Therapeutic drug monitoring of methotrexate in patients with Crohn's disease
Gerd Bouma and Maja Bulatović Ćalasan shared last authorship.
Preliminary data has been published on the European Crohn and Colitis Organisation Congress 2023 (Abstract number: P384).
The Handling Editor for this article was Professor Cynthia Seow, and it was accepted for publication after full peer-review.
Summary
Background
Therapeutic drug monitoring (TDM) has the potential to improve efficacy and diminish side effects. Measuring methotrexate-polyglutamate (MTX-PG) in erythrocytes might enable TDM for methotrexate in patients with Crohn's disease (CD).
Aim
To investigate the relationship between MTX-PGs and methotrexate drug survival, efficacy and toxicity
Methods
In a multicentre prospective cohort study, patients with CD starting subcutaneous methotrexate without biologics were included and followed for 12 months. Primary outcome was subcutaneous methotrexate discontinuation or requirement for step-up therapy. Secondary outcomes included faecal calprotectin (FCP), Harvey Bradshaw Index (HBI), hepatotoxicity and gastrointestinal intolerance. Erythrocyte MTX-PGs were analysed at weeks 8, 12, 24 and 52 or upon treatment discontinuation.
Results
We included 80 patients with CD (mean age 55 ± 13y, 35% male) with a median FCP of 268 μg/g (IQR 73–480). After the 12-month visit, 21 patients (26%) were still on subcutaneous methotrexate monotherapy. Twenty-one patients stopped because of disease activity, 29 because of toxicity, and four for both reasons. Five patients ended study participation or stopped methotrexate for another reason. A higher MTX-PG3 concentration was associated with a higher rate of methotrexate drug survival (HR 0.86, 95% CI 0.75–0.99), lower FCP (β −3.7, SE 1.3, p < 0.01) and with biochemical response (FCP ≤250 if baseline >250 μg/g; OR 1.1, 95% CI 1.0–1.3). Higher MTX-PGs were associated with less gastrointestinal intolerance. There was no robust association between MTX-PGs and HBI or hepatotoxicity.
Conclusions
Higher MTX-PG3 concentrations are related to better methotrexate drug survival and decreased FCP levels. Therefore, MTX-PG3 could be used for TDM if a target concentration can be established.
1 INTRODUCTION
Methotrexate (MTX) intramuscularly (IM) or subcutaneously (SC) is effective as maintenance therapy in patients with mild to moderate–severe Crohn's disease (CD)1, 2 The efficacy of MTX monotherapy for remission induction is not clearly established and high-quality studies are lacking.2, 3 In clinical practice, MTX is occasionally used for induction of remission in mild CD, alone or combined with prednisolone or budesonide.4 For maintenance treatment, the choice between MTX and thiopurines depends on careful consideration of the patient's preferences, clinical characteristics and safety profile.5 There are no biomarkers available to predict the response in order to aid drug selection or optimise MTX therapy.6, 7
Therapeutic drug monitoring (TDM) and patient-tailored dosing have the potential to improve efficacy and diminish adverse drug reactions.8 Intracellular methotrexate polyglutamates (MTX-PGs) are potential candidates for TDM since they are related to MTX efficacy in different immune-mediated inflammatory diseases (IMIDs).9 MTX-PG1-7 are formed by the enzyme folylpolyglutamate synthetase (FPGS), which catalyses the addition of up to seven glutamate residues to MTX intracellularly within 24 hours after administration.10 These MTX-PGs are potent inhibitors of key enzymes in folate metabolism and are thought to be responsible for the anti-inflammatory effects of MTX.11 Intracellular MTX-PGs in erythrocytes can be measured using mass spectrometry.12
Prior to implementing erythrocyte MTX-PGs as a TDM tool, at least two criteria should be assessed: (1) interindividual pharmacokinetic (PK) variability and (2) pharmacodynamic (PD) relationships.13 We have already demonstrated substantial interindividual variability of MTX-PG concentrations in patients with CD.14 The most important TDM criterion, the concentration–effect (PD) relationship, have been demonstrated in various IMIDs: higher concentrations of erythrocyte MTX-PGs are associated with lower disease activity in rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) and psoriasis.9 Data in patients with CD are lacking.9 Moreover, no clear relationship between erythrocyte MTX-PG levels and toxicity across IMIDs has been established.9 Therefore, we aimed to assess the relationship between erythrocyte MTX-PG concentrations and drug survival, efficacy and toxicity in a prospective cohort of patients with CD.
2 METHODS
2.1 Study design and patients
We performed a prospective longitudinal cohort study (“MTX-PG//CD”) in two university hospitals (Amsterdam University Medical Center (UMC), UMC Utrecht) and eight general hospitals (Sint Antonius Hospital, Hospital Gelderse Vallei, NoordWest Hospital Group, Elisabeth-Tweesteden Hospital, Spaarne Gasthuis, OLVG Hospital, Jeroen Bosch Hospital, Bravis Hospital) in The Netherlands representing a mixed population of gastroenterology patients. Patients ≥18 years old with an established diagnosis of CD starting MTX SC in routine care without concomitant biological treatment were eligible to participate. Patients were administered MTX as a primary treatment option or subsequent to the failure of prior therapies. The decision to start MTX therapy was at the discretion of the treating physician. All patients received an MTX dose (SC) of 25 mg/week with the option to taper to 15 mg/week (SC) after 8 weeks of treatment, with concurrent folic acid supplementation (5 mg, orally, once a week, 48 h after MTX administration).
We calculated the sample size based on a mean MTX-PG3 concentration (the most abundant PG species in erythrocytes) of 55 nmol/L with a standard deviation (SD) of 20 nmol/L. This was derived from data in our previous RA cohort study in which patients used 25 mg MTX/week orally.15 Although our CD patients used MTX SC, we anticipated based on our experience a minimal 15 nmol/L difference in levels between MTX responders and non-responders. We calculated the standardised difference to be 15/20 = 0.75. Based on the Altman nomogram, a calculated standardised difference of 0.75, a set power of 80% and significance level of 0.05, 55 patients were estimated to be needed.16 As we expected that our data would be non-normally distributed, with more MTX non-responders then responders, we decided to include 80 patients.
Ethical approval for this study was obtained from the ethics committees of the participating medical centres and conducted according to good clinical practice guidelines. All patients gave their informed consent before inclusion in the study.
2.2 Data collection
Prior to the start of MTX (baseline), at weeks 8, 12, 24 and 52 and when MTX SC monotherapy was stopped between the visits, we collected clinical data using electronic case report forms (Castor EDC, version 2019.1), filled out by the patient and treating physician. Clinical data included the Harvey Bradshaw Index (HBI), hospital admissions, IBD co-medication, adverse events (AE's), drug adherence (patients' self-reported by use of a Visual Analogue Scale17) and the Inflammatory Bowel Disease Questionnaire (IBDQ; only at baseline, week 24 and 52).18 Blood and faecal samples were collected at the same time points. As this was a real-world study, clinical and biochemical data were not always collected at the exact pre-defined time point. Therefore, time periods used for analyses were defined as the median week of actual data collection, along with the minimum and maximum week of data collection; for example week 8 (weeks 4–10), week 12 (weeks 10–14), week 25 (weeks 14–28) and week 51 (weeks 28–61).
2.3 Laboratory measurements
Blood (EDTA tubes) was drawn at the indicated time points, cooled on ice and centrifuged for 10 min at 1700g at 4°C.15 Whole blood, plasma and cell-pellet aliquots were stored at −80°C. Baseline cotinine was measured by a laboratory-developed test using LC–MS/MS. Non-detectable concentrations (below the limit of quantification, <5 μg/L) were imputed by half the limit of quantification (2.5 μg/L). Patients with levels >10 μg/L were considered smokers.19 Plasma levels of vitamin B12 and folate, and erythrocyte folate (corrected for haematocrit and plasma folate) were measured at baseline using routine electrochemiluminescence on a Roche Cobas platform (Roche).
Erythrocyte MTX-PGs were assessed from pellets collected at follow-up visits, employing ultra-high-performance liquid chromatography-electrospray ionisation-tandem mass spectrometry (UPLC-ESI-MS/MS), using stable-isotope-labelled internal standards, as described by Den Boer et al.12 If cell-pellet aliquots were unavailable, MTX-PGs were measured in whole blood with correction for haematocrit (7.5% of all samples, which showed comparable results with the erythrocyte measurements). Concentrations of MTX-PGs were reported in nmol/L packed erythrocytes. MTX-PGtotal is the sum of the five quantified species of MTX-PG (MTX-PGn). MTX-PG concentrations measured 4 weeks after the study endpoint were excluded from analysis.
Routine laboratory analysis, including standard biochemistry and haematology tests and faecal calprotectin (FCP), was measured at the local laboratory of the participating centres. Baseline FCP was measured at the earliest 4 weeks before and not after starting MTX.
2.4 Outcomes and definitions
The primary outcome was the duration of SC MTX monotherapy (drug survival). This was defined as the cumulative incidence of SC MTX monotherapy discontinuation during the first year due to toxicity and/or treatment failure (active disease). Reasons for discontinuation could be (1) switch of MTX to a different agent, (2) switch to oral MTX, as all patients needed to use the same route of MTX administration for proper PK/PD analyses and (3) step-up strategies: addition of systemic corticosteroids, start of a biologic drug and/or CD-related surgery. Topically applied corticosteroids (enema, suppository or oral budesonide) or dose escalation of MTX (from 15 mg/week back to 25 mg/week) were not classified as step-up therapy.
Secondary outcomes were efficacy and toxicity. Efficacy was defined as clinical remission (HBI ≤4), clinical response (decrease of HBI ≥3 compared to baseline), biochemical remission (FCP ≤250 μg/g) and biochemical response (FCP level ≤250 μg/g if baseline FCP was above 250 μg/g).20 Toxicity was defined as hepatotoxicity, gastrointestinal (GI) intolerance and other AE's. We defined hepatotoxicity as an increase of aspartate transferase or alanine transferase above 1.5 times the upper limit of normal. We assessed GI intolerance with the MTX Intolerance Severity Score (MISS).20 This questionnaire includes abdominal pain, nausea, vomiting and behavioural symptoms (amongst others, irritability) after MTX administration as well as anticipatory and associative complaints before and when thinking of MTX administration. GI intolerance was defined as a MISS ≥6, including at least one anticipatory or associative symptom of abdominal pain, nausea and/or vomiting, or one behavioural symptom.21 Additionally, we recorded other AE's as reported by the treating physician.
2.5 Statistical analysis
Descriptive statistics for demographic data were reported using means (±SD), medians (IQR) or percentages and analysed with the chi-square, Fisher's exact, Mann–Whitney-U or t-test, depending on the (non-)parametric distribution and type of variables. A two-sided p-value <0.05 was considered statistically significant. All analyses were performed using R statistical software.22 The relationship of the MTX-PG with the outcome was analysed separately for MTX-PGtotal, MTX-PG3-5 and each MTX-PG species in all analyses. MTX survival was assessed using Kaplan–Meier analyses. Censored cases were patients who discontinued MTX for undefined reasons or stopped study participation.
To determine the relationship of MTX-PGs with MTX survival, extended Cox regression analysis with MTX-PGs as time-varying covariates was employed (R packages survival, survminer). In this specific analysis, MTX-PG measurements were included up to 1 week after the study endpoint and missing MTX-PGs were imputed by informative imputation (using the last observed value for the missing value). In the simple model, analyses were corrected for prednisone/budesonide at start and budesonide use during follow-up (binary time-varying covariate). In the extensive model, we corrected for all predictors of the survival outcome as well. Predictors of MTX survival were the baseline variables with a p-value ≤0.1 on a univariate Cox regression analysis. The proportional hazards assumption was assessed for each variable and not violated. Area under the curves (AUCs) with bootstrapped 95% confidence interval (CI) were calculated for significant MTX-PGs to assess diagnostic accuracy. Discriminative MTX-PG concentrations (at week 8 or 12) for MTX drug survival in the first year were defined by recursive partitioning (R package rpart).
To assess the longitudinal relationships between MTX-PGs and efficacy/toxicity measures, we conducted linear mixed-model (LMM) analyses for continuous outcomes and generalised estimation equations (GEE) for dichotomous outcomes. In LMM, all variables were fixed, with time*MTX-PG as an interaction term, with a random intercept and maximum likelihood method. In GEE analysis, we used an exchangeable correlation structure. We corrected for the baseline values of respective outcomes (i.e. baseline HBI or FCP) in the “simple model” and for multiple variables in the “extensive model”. Multivariable analyses were performed using back- and forward-stepwise regression on variables with p ≤ 0.1 on univariate analysis.
Optimal target MTX-PG concentrations for disease activity measures at weeks 8 and 12 were determined by constructing receiver operating characteristic (ROC) curves with their respective AUCs. Target concentrations were determined by the Youden criteria (max(sensitivity + specificity − 1)) and Closest-top-left criteria (min((1 − sensitivity)2 + (1 − specificity)2)).
3 RESULTS
3.1 Cohort characteristics
3.1.1 Baseline characteristics and dosing
Eighty patients were included. Baseline characteristics are depicted in Table 1. The indication for starting MTX therapy was active disease in the vast majority of patients (72/80). The remaining patients were in remission and started MTX therapy because of side effects or a relative contraindication for the current therapy (thiopurines). Sixty out of 80 patients had used thiopurines and/or biologics previously.
| All patients (n = 80) | |
|---|---|
| Age in years, mean ± SD | 55.1 ± 12.5 |
| Male gender, n (%) | 28 (35) |
| BMI in kg/m2, mean ± SD | 26.2 ± 4.9 |
| Smoker, n (%) | |
| Yes | 30 (38) |
| No | 50 (63) |
| eGFR in mL/min/1.73/m2, mean ± SD | 97.4 ± 14.6 |
| Duration of CD in years, median (IQR) | 3.6 (0.6–11.1) |
| Age at diagnosis, n (%) | |
| <17 years | 3 (4) |
| 17–40 years | 25 (31) |
| >40 years | 52 (65) |
| CD disease location, n (%) | |
| Ileum | 38 (48) |
| Colon | 16 (20) |
| Ileum and colon | 24 (30) |
| Upper GI only | 1 (1) |
| Upper GI and colon | 1 (1) |
| Disease behaviour, n (%) | |
| Inflammatory disease | 52 (65) |
| Stricturing disease | 28 (35) |
| Penetrating disease | 3 (4) |
| Peri-anal disease, n (%) | 7 (9) |
| Prior intestinal resections, n (%) | 26 (33) |
| Prior peri-anal interventions, n (%) | 5 (6) |
| Enterostomy, n (%) | 3 (4) |
| Prior biological usea, n (%) | 23 (29) |
| Prior thiopurine useb, n (%) | 57 (71) |
| Concomitant medication, n (%) | |
| Prednisone | 8 (10) |
| Budesonide | 22 (28) |
| Mesalazine | 2 (3) |
- a All 23 patients used anti-TNF-α therapy, 5 of them ustekinumab as well, and 4 of them vedolizumab as well.
- b Three of the thiopurine naïve patients had received biological treatment before.
Treatment was initiated with SC MTX 25 mg/week, with the exception of two patients who received 15 mg/week. During follow-up, 56 patients switched from 25 mg/week to 15 mg/week, of whom 18 patients switched before week 8. Twenty-two patients used 25 mg/week during the whole study period. Only one patient switched back from 15 mg/week to 25 mg/week (at week 26). There was a 100% adherence to MTX in at least 75% of the patients. Nonadherence (i.e. less than 80% of the prescribed MTX administrations) was observed in one to three patients per time period.
3.1.2 MTX drug survival
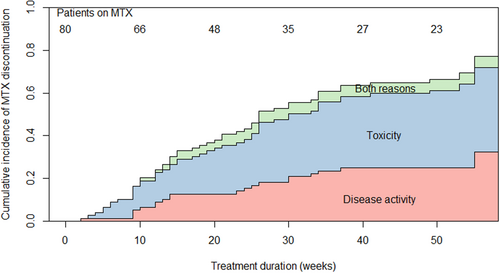
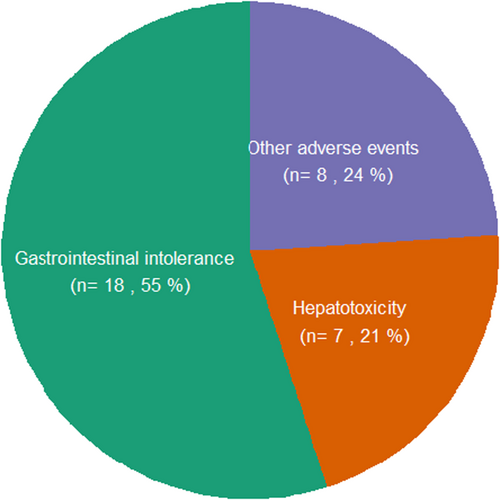
At the 12 months' visit, 25 patients were still using MTX. Twenty-one patients (26%) continued MTX SC monotherapy beyond this first year (Figure S1). The probability of MTX survival declined during follow-up from 76% at week 12, to 57% at week 24 and 34% at week 52. The cumulative incidence of MTX discontinuation over time was equal for both ‘failure’ and ‘toxicity’ causes (Figure 1). Twenty-one (26%) patients discontinued MTX monotherapy because of active disease (17 patients switched to other therapy, four patients started additional step-up therapy). Twenty-nine (36%) patients discontinued SC MTX because of toxicity (of whom one patient switched to oral MTX) and four (5%) patients stopped because of a combination of active disease and toxicity. Five patients were censored: four patients ended study participation and one patient stopped MTX for unknown reasons. Eighteen of 33 (55%) patients who stopped MTX because of (amongst others) toxicity did so because of GI complaints (Figure 2).
3.1.3 Disease activity
At baseline, the median HBI was 4 (IQR 3–7) and 49% of the patients had active disease (HBI >4) (Table S1). During the first year, the HBI decreased to a median of 1 (IQR 0–3.5) in patients still on MTX therapy. At baseline, the median FCP was 268 μg/g (IQR 73–480). Fifty-three per cent of the patients had biochemical active disease (FCP >250) at baseline, which declined to 9% (n = 2) at the last visit. Forty-two per cent of patients had a decreased quality of life (defined as IBDQ <168)23 at baseline, which was the case for only 16% of MTX-treated patients at the end of follow-up.
3.1.4 Adverse events and hospitalisation
GI intolerance was the most prevalent AE followed by hepatotoxicity (Table 2). The prevalence of GI toxicity did not change over the first year of MTX treatment and ranged between 25 and 28% at all time points. Significant baseline predictors of GI intolerance during follow-up were younger age (OR per 10 years 0.60, 95% CI 0.40–0.91), female sex (OR 3.3, 95% CI 1.1–10.0) and no alcohol use (OR 4.4, 95% CI 1.5–12.8). The MTX dose was not related to the occurrence of GI intolerance or hepatotoxicity, probably due to a low variation in dosing in our cohort. In addition, non-alcoholic fatty liver disease (n = 5) or the FIB4-score24 at baseline (70 patients <1.45, 9 patients 1.45–3.25, 1 patient 3.6) were not related to hepatotoxicity during follow-up or to hepatotoxicity as a reason for MTX discontinuation.
| Number of patients | |
|---|---|
| Gastro-intestinal intolerance (%) | 34 (42.5) |
| Hepatotoxicity (%) | 27 (33.8) |
| Other adverse events | |
| Infectionsa | 10 |
| Malaise and tiredness | 12 |
| Arthralgia, muscle pain and/or tendinopathy | 6 |
| Oral complaintsb | 6 |
| Alopecia | 4 |
| Injection pain and/or local skin reactions | 4 |
| Miscellaneousc | 18 |
- a Infections requiring antibiotics, antiviral medication or hospitalisation.
- b Aphtous ulceration, gingiva complaints and/or burning mouth sensation.
- c Globus sensation, vaginal bleeding, increased weight, dysgeusia, onchy-atrophia, cardiac events, skin complaints outside the injection site, edema, dyspnea, burnings eyes sensations and malignancy.
During the course of the study, four patients were hospitalised: one because of myocardial infarction and three because of disease activity, of whom one patient was admitted to the ICU because of E. coli sepsis, attributed to an underlying severe ileocolitis.
One patient was diagnosed with lung cancer and cerebral metastasis at week 12 and hence withdrawn from the study. Another patient was diagnosed with lung cancer after 12 months of MTX use. One patient died due to an out-of-hospital cardiac event.
3.1.5 Erythrocyte MTX-PG concentrations
A total of 213 MTX-PG concentrations were measured. MTX-PG measurements were not obtained in three patients (two patients stopped using MTX due to toxicity, one patient ceased study participation, and all three within 8 weeks). Amongst the different MTX-PG species, MTX-PG3 was the most abundant (Table 3), accounting for 33% of MTX-PGtotal at week 12.
| Week 8 (4–10) | Week 12 (10–14) | Week 25 (14–28) | Week 51 (28–61) | |
|---|---|---|---|---|
| PG 1 | 36 (26–57) | 36 (25–52) | 36 (20–44) | 41 (27–58) |
| PG 2 | 20 (14–22) | 20 (15–25) | 21 (13–26) | 24 (17–27) |
| PG 3 | 38 (33–51) | 51 (38–65) | 56 (38–66) | 63 (42–81) |
| PG 4 | 26 (18–34) | 29 (19–50) | 24 (16–38) | 26 (16–42) |
| PG 5 | 11 (7–16) | 12 (7–18) | 10 (4–18) | 7 (4–12) |
| PG total | 135 (112–194) | 146 (117–216) | 140 (106–188) | 160 (122–235) |
| n | 67 | 63 | 44 | 26 |
- Note: MTX-PG concentrations in nmol/L: median with interquartile range (first quartile–third quartile). Time periods: median (minimum – maximum) week of MTX-PG measurement. n = number of measurements included in time period. Thirteen measurements excluded as those were measured >4 weeks after MTX SC monotherapy discontinuation.
3.2 Pharmacodynamics
3.2.1 MTX-PG concentrations and drug survival
A higher MTX-PG3 concentration was associated with a higher MTX survival rate (Table 4). For every 10 nmol/L increase in the MTX-PG3 concentration, there was a 14% decrease in MTX discontinuation during the first year of MTX use. The AUC of MTX-PG3 was 0.71 [95% CI 0.66–0.81], showing good accuracy of the relationship between MTX-PG3 and MTX drug survival. The remaining MTX-PG species and MTX-PGtotal were not significantly related to MTX drug survival (Table S2).
| HR [95% CI] | p | AUC [95% CI] | |
|---|---|---|---|
| Simple modela | 0.86 [0.75–0.99] | 0.04 | 0.59 [0.52–0.70] |
| Extensive modelb | 0.84 [0.71–0.98] | 0.03 | 0.71 [0.66–0.81] |
- a Adjusted for usage of prednisone (at start) and budesonide (at start and/or during follow-up).
- b Adjusted for gender, alcohol use, eGFR, HBI and vitamin B12 at baseline plus usage of prednisone (at start) and budesonide (at start and/or during follow-up).
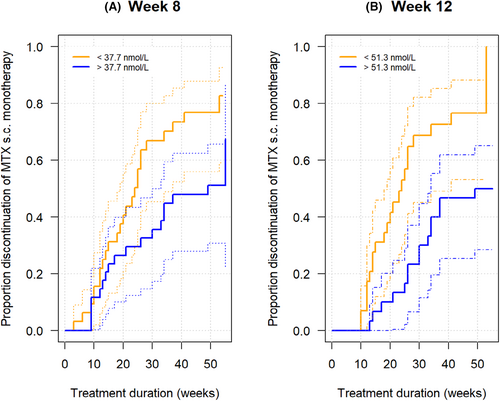
Moreover, MTX-PG3 concentrations measured at week 8 and at week 12 related to MTX drug survival during the first year of MTX use: an MTX-PG3 concentration above 37.7 nmol/L at week 8 and 51.3 nmol/L at week 12 were discriminative for MTX drug survival over the rest of the year (Figure 3).
3.2.2 MTX-PG concentrations and efficacy
Higher MTX-PG3,4,and 5 concentrations were associated with a lower FCP at both weeks 12, 25 and 51. At week 25 this association reached statistical significance (Table 5). For MTX-PG3 specifically, FCP decreased by 4 μg/g for every 1 nmol/L increase in MTX-PG3 (p < 0.01). In addition, higher MTX-PG1,3, and total concentrations were associated with biochemical response at week 12 (Table S3). For MTX-PG3 specifically, the odds for biochemical response increased by 1.13 (95% CI 1.02–1.27) for every 10 nmol/L increase in MTX-PG3.
| Week 8 (4–10) | Week 12 (10–14) | Week 25 (14–28) | Week 51 (28–61) | |
|---|---|---|---|---|
| PG 1 | −0.12 [0.27] | 0.14 [0.41] | −0.05 [0.53] | 0.27 [0.72] |
| p = 0.65 | p = 0.73 | p = 0.93 | p = 0.71 | |
| PG 2 | −1.68 [3.16] | −1.83 [2.45] | −2.24 [3.21] | −0.45 [1.66] |
| p = 0.60 | p = 0.46 | p = 0.49 | p = 0.79 | |
| PG 3 | 3.01 [2.02] | −0.67 [0.84] | −3.72 [1.28] | −0.89 [1.18] |
| p = 0.14 | p = 0.43 | p < 0.01 | p = 0.46 | |
| PG 4 | 1.19 [1.79] | −0.58 [0.95] | −3.98 [1.55] | −0.39 [2.37] |
| p = 0.51 | p = 0.55 | p = 0.01 | p = 0.87 | |
| PG 5 | −1.15 [2.84] | −1.48 [2.14] | −6.71 [2.71] | −0.98 [5.07] |
| p = 0.69 | p = 0.49 | p = 0.02 | p = 0.85 | |
| PG 345 | 0.57 [0.78] | −0.28 [0.39] | −1.66 [0.59] | −0.36 [0.74] |
| p = 0.47 | p = 0.47 | p = 0.01 | p = 0.62 | |
| PG total | −0.06 [0.24] | −0.06 [0.22] | −0.55 [0.33] | −0.01 [0.35] |
| p = 0.81 | p = 0.76 | p = 0.09 | p = 0.99 |
- Note: Beta [standard error] and p-value of MTX-PG (per 1 nmol/L) related to FCP in a linear mixed model with baseline FCP as covariate (simple model). Comparable results in the extensive model with correction for baseline FCP, sex, disease location and prednisone use at baseline (data not shown). Bold values are significant.
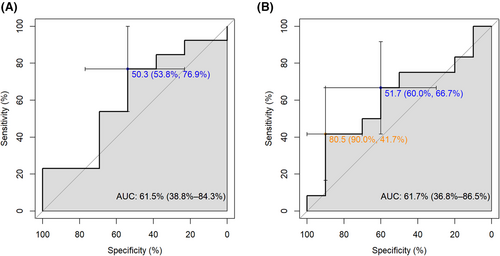
The target concentration of MTX-PG3 for biochemical response in the first 3 months was calculated based on the ROC curve presented in Figure 4. The sensitivity and specificity of the proposed target concentrations displayed large confidence intervals as our study was underpowered for this analysis. Taking these uncertainties into account, the target MTX-PG3 concentration for biochemical response with a sensitivity >0.5 and specificity >0.6 was estimated at 45 nmol/L at week 8 and 51 nmol/L at week 12. The proposed target concentrations of MTX-PG3 for biochemical response were reached by 38% (week 8) and 51% (week 12) of the patients.
No significant relation between MTX-PG and HBI was found (Table S4).
3.2.3 MTX-PG concentrations and hepatotoxicity
Next, we determined if MTX-PG levels were associated with liver toxicity. Overall, no clear association was found. For MTX-PG2 and MTX-PG345 some associations were found: higher levels were significantly associated with hepatotoxicity at week 25 and 51 respectively in a multivariable analysis (OR per 5 nmol/L MTX-PG2 week 25: 2.2 [95% CI: 1.2–3.9], OR per 1 nmol/L MTX-PG345 at week 51: 3.0 [95% CI 1.2–7.7], Table S5). Hepatotoxicity occurred in 7% of the patients with the lowest MTX-PG2 concentrations (the first quartile with concentrations of 5.7–15.2 nmol/L) compared to 24% in patients with the highest MTX-PG2 concentrations (the fourth quartile (>25.1 nmol/L), p = 0.03). This trend was not observed for MTX-PG345. However, we were unable to detect a decrease in MTX-PG2 or MTX-PG345 concentrations in patients in whom transaminases normalised or a continuously high MTX-PG2 or MTX-PG345 concentration in patients with ongoing hepatotoxicity.
3.2.4 MTX-PG concentrations and GI intolerance
Higher MTX-PGs were associated with less GI complaints. Specifically, a higher MTX-PG3 concentration was associated with less GI intolerance (OR per 10 nmol/L at week 51: 0.65 [0.50–0.84], p < 0.01). This was also observed for the other MTX-PG species, but did not yield significance at all time points during follow-up (Table S6). In line with this observation, MTX-PG3, MTX-PG345 and MTX-PGtotal concentration were higher in GI tolerant patients compared to intolerant patients (Table 6).
| Time | GI tolerant | GI intolerant | p | |
|---|---|---|---|---|
| Week 12 (10–14) | n | 44 | 16 | |
| MTX-PG3 | 56 (41–74) | 43 (36–53) | 0.06 | |
| MTX-PG345 | 97 (77–148) | 83 (52–109) | 0.10 | |
| MTX-PGtotal | 161 (128–200) | 122 (99–150) | 0.01 | |
| Week 51 (28–61) | n | 19 | 4 | |
| MTX-PG3 | 64 (50–98) | 35 (28–44) | 0.01 | |
| MTX-PG345 | 116 (71–152) | 54 (46–65) | 0.03 | |
| MTX-PGtotal | 166 (151–254) | 91 (75–118) | 0.01 |
- Note: MTX-PG concentrations (nmol/L) are medians with interquartile range (first quartile–third quartile). Other MTX-PGs or other time points not significant (not shown).
4 DISCUSSION
In this prospective cohort study, we set out to determine the relationship between MTX-PGs levels and clinical outcomes in CD. We observed a high discontinuation rate in CD patients (75% after failing previous therapy) in whom MTX monotherapy was initiated: only one quarter continued MTX beyond 1 year. A higher erythrocyte MTX-PG3 concentration was associated with better MTX drug survival and efficacy and lower rates of GI toxicity. These concentration-effect relationships suggest that MTX-PG3 holds potential for TDM in CD. We demonstrated that a 10 nmol/L increase in MTX-PG3 resulted in 14% reduction in the rate of discontinuation in the first year (AUC 0.71). Also, a higher MTX-PG3 was associated with a decrease in FCP and higher chance of biochemical response.
Our findings in this CD cohort are in line with findings in other IMIDs showing a concentration-effect relationship between MTX-PG3 and efficacy.13 However, prior to implementation of erythrocyte MTX-PG3 as a TDM tool, a target concentration for efficacy should be defined. Using biochemical response as outcome, we estimated the optimal effective threshold concentration of 50 nmol/L at week 12. A similar discriminative concentration was observed for drug survival at week 12. This is somewhat higher than established in RA patients (32 nmol/L),15 which could be due to lower dosing, especially in the first weeks of RA treatment. Of note, our cohort was underpowered (power = 0.15) to find a target concentration for biochemical response, resulting in a wide confidence interval and a lower AUC (0.55) compared to that of MTX drug-survival (AUC 0.71). Validation in a larger cohort of an estimated 300 patients is warranted to confirm the optimal target concentrations of MTX-PG3.
Our study shows that the current dosing regimen is not well maintained in patients with mild to moderate CD, with only 34% still using MTX at week 51. This is in line with data from two recent retrospective cohorts of CD patients, which reported MTX drug-survival rates of 50% (week 44) and 22% (week 104) on MTX monotherapy.25, 26 However, our MTX drug-survival rate is lower compared to a nationwide cohort of Dutch CD patients starting MTX monotherapy after thiopurine therapy (63% 1 year, 49% 2 years).27 Higher MTX discontinuation rate could be explained by the option to initiate thiopurine in 23 thiopurine-naïve patients in our cohort as well as by the changes in CD treatment over time: reduced tolerance for side effects, increased accessibility to biologics and ongoing development of new drugs, leading to early therapy switches.
Twenty-six per cent discontinued MTX because of disease activity. To improve MTX drug-survival in patients with active disease, higher concentrations of erythrocyte MTX-PG3 should be achieved. In those patients with low MTX-PG3 levels (mostly younger patients in our cohort (data not shown)) despite treatment with a maximal dosage of 25 mg/week MTX SC, alternative approaches to MTX administration are currently being explored. These include daily rather than weekly dosing schemes (5 days per week, divided weekly dose),28 pH-sensitive and colon-specific nanoparticles loaded with MTX to target the gut specifically,29 and addition of PK enhancers to MTX, such as efflux transporter blockers (MTX Forte, Amplio Pharma, Sweden). Further research is necessary to evaluate the added value of these new MTX administration strategies.
In our cohort, toxicity was the main reason for low MTX drug-survival rates as 36% of patients stopped MTX because of AEs. Published discontinuation rates due to AEs are highly variable (0–30%)30 although two recent retrospective CD cohorts found rates comparable to our cohort of approximately 40%.25, 26 GI intolerance was the most reported AE in our cohort. GI intolerance was reported by patients instead of physicians which may explain the high number of patients detected with GI intolerance, as normally, physicians report and tend to underreport these complaints.31 Other AEs, such as hepatotoxicity, infections and malaise (Table 2) occurred as well during follow-up, leading to a quite high number of patients with significant side effects.
Studies so far have not found a relationship between MTX-PG concentrations and GI intolerance, with the exception of one cross-sectional study.9, 32 We did observe that higher MTX-PG levels were associated with a lower incidence of GI intolerance. This remarkable observation is unexplained and needs further research. Weekly recurring complaints of nausea around MTX administration cannot rationally be explained by continuously high intracellular PG levels but more likely by transiently high MTX plasma concentrations. This is underscored by the observation that higher systemic plasma exposure, due to specific single nucleotide polymorphisms (SNPs), as well as SC administration, and in turn higher systemic plasma exposure, are associated with an increased likelihood of MTX-induced nausea.33, 34 A possible explanation for the unexpected inverse relationship between MTX-PGs and GI intolerance may be attributed to intracellular folate metabolism (confounder). Folate and MTX utilise similar cellular receptors and enzymes for transport and glutamylation. Consequently, patients with highly active intracellular folate metabolism exhibit increased MTX-PG levels due to enhanced MTX conversion.35 Additionally, folate supplementation effectively mitigates GI intolerance associated with MTX therapy.36 In that line, heightened intracellular folate metabolism may offer a protective effect against GI intolerance (as well as it leads to higher MTX-PG concentrations).
MTX is known to cause hepatotoxicity.37 In our study, hepatotoxicity incidence was high (38% transient) compared to RA patients (18%).38 A meta-analysis confirmed higher prevalence of MTX-related liver injury in IBD patients, compared to RA and psoriasis patients.39 We expected that long-chain MTX-PGs, with longer cellular retention, would be most harmful. Indeed, we found that the MTX-PG345 (as well as MTX-PG2) concentration was associated with hepatotoxicity. Caution is needed in interpreting this potentially false positive finding as those relationships could not be confirmed in additional analyses (for example no decrease in MTX-PGs when transaminases normalised).
This study has several strengths. First, it is the largest prospective cohort of CD patients starting MTX SC without biologicals, thus far. Previous studies on MTX-PG in CD patients were underpowered (maximum 21 patients), included both patients with ulcerative colitis and CD, did not exclude patients on biological co-treatment and often had a cross-sectional design.32, 40-43 Second, our cohort yielded valuable real-life data on the performance of MTX in patients with CD. Real-world data are important because they represent the general CD population and take clinical decision-making into account.44 Third, we used a validated mass-spectrometry method for erythrocyte MTX-PG measurements with a stable isotope as an internal standard. Fourth, we collected various disease outcome parameters to analyse the relationship between MTX-PG concentration and efficacy/toxicity.
There are also limitations to our study. First, MTX response was based on clinical and biochemical parameters and not routinely confirmed using endoscopy. Second, most (75%) of our patients were not therapy-naive (previously used thiopurines and/or biologics). Difficult-to-treat patients could have lowered the MTX drug-survival rate more compared to therapy-naive patients. Third, we had missing data, amongst others because of public measures during the COVID-19 pandemic. No MTX-PG measurements were available from three patients (various reasons) and another four patients were missing in the extended Cox model as those MTX-PG measurements were more than 1 week after MTX discontinuation (all these patients stopped before week eight). Of note, these MTX-PG measurements were in the lower concentration range of all MTX-PG concentrations. Therefore, we assume that even if those missing values would have influenced our results, it would have led to an underestimation of the effect of MTX-PG3.
5 CONCLUSION
The rate of MTX SC monotherapy discontinuation in CD patients during the first year is high. Amongst the different MTX-PGs, higher erythrocyte MTX-PG3 concentrations are associated with increased MTX drug-survival, lower FCP levels, a higher biochemical response and less GI intolerance. MTX-PG3 levels may therefore be used to increase clinical efficacy and reduce drug-associated side effects in patients with CD. TDM by erythrocyte MTX-PG3 could be an option if future studies determine a robust therapeutic target concentration and MTX administrations are developed which lead to higher drug levels.
AUTHOR CONTRIBUTIONS
Maartje M. van de Meeberg: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – original draft (lead); writing – review and editing (equal). Herma H. Fidder: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing – review and editing (equal). Bas Oldenburg: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Janani Sundaresan: Formal analysis (supporting); writing – review and editing (equal). Eduard A. Struys: Formal analysis (equal); writing – review and editing (equal). Nahid S. M. Montazeri: Methodology (equal); writing – review and editing (equal). Wout G. N. Mares: Writing – review and editing (equal). Nofel Mahmmod: Writing – review and editing (equal). Dirk P. van Asseldonk: Writing – review and editing (equal). Maurice W. M. D. Lutgens: Writing – review and editing (equal). Johan P. Kuijvenhoven: Writing – review and editing (equal). Svend T. Rietdijk: Writing – review and editing (equal). Loes H. C. Nissen: Writing – review and editing (equal). Parweez Koehestanie: Writing – review and editing (equal). Nanne K. H. de Boer: Writing – review and editing (equal). Robert de Jonge: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing – review and editing (equal). Gerd Bouma: Conceptualization (equal); funding acquisition (lead); supervision (equal); writing – review and editing (equal). Maja Bulatovic Calasan: Conceptualization (equal); funding acquisition (equal); supervision (lead); writing – review and editing (equal).
ACKNOWLEDGEMENTS
Declaration of personal interests: We thank Rik van Eekelen (Department of Epidemiology & Data Science, Amsterdam UMC, Amsterdam, the Netherlands) for his statistical support.
FUNDING INFORMATION
This study was funded by the Dutch gastroenterology foundation (“Maag Lever Darm Stichting”). The funding body had no role in data collection, analysis, and interpretation or in writing the manuscript.
CONFLICT OF INTEREST STATEMENT
All conflicts are outside the submitted work: H.H.F. has served as a speaker for both Janssen and Takeda. She has served as a consultant for Takeda, Galapagos, and Ferring. She has received a research grant from Takeda. B.O. has served as a speaker for Takeda, Galapagos, and MSD. He has received grants from Pfizer, Takeda, and Ferring. He has served as a consultant/on the advisory board for Janssen, Pfizer, Takeda, and Cablon. G.B. has served as the speaker for Janssen and Takeda. He has served as a consultant for Roche, Takeda, and Calyps Biotech. N.K.H.d.B. has served as a speaker for AbbVie and MSD. He has also served as a consultant and/or principal investigator for TEVA PharmaBV and Takeda. He has received a (unrestricted) research grant from DrFalk, TEVA PharmaBV, MLDS and Takeda. L.H.C.N. has served on the advisory board for AbbVie and Ferring. M.B.C. has received an unrestricted grant from Takeda, CSL Behring and Pharming. The other authors have nothing to declare.




