Editorial: inaccuracies in attribution of symptoms due to gluten—not just in those with self-reported noncoeliac gluten sensitivity. Authors' reply
AP&T invited editorial columns are restricted to discussing papers that have been published in the journal. An editorial must have a maximum of 500 words, may contain one table or figure, and should have no more than 10 references. It should be submitted electronically to the Editors via http://mc.manuscriptcentral.com/apt
Abstract
LINKED CONTENT
This article is linked to Daveson et al and Gibson papers. To view these articles, visit https://doi.org/10.1111/apt.15551 and https://doi.org/10.1111/apt.15620.
Gluten-related disease has been confusing for patients with coeliac disease as well as clinicians and researchers seeking evidence linking symptoms with pathophysiology. The editorial by Professor Gibson summarises the challenges of attributing symptoms to gluten when other components in the food challenge may also be causing symptoms.1 Wheat flour has dual effects due to gluten protein providing peptides that activate T cells in coeliac disease, and fructans, a type of fermentable oligo-, di-, monosaccharides and polyol (FODMAP) that Gibson's group and others have implicated as a common cause of IBS-like symptoms in individuals unaffected by coeliac disease.2 This point is yet to be integrated into guidelines for clinicians undertaking gluten challenge in patients on gluten-free diet for the purpose of diagnostic testing for coeliac disease. For researchers, the results underscore the need to standardise guidelines for gluten challenges especially when gluten-induced symptoms are the primary measure of efficacy for novel therapies.
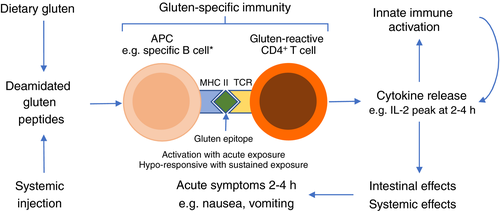
Systemic cytokine release occurs after gluten ingestion in patients with coeliac disease, which is coincident with and proportionate to early upper gastrointestinal symptoms,3 but not in patients with self-reported non-coeliac gluten sensitivity after FODMAPs or gluten.4 This is important for several reasons. It highlights the segmentation of the current market for ‘gluten-free’ food comprising those with coeliac disease who need to strictly avoid gluten, those who have IBS and may need to avoid FODMAPs but not gluten, and some patients with coeliac disease and IBS who need to avoid both. This new model for understanding the immuno-clinical effects of gluten (shown in Figure 1) directly implicates the central role of gluten-reactive CD4+ T cells as the drivers of acute digestive symptoms after gluten ingestion (or injecting immunogenic gluten peptides).3, 5 The link between serum levels of interleukin-2, a classic marker of T cells recently activated by antigen, with symptoms of nausea and vomiting after gluten indicates it could serve as an objective biomarker for researchers, drug developers and clinicians seeking to monitor or diagnose coeliac disease without prolonged gluten challenge.6, 7 In addition, this new model offers an explanation for symptoms being mild or absent in patients with coeliac disease regularly consuming gluten, since chronic antigen exposure renders CD4+ T cells hyporesponsive to further antigen stimulation.3 The first proof of concept that this strategy might also offer a therapeutic approach without causing intestinal mucosal injury was provided by studies using repeated injections of short deamidated gluten peptides.5 Several other immunotherapies comprising gluten antigen in different delivery systems are showing promise,8 and may become adjuncts to gluten-free diet to address both acute gluten-mediated symptoms and persistent mucosal inflammation.9 However, without understanding the basic issues such as symptoms caused by gluten, and having biomarkers such as interleukin-2 that we have now reported using masked gluten food challenge low in FODMAPs,10 the substantial investments needed to explore potential therapies to better manage coeliac disease may be deterred.
ACKNOWLEDGEMENT
The authors' declarations of personal and financial interests are unchanged from those in the original article.7




