Randomised clinical trial and meta-analysis: mesalazine treatment in irritable bowel syndrome—effects on gastrointestinal symptoms and rectal biomarkers of immune activity
The Handling Editor for this article was Professor Alexander Ford, and it was accepted for publication after full peer-review.
Summary
Background
Low-grade immune activation in the gut is a potential treatment target in irritable bowel syndrome (IBS).
Aims
To determine improvement in IBS symptoms after mesalazine treatment, and the utility of measures of immune activity in the rectal mucosa
Methods
This was a randomised, double-blind, placebo-controlled, parallel-arm, multicentre trial in subjects with IBS (Rome III criteria), with an eight-week treatment period of mesalazine 2400 mg or plcebo once-daily. The primary endpoint was the global assessment of satisfactory relief of IBS symptoms in ≥50% of weeks during intervention. IBS symptoms were also measured with the IBS severity scoring system; immune activity was measured by mucosal patch technology. A post hoc meta-analysis of randomised placebo-controlled trials of mesalazine in IBS was added.
Results
Of 181 included patients, 91 received mesalazine and 90 received placebo. The primary endpoint was met by 32 (36%) patients after mesalazine and 27 (30%) after placebo (p = 0.40). There were no differences in response rates related to IBS subtype or post-infection symptom onset. More reduction of abdominal bloating was noted in the mesalazine group (p = 0.02). The meta-analysis showed no effect of mesalazine on IBS symptoms. No mucosal patch technology measure could predict response to mesalazine, and found no differences in the effects of intervention on levels of immune markers.
Conclusions
Mesalazine is ineffective in reducing IBS symptoms. Rectal measures of immune activity by the mucosal patch technology cannot predict a higher chance of response to mesalazine.
1 INTRODUCTION
Irritable bowel syndrome (IBS) is a functional bowel disorder, currently defined by the coexistence of abdominal pain and altered bowel habits that persist for at least 6 months, but without objective biomarkers.1 Although the disorder is not associated with increased mortality,2, 3 it causes significant morbidity reflected by reduced quality of life,4 decreased work productivity4, 5 and increased healthcare costs.6 Considering the high prevalence of IBS,7, 8 there is a need for improved treatment options, even if there are good clinical guidelines to follow, based on predominant symptoms9 and a multidimensional clinical profile.10
Our current understanding of IBS pathophysiology is that of a complex disorder with several interacting factors resulting in an aberrant bowel function where the number of factors that can be detected is associated with the intensity of IBS symptoms.11 No individual pathophysiological factor is universal, even if a bidirectionally disordered interaction between the gut and the brain has evolved as a central phenomenon.12 Among the putative pathophysiological mechanisms, a persisting low-grade immune activation in the gut has received much attention. The evidence comes from reports of gastrointestinal infections being the strongest risk factor for developing IBS,13 and from a higher-than-expected rate of IBS-like symptoms in patients with inflammatory bowel disease in remission.14, 15 This has fuelled interest in treatments aiming at dampening inflammatory mechanisms also in IBS. Disappointingly, neither treatments with prednisolone in patients with post-infection IBS16 nor treatment with mesalazine in patients suffering from IBS with diarrhoea (IBS-D)17 or IBS including all subtypes18 was superior to placebo with symptom ratings as the outcome assessment, but with some indications of a favourable response in subsets of patients.
However, the identification of IBS patients predisposed to respond to anti-inflammatory treatment should potentially be based on the local immune activity in the gut rather than on the predominant bowel habit or mode of symptom onset. A small number of studies have used such an approach although with little success, as mucosal immune cell counts did not allow identification of IBS patients responding to anti-inflammatory treatment.17, 19 Furthermore, faecal calprotectin is used for detecting and monitoring acute gastrointestinal inflammation among patients with inflammatory bowel disease, but is not sensitive enough to detect low-grade inflammation in IBS.20 The mucosal patch technology is potentially an alternative to measure local gut immune activity, showing noticeable difference in a subset of IBS patients when compared to healthy subjects.21 The mucosal patch technology was described as simple to use, safe and reliable, which together with the above-described need for a surrogate marker of low-grade gut immune activity fits well with a clinically useful tool to further our understanding about immunologic abnormalities in IBS and the effects of anti-inflammatory treatment.
In this study, we therefore tested the hypothesis that at least a subset of subjects with IBS respond clinically to anti-inflammatory treatment, and that this is associated with a specific immune activation profile at baseline and/or a clear effect on gut immune activity. The primary study aim was to determine if mesalazine (Asacol) treatment was superior to placebo in improving IBS symptoms. The secondary aims were to investigate if improvement in specific IBS symptoms with mesalazine could be detected, and to establish if mucosal patch technology measures of immune activity in the rectal mucosa could identify specific IBS symptoms or patients more prone to respond to mesalazine treatment. As a post-hoc analysis, our data were added to a meta-analysis that included all randomised, double-blind, placebo-controlled trials (RCT) comparing the effect on IBS symptoms of mesalazine versus placebo.
2 MATERIALS AND METHODS
2.1 Study design and study population
This was a randomised, double-blind, placebo-controlled, parallel-arm, multicentre trial in patients with IBS defined by the Rome III criteria.22 Two Swedish and three Norwegian hospitals participated in the recruitment of patients. The study included a 3-week screening period, an 8-week treatment period with oral mesalazine (Asacol, 800 mg tablets, Tillotts Pharma AG) 2400 mg once daily or matching placebo tablets (Tillotts Pharma AG) once daily (1:1 ratio), and a two-week safety follow up-period (Figure 1). At the screening visit, the patients were assessed for eligibility. The inclusion criteria were: Age ≥ 18 years, already diagnosed with IBS (Rome III criteria), IBS severity scoring system (IBS-SSS)23 ≥175, and having provided a signed informed consent to participate. All IBS subtypes were eligible for inclusion; IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D), and IBS with mixed bowel habits or unsubtyped IBS. Already prescribed IBS medications were allowed during the study if they had been used >3 months and at a stable dose. Exclusion criteria were: presence of a systemic inflammatory disease, other gastrointestinal disease likely to explain the IBS symptoms or other severe diseases as judged by the investigator; treatment with non-steroidal anti-inflammatory drugs, opioid analgesics or acetylsalicylic acid within 7 days prior to screening; treatment with antibiotics, immunosuppressive drugs or other significant medical treatment that could compromise the safety or efficacy objectives of the study within 28 days prior to screening; previously confirmed allergy towards mesalazine or acetylsalicylic acid; current infection; being pregnant or lactating; a history of or current drug or alcohol dependence; women of childbearing potential with unwillingness to use adequate contraceptive measures throughout the duration of the study. At visit 1, the screening visit, after the IBS-SSS questionnaire was completed, a review of the medical history and concomitant medication as well as a physical examination including vital signs was performed. A blood sample for analysis of haematology and clinical chemistry, a faecal sample for calprotectin, and in females of childbearing potential also a urine pregnancy test, was checked. Finally, the rectosigmoid colon was investigated with flexible sigmoidoscopy, and thereafter, a mucosal patch technology procedure was performed. At visit 2, the randomisation visit, the IBS-SSS questionnaire was once more completed, where the result still needed to be ≥175 to be eligible for randomisation into the intervention period, and the hospital anxiety and depression scale questionnaire24 was completed. After a symptom-directed physical evaluation including vital signs, the investigator assigned a consecutive randomisation number to the patient that corresponded to a study medication kit number available at the site and study medication was dispensed. The randomisation numbering of medication kits in blocks of four was done by use of a computer-generated list by the company providing mesalazine and placebo. The number code was kept confidential until after the end of the study and was broken after database lock. The patients were instructed to answer the weekly question about global symptom relief and complete the IBS-SSS questionnaire biweekly during the full intervention period. At visit 3 (day 28 ± 2 after randomisation), a symptom-directed physical examination was performed if needed, vital signs checked, and a blood sample for analysis of haematology and clinical chemistry was taken. Study medication was reviewed for compliance and any adverse event was noted. At visit 4 (day 56 ± 2 after randomisation), the end-of-treatment visit, a physical examination was performed, vital signs were checked, a blood sample for analysis of haematology and clinical chemistry was taken, and a faecal sample for calprotectin was collected. Remaining study medication was returned and reviewed for compliance and any adverse events were noted. Questionnaires on treatment satisfaction and IBS-SSS were collected, and the hospital anxiety and depression scale questionnaire was completed. Finally, the rectosigmoid colon was investigated for normality with flexible sigmoidoscopy, and thereafter, a mucosal patch technology procedure was performed. A telephone follow-up was done 2 weeks after the end of the intervention period to review any new adverse events or changes to pre-existing adverse events.
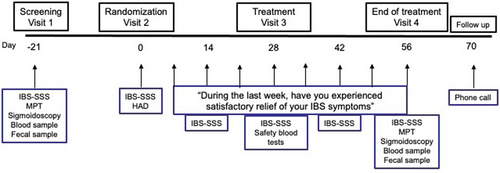
The study was conducted in accordance with the Declaration of Helsinki. All aspects of the study had been approved by Swedish and Norwegian Regional Ethical Review Boards (2011/1793–31/2, 2013/2032) and Medical Products agencies (2011–003418-18, 13/16072). The study was registered at ClinicalTrials.gov, identifier NCT01699438.
2.2 Mucosal patch technology
The mucosal patch technology procedure21 for sampling of rectal mucosal fluid was done at the screening visit (visit 1) and at the end-of-treatment visit (visit 4). Before the mucosal patch technology procedure, a flexible sigmoidoscopy was performed to rule out rectosigmoid pathology. There was a separation in time between these investigations and randomisation to avoid procedure-related effects on IBS symptoms during the intervention period. The mucosal patch technology device consists of a plastic catheter with a silicon balloon at the end with three patches made of highly absorptive cellulose material attached on the balloon (Alimenta Medical AB). After positioning of the catheter in the rectum with the patient lying in the left lateral position with an intubation technique identical to rigid sigmoidoscopy, the balloon was inflated with 80 ml of air and kept inflated for 10 min. If the patient reported intolerable discomfort or pain intolerable, the volume of air was reduced in 5–10 ml steps until tolerated. Since the minimum volume assuring patches adhering to the rectal mucosa is 50 ml, the procedure was stopped if this volume could not be tolerated, or the patient otherwise judged the investigation to be intolerable. In those with a successful mucosal patch technology procedure, the instrument was retracted after balloon deflation, patches were cut off and placed in buffer solution in room temperature for 1 h. As last steps, the extraction solution was manually squeezed out of the patches, centrifuged at 2000–3000 g for 10 min and kept frozen at −80°C until analysis.
2.3 Study assessments
2.3.1 IBS symptom assessments
To assess the overall effect of the intervention, the patients were asked the following question on a weekly basis during the intervention period: “During the last week, have you experienced satisfactory relief of your IBS symptoms”. In addition to this, the patient completed the IBS-SSS questionnaire at the screening and randomisation visits, and biweekly during the intervention period. This is a validated retrospective recall questionnaire for the assessment of IBS symptom severity. It includes four questions, intensity of abdominal pain, severity of abdominal bloating, dissatisfaction with bowel habits and the daily-life interference of IBS in general during the last week, for which answers are given on visual analogue scales (0–100). A fifth question, frequency (number of days) of abdominal pain during the last 10 days, is answered with the outcome multiplied by 10. IBS-SSS has a maximum aggregated score of 500. Severity is defined as moderate if the sum is ≥175 and severe if ≥300. It has been shown to be sensitive for assessment of a clinically relevant improvement of IBS symptoms if the reduction over time is ≥50.23
2.3.2 Hospital anxiety and depression scale
This is a 14-item questionnaire measuring symptoms of anxiety and depression and intended for use in non-psychiatric populations.24 The maximum score is 21 on each of the two subscales. A higher score indicates higher levels of psychological distress within this specific domain.
2.3.3 Markers of immune activity
From the mucosal patch technology extracts, the following analyses were performed: Myeloperoxidase and human neutrophil lipocalin as markers of neutrophil activity,25, 26 eosinophil cationic protein as marker of eosinophil activity27 and human phospholipase B-precursor as marker of neutrophil and eosinophil activity and a non-specific epithelial cell factor.28 These were all measured by ELISA-kits provided by Diagnostics Development, Uppsala, Sweden. Tryptase measures were done by the Pharmacia ImmunoCAP assay to reflect mast cell activation. Faecal calprotectin was measured as a regular clinical assay at each study site according to the instructions given by the provider (Bühlmann Laboratories, Switzerland) and with lowest detection limit 15 mg/g.
2.4 Compliance
The definition of satisfactory compliance to treatment was defined a priori as intake of ≥80% of the prescribed number of mesalazine/placebo doses. The medication was dispensed at visit 2 in identical packages labelled with a unique study ID and all packages were brought back to the hospital for medication count at visits 3 and 4.
2.5 Data analysis and statistics
The global assessment of satisfactory relief of IBS-symptoms was used as the response parameter to define the primary endpoint. Response to treatment, that is, the primary endpoint, was defined as answering “yes” to the weekly question “During the last week, have you experienced satisfactory relief of your IBS symptoms” at least out of 8 weeks (≥50%). Two symptom-based secondary endpoints were used: response to treatment defined by giving the answer “yes” to the weekly question at least 6 out of 8 weeks (≥75%), or a reduction of IBS-SSS by ≥50 from randomisation (visit 2) to end of treatment (visit 4). Furthermore, both between- and within-group comparisons were made for the change in IBS-SSS and for the mucosal patch technology measures from visit 2 to visit 4. Finally, we also evaluated the response to treatment over time by use of the biweekly IBS-SSS data. All patients who received treatment after randomisation were included in the intention-to-treat analysis if any efficacy of response data was available. For the primary analysis, values of no symptom relief were imputed using the last-data-carried-forward principle when data was missing due to withdrawal. All patients who received treatment were also included in the safety population. With an expected 50% of patients in the mesalazine group and 30% of the patients in the placebo group achieving satisfactory symptom relief ≥50% of the time, the study needed to include 93 patients in each treatment group to detect a statistically significant difference between the two treatments on a 5% level with 80% power. With an expected 7% of patients being excluded from the primary analyses, a total of 200 patients were planned for randomisation.
Categorical data are summarised and presented as total numbers and percentages, with comparison between groups performed using the chi-squared test. Continuous data are presented as mean and standard deviation for symptom data (IBS-SSS) and demographics, or as median with interquartile range for mucosal patch technology data. For comparisons of continuous data between the two treatment groups, the Mann–Whitney U-test was used, and for comparisons of mucosal patch technology data between IBS subtypes, the Kruskal–Wallis test was used. For all comparisons of paired samples of data, we used the Wilcoxon signed-rank test. Response to treatment over time was compared between mesalazine and placebo by use of repeated-measures ANOVA with treatment as independent variable and the symptom scores as outcome variable. Correlations between symptom scores and mucosal patch technology measures were determined with Spearman's rank-order correlation. The statistical program SPSS version 27.0 (SPSS) was used for calculations. p-values less than 0.05 were used to define statistical significance.
2.6 Meta-analysis of randomised clinical trials comparing effects on IBS symptoms from mesalazine vs. placebo
A meta-analysis of previous randomised clinical trials17-19, 29, 30 and the current trial was undertaken using satisfactory relief of IBS symptoms as the endpoint. Data were pooled and analysed with a random-effects model. Statistical heterogeneity was evaluated using I2 statistic. Differences were reported with standardised mean difference and values of 0.2–0.5 were considered small, 0.5–0.8 medium and >0.8 large. To represent satisfactory relief of IBS symptoms in studies with multiple endpoints,19, 29, 30 the endpoints abdominal pain,19, 29, 30 abdominal bloating,19, 29, 30 stool frequency,19, 29, 30 urgency,29, 30 and stool consistency29 were aggregated using the “MAd” package31 in R (version 4.0.3 – R Foundation, Vienna, Austria). Estimated correlation among within-study endpoints was set at 0.9. In studies with a categorical endpoint, odds ratios and their confidence intervals were converted to standardised mean differences as recommended in the Cochrane Handbook.32 These endpoints included global assessment of satisfactory relief of IBS symptoms ≥50% of weeks during intervention (current study), satisfactory relief of overall IBS symptoms in at least 50% of weeks over a three-month period18 and number of patients with satisfactory relief of IBS symptoms at 12 weeks.17
3 RESULTS
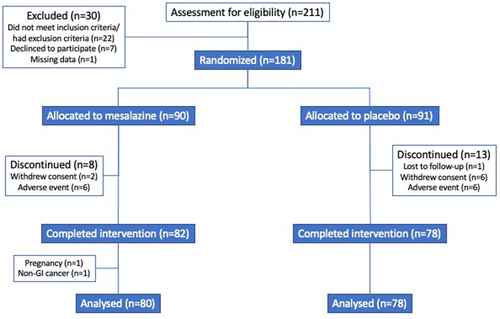
The first patient entered the trial in July 2012 and the study was completed in January 2017. In total, 211 patients were screened for eligibility into the study until the study was stopped due to the study exceeding the funding period. Of these, 181 eligible IBS patients (70% females) were randomised and 90 received mesalazine 2400 mg/day and 91 received placebo. In total, 158 patients completed the entire intervention period and met the compliance criteria for the study medication. A study flow chart including screening failures and withdrawals from the study is outlined in Figure 2. The mean age for those randomised was 45.3 (range 20–72) years, where 28 (15%) had IBS-C, 72 (40%) had IBS-D and 81 (45%) had IBS with mixed bowel habits or unsubtyped IBS, without any difference in subtype distribution between the treatment groups. IBS severity was moderate in 69 (38%) patients and severe in 112 (62%) with a total range of IBS-SSS of 177–470. Demographics in the two treatment groups are summarised in Table 1. Both groups were comparable, except for a higher abdominal pain intensity in the placebo group, although the overall IBS symptom severity was similar.
| Mesalazine (n = 90) | Placebo (n = 91) | p-value | |
|---|---|---|---|
| Age, years (SD) | 46.2 (14.2) | 44.2 (14.9) | 0.37 |
| Females, n (%) | 64 (71) | 63 (69) | 0.79 |
| BMI (SD) | 25.1 (4.7) | 24.5 (5.0) | 0.42 |
| IBS-SSS, Total (SD) | 319 (70) | 327 (72) | 0.47 |
| Abdominal pain intensity (SD) | 46 (25) | 53 (24) | 0.02 |
| Abdominal pain frequency (SD) | 66 (31) | 70 (28) | 0.44 |
| Abdominal bloating (SD) | 62 (24) | 60 (27) | 0.71 |
| Dissatisfaction with bowel habit (SD) | 70 (23) | 70 (23) | 0.82 |
| General life interference (SD) | 75 (18) | 75 (18) | 0.80 |
| IBS-C, n (%) | 13 (14) | 15 (16) | 0.90 |
| IBS-D, n (%) | 37 (41) | 35 (38) | |
| IBS-nonCnonD, n (%) | 40 (44) | 41 (45) | |
| Sudden onset, n (%) | 25 (28) | 23 (25) | 0.70 |
| Anxiety, HAD (SD) | 7.3 (4.2) | 8.1 (4.1) | 0.23 |
| Depression, HAD (SD) | 4.1 (3.1) | 4.5 (3.4) | 0.46 |
- Abbreviations: BMI, Body Mass Index; HAD, Hospital Anxiety and Depression scale; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhoea; IBS-nonCnonD, irritable bowel syndrome with mixed or unsubtyped bowel habit; IBS-SSS, Irritable bowel syndrome severity scoring system; SD, Standard deviation.
3.1 Effect on symptoms
3.1.1 Primary endpoint
Satisfactory relief of IBS symptoms at least 50% of the weeks during treatment was reported by 32 (36%) patients in the mesalazine group compared to 27 (30%) in the placebo group (p = 0.40). There were no differences in response rates among IBS subtypes: IBS-C; 6 (46%) versus 6 (40%) (p = 0.74), IBS-D; 14 (38%) versus 11 (31%) (p = 0.57), or IBS with mixed bowel habits or unsubtyped IBS; 12 (30%) versus 10 (24%) (p = 0.57). A sudden or post-infection onset of the IBS symptoms, which was reported by 48 patients, did not influence the treatment outcome; 8 (32%) versus 7 (30%) of those subjects were classified as responders to mesalazine and placebo respectively (p = 0.91). Also, in the per-protocol analysis (n = 158), the outcome was similar between the groups receiving mesalazine and placebo; 32 (40%) versus 26 (33%) (p = 0.38).
3.1.2 Secondary endpoints
The secondary endpoint, reporting satisfactory relief of IBS symptoms at least 75% of weeks during treatment, was reported by 15 (17%) patients in the mesalazine group and 16 (18%) in the placebo group (p = 0.87). The proportions of responders to treatment were similar also when a reduction in IBS-SSS ≥50 was used as the response definition: 42 (47%) patients in the mesalazine group compared with 37 (42%) in the placebo group (p = 0.45). In both treatment groups, there was a significant improvement in IBS-SSS after the intervention period compared with baseline (mesalazine; baseline 319 (70) vs. after treatment 244 (107) (placebo; baseline 327 (72) vs. after treatment 270 (120), p > 0.001 for both). This was also noted for all the five individual IBS-SSS domains with similar improvements in both groups, except for abdominal bloating, where the improvement was greater in the mesalazine group than in the placebo group (p = 0.02), but other domains were similar in the treatment groups (Table 2). Both treatment groups showed a significant effect of time on IBS-SSS (p < 0.001), but there was no effect of the treatment (p = 0.33) and no time × treatment interaction effect (p = 0.60) indicating a similar symptom response during the 8-weeks intervention period regardless of time point. In both treatment groups, there was a significant improvement in symptoms of anxiety after the intervention period (mesalazine; baseline 7.3 [4.2] versus after treatment; 6.5 [3.7], p = 0.04, placebo; baseline 8.1 [4.1] vs 6.8 [4.0] p < 0.001), but without any difference between groups (p = 0.61). Depressive symptoms were unchanged in the mesalazine group (baseline 4.1 [3.1] vs after treatment; 3.9 [3.2], p = 0.41), but reduced in the placebo group (baseline 4.5 [3.4] vs after treatment 3.5 [3.2] p < 0.001), but without any difference between groups (p = 0.39).
| IBS-SSS | Mesalazine | Placebo | p-value—between groups | ||
|---|---|---|---|---|---|
| Baseline (n = 90) | End of treatment (n = 82) | Baseline (n = 91) | End of treatment (n = 78) | ||
| Total | 319 (70) | 244 (107)*** | 327 (72) | 270 (120)*** | 0.13 |
| Abdominal pain intensity | 46 (25) | 37 (29)* | 53 (24) | 40 (29)*** | 0.42 |
| Abdominal pain frequency | 66 (31) | 48 (36)*** | 70 (28) | 49 (33)*** | 0.79 |
| Abdominal bloating | 62 (24) | 44 (30)*** | 60 (27) | 55 (35)* | 0.01 |
| Dissatisfaction with bowel habit | 70 (23) | 54 (28)*** | 70 (23) | 60 (29)*** | 0.19 |
| General life interference | 75 (18) | 60 (27)*** | 75 (18) | 65 (29)*** | 0.23 |
- Note: IBS-SSS = irritable bowel syndrome severity scoring system. Data are presented as mean with standard deviations.
- * <0.05 vs baseline
- *** <0.001 vs baseline.
3.2 Markers of immune activity
The mucosal patch technology procedure was performed with complete results at the screening visit in 162 (90%) of the patients who were later randomised at visit 2, and at the end-of-the-treatment visit (visit 4) in 141 patients. In patients missing data from one or both mucosal patch technology procedures, this was due to intolerance to the procedure (n = 5), the equipment being temporarily unavailable during a part of the study period (n = 12), post-procedure handling of material that did not fulfil quality standards for reliable data outcome (n = 7) and the remaining for not finishing the study.
Baseline measures of mucosal biomarkers in mucosal patch technology fluids did not differ between the two treatment groups (Table 3). Less than 10% of the samples had detectable tryptase levels. When comparing the levels of the four mucosal biomarkers with measurable levels in the mucosal patch technology fluid, no difference could be noted based on IBS subtype. There were no correlations between the immune markers and IBS symptoms observed at baseline (Table 4).
| Mesalazine | Placebo | p-value—between groups | |||
|---|---|---|---|---|---|
| Baseline (n = 81) | End of treatment (n = 73) | Baseline (n = 81) | End of treatment (n = 68) | ||
| MPO | 19 (10–29) | 17 (10–29) | 17 (12–38) | 15 (10–28) | 0.95 |
| ECP | 20 (9–62) | 15 (9–42) | 18 (9–66) | 16 (8–45) | 0.64 |
| HPLB-P | 58 (36–78) | 42 (32–64)** | 51 (35–68) | 48 (32–64)* | 0.65 |
| HNL | 28 (20–37) | 24 (18–38) | 28 (23–39) | 28 (21–36) | 0.36 |
- Note: Data are presented as μg/L, median (interquartile range).
- Abbreviations: ECP, Eosinophil cationic protein; HPLB-P, human phospholipase B-precursor, HNL, human neutrophil lipocalin; MPO, myeloperoxidase.
- * <0.05 vs baseline
- ** <0.01 vs baseline.
| IBS-SSS screening visit | HPLB-P | MPO | ECP | HNL |
|---|---|---|---|---|
| Total | −0.008 | 0.020 | 0.005 | 0.001 |
| Abdominal pain intensity | −0.023 | 0.005 | −0.019 | 0.014 |
| Abdominal pain frequency | 0.024 | 0.036 | 0.066 | −0.053 |
| Abdominal bloating | 0.006 | −0.040 | −0.005 | −0.088 |
| Dissatisfaction with bowel habit | −0.115 | 0.009 | −0.021 | 0.139 |
| IBS-SSS end of treatment | HPLB-P | MPO | ECP | HNL |
|---|---|---|---|---|
| Total | −0.032 | 0.119 | −0.143 | 0.275** |
| Abdominal pain intensity | 0.035 | 0.067 | −0.072 | 0.218** |
| Abdominal pain frequency | −0.146 | 0.094 | −0.098 | 0.126 |
| Abdominal bloating | 0.058 | 0.081 | −0.160 | 0.224** |
| Dissatisfaction with bowel habit | −0.121 | 0.072 | −0.030 | 0.247** |
- Abbreviations: ECP, eosinophil cationic protein; HPLB-P, human phospholipase B-precursor; HNL, human neutrophil lipocalin; IBS-SSS, Irritable bowel syndrome severity scoring system; MPO, myeloperoxidase.
- ** p < 0.01.
Of the four immune markers measured by the mucosal patch technology, only human phospholipase B-precursor was reduced after the intervention, and this was seen in both the mesalazine and placebo groups. However, there were no differences between the intervention groups regarding the change in the immune markers before versus after treatment (Table 3). There were weak, but significant positive correlations between levels of human neutrophil lipocalin and severity of abdominal pain, severity of abdominal bloating, dissatisfaction with bowel habits and total IBS-SSS at the end of the treatment period, but for the other immunologic measures, no significant correlations to IBS symptoms were noted (Table 4). None of the immune measures at baseline differed between responders and non-responders to the treatment options (Table 5).
| Mesalazine | p-value | Placebo | p-value | |||
|---|---|---|---|---|---|---|
| Responders to treatment (n = 29) | Non-responders to treatment (n = 52) | Responders to treatment (n = 24) | Non- responders to treatment(n = 57) | |||
| MPO | 17 (10–30) | 19 (10–29) | 0.75 | 17 (14–42) | 17 (10–36) | 0.45 |
| ECP | 24 (10–52) | 18 (9–68) | 0.82 | 20 (10–45) | 18 (8–71) | 1.00 |
| HPLB-P | 42 (32–78) | 58 (39–78) | 0.19 | 44 (31–61) | 54 (36–70) | 0.38 |
| HNL | 26 (18–32) | 30 (22–38) | 0.14 | 29 (26–44) | 28 (22–37) | 0.31 |
- Note: Data are presented as μg/l, median (interquartile range).
- Abbreviations: ECP, eosinophil cationic protein; HPLB-P, human phospholipase B-precursor; HNL, human neutrophil lipocalin; MPO: Myeloperoxidase.
Faecal calprotectin measures were available in 178 patients of those randomised (98%), and in 153 patients who completed the study (97%). There were two and three patients in the mesalazine group, and three and two patients in the placebo group with a calprotectin level >100 g/mg at screening and at the end of study. None of these had signs of mucosal inflammation at sigmoidoscopy or signs of inflammation in blood tests. Since 70% of tests at the screening visit and 72% of tests at end of study were below the detection level, this immune parameter was not used for further analysis.
3.3 Adverse events
In total, 123 (68%) reported one or more adverse events (Table 6). The judged causality between treatment and adverse events was similar in the two treatment groups as were the number of patients that discontinued treatment because of adverse events.
| Adverse event, n (%) | Mesalazine (n = 90) | Placebo (n = 91) |
|---|---|---|
| Any AE | 62 (69) | 61 (67) |
| Treatment-related AE | 20 (22) | 17 (18) |
| Serious AE | 1 (1.1)a | 0 |
| AE leading to study drug discontinuation | 4 (4.4) | 5 (5.5) |
| AE by categories | ||
| Gastrointestinal | 24 (27) | 23 (25) |
| Musculoskeletal | 18 (20) | 12 (13) |
| Infections | 29 (32) | 31 (34) |
| Dermatological | 9 (10) | 10 (11) |
| Headache | 8 (8.9) | 11 (12) |
- a Pregnancy during study.
One patient treated with mesalazine became pregnant during the trial and was therefore excluded. Another subject who received mesalazine treatment was diagnosed with a non-GI cancer after the trial start and excluded from analysis, although this was judged without causality to the treatment.
3.4 Meta-analysis of randomised clinical trials comparing effects on IBS symptoms from mesalazine vs. placebo
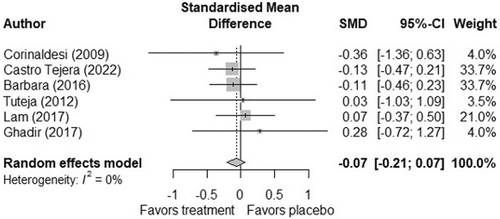
In the meta-analysis, the pooled standardised mean difference did not indicate any clear effect of mesalazine treatment or placebo. The pooled standardised mean difference slightly favoured mesalazine (−0.07 [−0.21; 0.07]), but the estimate was close to zero and its confidence interval included both negative and positive values (Figure 3). The I2 was 0%, indicating low heterogeneity.
4 DISCUSSION
In this randomised, double-blind, placebo-controlled, multicentre trial, the effect of 8-weeks mesalazine treatment was not superior to placebo treatment for global improvement of IBS symptoms. Furthermore, no clear effects of mesalazine as compared with placebo were noted on individual IBS symptoms or gut immune activity measured by the mucosal patch technology, and gut immune activity measured with the mucosal patch technology could not predict the response to mesalazine treatment.
With the outcome of our study at hand supporting previous reports,17, 18 the evidence that treatment with mesalazine for symptom relief in patients with IBS is ineffective is now convincing. We used a treatment period of 8 weeks, and two other major studies with negative outcome both had 12 weeks of treatment.17, 18 With the current understanding of the disorders of gut–brain interaction,33 an even longer treatment period would perhaps be needed to fully rule out mesalazine treatment in IBS. However, none of the so far conducted studies has identified any tendencies suggesting that certain patient subsets would clearly benefit from long-term mesalazine treatment. This is further strengthened by our post-hoc meta-analysis of randomised clinical trials comparing effects on IBS symptoms from mesalazine versus placebo.17-19, 29, 30
A justification for repeating a study of mesalazine in IBS would have been valid if we had identified a specific subgroup with immunologic activation with a more favourable treatment outcome. This fell short, both by prediction of outcome from pre-treatment levels of immunologic markers, and by not showing any significant effects comparing the change in immunologic markers between treatment groups. Now almost 20 years ago, when the suspicion of low-grade mucosal inflammation being a significant factor in the pathophysiology of IBS still was novel, a pioneering study in patients with post-infection IBS was presented, where the effects of 30 mg prednisolone/day for 3 weeks on IBS symptoms were evaluated in a randomised controlled design on 29 patients. No effect on symptoms was evident, but a reduction in mucosal lymphocyte count was noted after treatment.16 Later, in a proof-of-concept study including the effects of mesalazine treatment on colonic immune cell counts and IBS symptoms, the same pattern was noted, with a reduction in lymphocyte counts that was not accompanied by any discernible symptom-reducing effects compared with placebo.19 These data speak against the lymphocytes being the major immunologic factor to address in anti-inflammatory treatments in IBS. Added to this, several similar attempts have been made to find a niche of IBS patients responsive to treatment with mesalazine,12, 18, 29, 30 all without success in defining any subgroup of relevance.
Our hypothesis that mucosal patch technology measures could provide one or more sensitive biomarkers of low-grade immune activity of relevance in IBS was based on the previous report of elevated levels of human neutrophil lipocalin and myeloperoxidase, potentially reflecting neutrophil activity, in a small IBS sample.21 In our current study, we noted a signal after treatment with levels of human neutrophil lipocalin weakly correlated with IBS symptom severity. However, no similar correlations were seen before the intervention, which makes the relevance of the post-intervention findings of correlations uncertain. Based on a rather convincing evidence that mast cell activity is of putative importance in the pathogenesis of IBS34 and also has been linked to the intensity of abdominal pain in a pivotal study,35 we aimed to measure tryptase levels in the mucosal patch technology fluids. Unfortunately, the mucosal patch technology procedure as used by us during this study was not able to detect tryptase in the analyses from most participants. Whether this depends on a flaw in any step of our protocol or reflects truly low levels cannot be answered with certainty. Considering the recent study that linked a mast cell-dependent mechanism to the development of visceral hypersensitivity after an inflammatory immune response,36 this aspect of the putative role of a local measure is still highly relevant and where methods like the mucosal patch technology could be useful. In this context, the time window and characteristics in relation to a putative infectious provocation in humans need further studies. We still do not know if tryptase activity is relevant for symptom progression over time in humans after visceral hypersensitivity has been established and the prerequisite of an IBS diagnosis of at least 6 months symptom duration is fulfilled.
Another positive finding from the mucosal patch technology measures, but with unclear relevance, is the reduced levels of human phospholipase B-precursor after the treatment period in both groups, but with no difference between the treatments. This phospholipase B-precursor is expressed in neutrophils, eosinophils and by gut epithelial cells,28, 37 but with an unclear relation to disease that in theory could reflect both immune activity and some aspect of epithelial cell health. Fritcher-Ravens et al. reported in 2014 that in IBS patients with suspected food intolerance, candidate food antigens caused rapidly occurring objective mucosal damage after local gut exposure in the form of epithelial leaks and gaps and widened intervillous spaces, observed by use of confocal laser endomicroscopy.38 It is tempting to speculate that human phospholipase B-precursor measured by the mucosal patch technology could be an alternative biomarker of gut mucosal integrity or “health”, but as stated above, the relevance of our findings remains speculative. The rationale for exploring mesalazine in the treatment of a lymphocyte and mast cell predominant immune activation as reported in IBS was among other things motivated by the peroxisome proliferators-activated receptor-γ binding properties of mesalazine.39 Since peroxisome proliferators-activated receptor-γ is widely expressed on many cell types, including colonic epithelial cells, lamina propria T and B cells,40 and also mast cells,41 an effect of mesalazine on various immune cells can be expected, which may theoretically be advantageous in IBS, where different immune alterations have been suggested.
The major strength of our study is the number of participants and the randomised, double-blind, placebo-controlled design. Even if study recruitment was stopped prematurely and the number of dropouts was higher than expected, meaning that the study is formally underpowered, we can conclude that the results make it highly unlikely that we have missed a relevant role for mesalazine treatment in IBS in clinical practice, regardless of subtype or whether having characteristics of post-infection IBS. The multicentre recruitment also meant that the participants were representative for at least a Scandinavian adult population. The old definition of satisfactory relief of IBS symptoms can also be argued as clinically relevant, even if this is not being the current gold standard in pharmacological interventions in IBS patients. Since we also report on abdominal pain and dissatisfaction with bowel habit, we can conclude it as highly unlikely that the current recommended composite endpoint for pharmacologic interventions in IBS would have been met.42 The novel inclusion of biomarkers of rectal mucosal integrity and immune function also adds to the importance, even if the outcome is largely negative.
One weakness in any study design that includes invasive procedures, such as sigmoidoscopy and the mucosal patch technology procedure in our study, is that they can affect symptom reports. The possibility of anxiety affecting symptom intensity in the period before each measurement, but also for a period after each measurement, can also not be excluded. However, both treatment groups had similar baseline characteristics regarding psychological symptoms, and the randomised, double-blind, placebo-controlled design should prevent this from being a major limitation. We also believe that separating the screening visit where these investigations were done from the randomisation visit prevented post-procedural symptoms to affect baseline symptom reports.
To conclude, mesalazine is ineffective in reducing IBS symptoms after an 8-week treatment period. Mucosal patch technology measures are not able to detect biomarkers of immune activity that can predict a higher chance for response to mesalazine treatment.
AUTHOR CONTRIBUTIONS
Valeria Castro Tejera: Data curation (lead); formal analysis (equal); investigation (equal); project administration (equal); writing – original draft (lead). Lena Öhman: Conceptualization (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); project administration (supporting); writing – review and editing (equal). Lars Aabakken: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). Bengt Fellström: Funding acquisition (supporting); investigation (supporting); methodology (supporting); writing – review and editing (supporting). Trygve Hausken: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). Oistein Hovde: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). Johann P Hreinsson: Formal analysis (supporting); methodology (supporting); writing – review and editing (supporting). Greger Lindberg: Investigation (supporting); project administration (supporting); writing – review and editing (supporting). Per Venge: Conceptualization (supporting); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); writing – review and editing (equal). Magnus Simrén: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); project administration (equal); writing – review and editing (equal). Hans Törnblom: Conceptualization (lead); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); supervision (lead); writing – original draft (supporting); writing – review and editing (lead).
ACKNOWLEDGEMENT
Declaration of personal interests: VCT, LA, GL and JPH declare no conflict of interest. LÖ has served as Consultant/Advisory Board member for Genetic Analysis AS, has received unrestricted research grants from AstraZeneca and as a speaker for Takeda, AbbVie and Meda. BF has served as Consultant for BMS, AstraZeneca, Calliditas, Pharmalink, Astellas, ALEXION, CSL Behring, SANDOZ, has ownership interest in Calliditas (<1%), has received research funding from BMS, Pharmalink, Astellas, SANDOZ, CSL Behring, has received honoraria from BMS, NOVARTIS, Sandoz, Astrazeneca, Roche, Calliditas, ALEXION, and has personal pending patent and Advisory or Leadership Role: Calliditas, Astellas, ALEXION,CSL Behring, Sandoz, BioAnalogica AB, Transcutan AB, BioConcept AB. ØH has served as Consultant/Advisory Board member/speaker for Jansen-Cilag. PV is the major owner of Diagnostics Development and owns worldwide patents of the immunoassay of human neutrophil lipocalin and phospholipase B-precursor. MS has received unrestricted research grants from Glycom and Danone Nutricia Research and served as advisory board member/consultant and/or speaker for Biocodex Glycom, Danone Nutricia Research, Ironwood, Genetic Analysis AS, Kyowa Kirin, Menarini, Arena, Adnovate, Tillotts, Takeda, Alimentary Health, AlfaSigma, Falk Foundation and Shire. HT has served as a speaker for Biocodex, Takeda and Tillotts.
FUNDING INFORMATION
This study was funded by Eurostars project grant E!5691, an unrestricted grant from Tillotts Pharma AB (mesalazine (Asacol) and placebo), and by grants from the Swedish state under the agreement between the Swedish government and the county councils ALF-agreement (ALFGBG 295071, 620,221, 726,561, 875,581).
AUTHORSHIP
Guarantor of the article: Hans Törnblom. All authors approved the final version of the manuscript.




