course at diagnosis, ROC curves were established. The neutrophil markers FC, MPO and HNL demonstrated a moderate capacity for predicting future disease course in patients diagnosed with IBD, with significant AUCs for FC (0.64) and MPO (0.65) (both p < 0.01) (Table S6A). Numerically lower but statistically significant AUCs were observed for HNL pAb/765 (0.62) and HNL 763/764 (0.63) (both p < 0.05), whereas the corresponding estimated AUCs for ECP and EDN were not significant. After stratification by subtype of IBD, the models for future course of UC based on HNL 763/764 yielded an AUC of 0.74 (p < 0.01) followed by HNL pAb/765, MPO, FC and ECP with AUCs ranging from 0.69 to 0.72 (p between <0.05 and <0.01) (Table S6B). In contrast, in the corresponding models for CD, none of the markers yielded significant results.
The association of markers at inclusion and an aggressive IBD disease course (n = 44) was analysed by an alternative statistical method including adjustment for clinical characteristics. Levels of FC, MPO and HNL subtypes at inclusion were associated with an aggressive course, and the strongest association was seen for MPO (p = 0.006; Table 6). When analysing IBD subgroups separately, associations were seen only for patients with UC and not for CD (Table S7).
| Faecal marker IBD | n | χ 2 | df | p | p adj |
|---|---|---|---|---|---|
| FC | 152 | 8.47 | 2 | 0.014 | 0.028 |
| MPO | 153 | 11.8 | 2 | 0.003 | 0.006 |
| HNL pAb/765 | 152 | 9.11 | 2 | 0.011 | 0.023 |
| HNL 763/764 | 152 | 9.71 | 2 | 0.008 | 0.018 |
| ECP | 152 | 4.28 | 2 | 0.118 | 0.120 |
| EDN | 152 | 5.73 | 2 | 0.057 | 0.089 |
4 DISCUSSION
In this study, we performed a comprehensive analysis of faecal markers with different cell origins in an inception cohort of treatment naïve patients with IBD. Our results demonstrated that the neutrophil markers MPO and FC exhibited the highest diagnostic capability in differentiating patients with IBD from symptomatic non-IBD patients. Moreover, assessing the capacity of markers to differentiate patients with an aggressive disease course from those with an indolent course, we found that levels of MPO, FC and HNL at the time of diagnosis were associated with an aggressive disease course in patients with UC. However, the overall prognostic capacity of these markers alone must be considered as limited in a clinical context.
In IBD, innate immune cells such as neutrophil and eosinophil granulocytes contribute to the inflammatory process in the intestinal mucosa by the release of cytotoxic proteins, which can be measured in faeces and serve as biomarkers. Most of the previous studies examining faecal proteins from inflammatory cells have focused on FC. Tibble et al. demonstrated 100% sensitivity and 97% specificity using FC to discriminate CD from IBS,21 and multiple studies have confirmed FC to be a robust screening instrument for identifying patients who are likely to need an endoscopy for suspected IBD.22, 23 However, other faecal proteins remain to be evaluated, either as single markers or in combination with FC. In this study, we demonstrated that levels of FC, MPO, two variants of HNL, ECP and EDN at baseline were elevated in patients with IBD compared with HC, which is in line with previous findings.13, 24 However, the challenging part in clinical practice is not to separate IBD from healthy but to distinguish IBD from symptomatic non-IBD patients. With this in mind, we included a control group of patients who were referred for suspected IBD but were ultimately diagnosed with non-IBD gastrointestinal disorders. We found that MPO and FC exhibited a high diagnostic capability in differentiating patients with IBD from SC, whereas the capacity of HNL pAb/765, HNL 763/764, ECP and EDN was lower. Thus, faecal biomarkers may play an important role as a helping tool in clinical decision-making, as suggested by other investigators.25
The discriminative ability of FC in separating SC from IBD was comparable with previous studies, although the compositions of the control groups differed.22 The capacity of HNL in the discrimination of IBD from patients with IBS has previously been examined,11 as was ECP in a cohort of patients with IBD and a mix of healthy and SCs.14 However, corresponding data on MPO and EDN are scarce. MPO is a granule protein of neutrophils, but may also, to a minor extent, be released by monocytes, either as a phagosome or via degranulation.26 Our results highlight MPO as a diagnostic biomarker of IBD with a capacity comparable to FC. Like FC, the diagnostic performance of MPO was slightly better in CD than in UC. This finding is perhaps somewhat unexpected since neutrophils generally are described as the hallmark of inflammation in UC. However, no other studies have analysed this relationship in newly onset and treatment naïve IBD patients. The disease extension in patients with UC was equally distributed between E1 and E3, and it is plausible that the biomarkers would have performed better in UC if the assay had been conducted in a cohort with dominating extended colitis (E3).
HNL is released from activated neutrophils but also from epithelial cells, and recent data indicate that HNL may be a marker of low-grade inflammation.10 In the current study, two different antibody combinations directed towards different HNL epitopes were analysed: HNL pAb/765 and HNL 763/764, assessing HNL derived from both neutrophils and epithelial cells to varying degrees. When compared with more neutrophil-specific markers such as FC or MPO, these HNL variants would therefore also reflect the impact of epithelial cells. In our analysis, both variants of HNL showed slightly lower capacity in distinguishing IBD from SC than FC and MPO. One possible explanation for this finding is that HNL is released by intestinal epithelial cells in some non-IBD conditions, such as gastroenteritis27 and functional disorders with altered microbiota,28 which could have acted as a confounding factor.
ECP and EDN are granule proteins selectively released from activated eosinophils by piecemeal degranulation, cytolysis, classical or compound exocytosis upon specific stimulation.29 In the current study, ECP was superior to EDN in diagnostic accuracy and showed a more pronounced elevation in patients with IBD, especially in CD. In previous studies, EDN has been proven a stable biomarker correlating with IBD disease activity,12 useful for the evaluation of response to IBD treatment30 and prediction of flares in UC.31 The difference between the two eosinophil proteins found in this study may be attributed to their storage in different granule populations or to various degranulation mechanisms.29, 32 One interesting possibility is that ECP and EDN have different tasks and that in this cohort of patients with newly onset IBD, EDN is not as active as in patients with a longer disease duration. Instead, it is conceivable that EDN plays a role in chronic inflammation with fibrosis development and tissue repair, as has been suggested before.33
A combination of several biomarkers could potentially sharpen the precision of non-invasive IBD diagnostics. Recently, Gacesa et al. demonstrated an improved discrimination between IBD and IBS by combining FC with faecal human beta-defensin 2.34 We assessed the impact of a combination of markers in a diagnostic model of IBD and SCs by starting with FC, as it is currently the most established marker in clinical use, and adding a second marker. Although we demonstrated a considerable correlation between FC and MPO, combining these two markers yielded 9% new information and significantly improved diagnostics. This may indicate that the release of these two neutrophil markers is not completely overlapping and may differ between patients. In a clinical setting, where a borderline FC needs to be verified for IBD diagnostics, a combined test including dual marker analysis could be feasible. Combining FC with faecal HNL, ECP or EDN did by contrast not improve the diagnostic capacity of the model, suggesting that biomarkers of eosinophil and epithelial cell origin may not add further to the diagnostic accuracy of neutrophil markers.
When evaluating the diagnostic ability of biomarkers, the composition of control groups is critical. Many of the SCs in our study were referred for endoscopic evaluation as suspected cases of IBD, displaying elevated levels of FC assessed in primary care. This group comprised patients with inter alia microscopic colitis, infections and ischemic colitis; however, about half of them were patients with IBS. Previous research from other groups has shown increased levels of FC in IBS—especially in IBS with predominate diarrhoea (IBS-D)35 as well as in microscopic colitis.36 The slightly increased levels of neutrophil markers (FC, MPO and both variants of HNL) but not of eosinophil markers seen in SC compared with HC are reasonable bearing in mind the pre-endoscopic selection and the great heterogeneity of diagnoses represented.
An important aspect of precision medicine is the prediction of disease course, as this may facilitate early advanced treatment in patients at a high risk of severe disease to prevent further complications of IBD. Recently, a promising serum protein profile with potential prognostic properties has been identified and is under validation (NORDTREAT, Nordic IBD treatment strategy trial).37 Several other blood-based biomarkers38, 39 as well as clinical factors40, 41 have been proposed to be associated with prognosis, but so far no such tool has been implemented in clinical practice. Faecal markers have in several studies been demonstrated to function as predictive tools. FC seems to have the potential to predict relapse in both UC and CD,42-44 and ECP and EDN are predictive of flares in UC but not in CD.14, 31 Also, for the prediction of disease progression, an association between index levels of FC and ECP has been demonstrated.14, 45
We assessed the clinical course of patients with IBD and examined the capacity of the faecal markers to differentiate patients with an aggressive disease course from those with an indolent course. Our data indicate that high levels of the neutrophil markers HNL, MPO and FC, at the time of diagnosis, may be predictive of an aggressive disease course with MPO showing the strongest association. In line with this, Swaminathan et al. recently conducted two studies including a validation of the MPO assay, demonstrating MPO and FC to be predictive of a complicated IBD disease course.9, 46 However, an advantage of our study is that these results now have been confirmed in a cohort of active and treatment-naive patients. Subgroup analysis revealed significantly elevated biomarkers in patients with UC and aggressive course compared to those with indolent course, whereas no differences were seen in CD. The limited number of patients with CD may have contributed to impaired analysis, but it could also be true that these biomarkers are not associated with disease course in CD. Possibly other markers, reflecting fibrosis and remodelling events that normally occur in CD complications, would be more sensitive markers within the CD group. One may also argue that the observational time for developing complications in CD in our study is short. However, Yanai et al. recently reported that events defining a complicated disease course appear early, with a plateau about 6 months from CD diagnosis.47 Given the rather modest AUCs generated in our study, and in light of the recent failure of the PROFILE study48 to find predictive biomarkers for CD, there is still an unmet need for identifying predictive biomarkers in IBD.
The major strength of this study is the inception cohort of well-characterised newly onset patients with IBD. The treatment-naïve patients and the prospective design make this cohort highly suitable for diagnostic as well as prognostic studies. Another strength is the study design, which included symptomatic patients as a control group and made it possible to evaluate biomarkers in a real-world scenario. Furthermore, also reflecting real-world practice and considered as a strength, we used objective measurement based on clinical aspects to define the outcome of an aggressive disease course.18 Some limitations need to be emphasised. Due to the exploratory nature of this study, no validation of data has been executed. To confirm the results, biomarkers need to be re-analysed in a validation cohort. However, some of our findings, such as the prominent role of MPO in forecasting disease course, have been confirmed by recent studies reporting similar results.9 In addition, the limited number of included patients prevented us from further analysis of IBD subgroups in some respects.
In summary, we have explored the diagnostic potential of a number of neutrophil and eosinophil faecal markers in a cohort of newly onset and treatment-naïve patients with IBD. MPO exhibited a diagnostic capability comparable to FC, and a combination of these two markers further improved the diagnostic capacity. Interestingly, we noticed that the overall diagnostic capacity of markers differed between IBD subtypes. Elevated levels of neutrophil markers FC, MPO and HNL at diagnosis were predictive of an aggressive disease course at 12 months in UC, with MPO showing the strongest association. This study provides new insight into the diagnostic and prognostic capability of potential faecal biomarkers in IBD and suggests that MPO alone or in combination with FC may be an interesting alternative for future use.
AUTHOR CONTRIBUTIONS
Maria Ling Lundström: Data curation; investigation; methodology; software; writing – original draft; formal analysis; writing – review and editing. Christer Peterson: Investigation; methodology; software; writing – review and editing; formal analysis; visualization. Charlotte R. H. Hedin: Investigation; writing – review and editing. Daniel Bergemalm: Investigation; writing – review and editing. Maria Lampinen: Methodology; writing – original draft. Maria K. Magnusson: Investigation; writing – review and editing. Åsa V. Keita: Investigation; writing – review and editing. Robert Kruse: Data curation; writing – review and editing. Carl Mårten Lindqvist: Data curation; writing – review and editing. Dirk Repsilber: Data curation; writing – review and editing. Mauro D'Amato: Data curation; writing – review and editing. Henrik Hjortswang: Investigation; writing – review and editing. Hans Strid: Investigation; writing – review and editing. Johan D. Söderholm: Investigation; writing – review and editing. Lena Öhman: Investigation; writing – review and editing. Per Venge: Methodology; resources; writing – review and editing. Jonas Halfvarson: Conceptualization; funding acquisition; investigation; methodology; supervision; writing – original draft. Marie Carlson: Conceptualization; funding acquisition; investigation; methodology; resources; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
We thank Catarina Lövgren, Uppsala University Hospital, for her diligent work with sample collection and Malin Olsson, Linköping University Hospital, for supplementary data collection. We also acknowledge the skilful laboratory assistance of David Amcoff, Eugenia Kalliontzi and Ann-Katrin Eriksson, Uppsala University Hospital. Finally, we thank Johan Westerbergh at Uppsala Clinical Research Center, Uppsala, Sweden, for excellent statistical support.
Declaration of personal interests: CRHH has received speaker fees from Takeda, Ferring, AbbVie and Janssen and consultancy fees from Pfizer. She has acted as the local principal investigator for clinical trials for Janssen and GlaxoSmithKline. She has received project grants from Takeda and Tillotts. JH served as speaker and/or advisory board member for AbbVie, Aqilion, BMS, Celgene, Celltrion, Dr. Falk Pharma and the Falk Foundation, Eli Lilly, Ferring, Galapagos, Gilead, Hospira, Index Pharma, Janssen, MEDA, Medivir, Medtronic, MSD, Novartis, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, UCB and received grant support from Janssen, MSD and Takeda. MC has received speaker's fees from Vifor Pharma. She is the national PI for clinical trials for AstraZeneca. None of these activities have any relation to the present study.
FUNDING INFORMATION
This work was supported by the Swedish Foundation for Strategic Research (grant number RB13-016) (J.H.), Swedish Research Council (grant number 2020–02021) (J.H.), the Örebro University Hospital Research Foundation (grant numbers OLL-890291) (J.H.) and Medical Faculty, Uppsala University, Uppsala, Sweden M.C.
AUTHORSHIP
Guarantor of the article: Marie Carlson, MD, PhD.
Open Research
DATA AVAILABILITY STATEMENT
The data set underlying this article cannot be shared directly under current legislation for data protection and must be requested directly from the respective holders, after approval by the Swedish Ethical Review Authority.




