Increased susceptibility to azithromycin of Pseudomonas aeruginosa biofilms using RPMI 1640 testing media
Abstract
Azithromycin (AZM) is efficient for treatment of chronic Pseudomonas aeruginosa biofilm lung infections, despite of resistance in conventional susceptibility testing. It has been shown that planktonic P. aeruginosa are more susceptible to AZM when tested in RPMI 1640 medium. The aim of the study was to test the susceptibility to AZM of P. aeruginosa biofilms in LB vs RPMI 1640 media. We investigated the effect of AZM on planktonic and biofilms of (WT) P. aeruginosa (PAO1), the hypermutable (ΔmutS) and the antibiotic-resistant phenotype(ΔnfxB) mutants. The effect of AZM on young and mature biofilms was investigated in the modified Calgary Biofilm Device by estimation of the minimal biofilm inhibitory concentration (MBIC). The AZM MBIC90 in LB/RPMI1640 on young biofilms treated for 24 h was 16/4 μg/mL for PAO1, 32/8 μg/mL for ΔmutS, and 256/16 μg/mL for ΔnfxB, while in mature biofilms was 256/2 μg/mL for PAO1 and ΔmutS and 16/1 μg/mL for ΔnfxB. The effect of AZM was improved when the treatment was prolonged to 72 h, supporting the intracellular accumulation of AZM. An increased susceptibility of P. aeruginosa biofilms to AZM was observed in RPMI 1640 than in LB medium. Our results might improve susceptibility testing and dosing of AZM for treatment of biofilm infections.
Standard antibiotic susceptibility testing fails sometimes to detect antibiotics that are effective in vivo [1]. This is the case with azithromycin (AZM), a macrolide toward which Pseudomonas aeruginosa shows resistance in the standard antimicrobial testing media recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; Ca++ adjusted Mueller-Hinton) but that proved to be effective in patients with P. aeruginosa chronic biofilm lung infections, such as patients with cystic fibrosis (CF) [2, 3], patients with non-CF bronchiectasis [4], diffuse panbronchiolitis [5], and primary cell diskinesia [6]. Apart from Salmonella typhi and S. paratyphi, AZM [7] is not a standard treatment option for Gram-negative infections, susceptibility data and clinical breakpoints have not been published by EUCAST.
The mechanism explaining the good clinical effect of macrolides has been attributed to the immune-modulating effects of the host response [8, 9] and regulation of the P. aeruginosa virulence factors, including toxin production, biofilm establishment, and quorum sensing (QS) [10].
Acquired resistance to AZM of P. aeruginosa from CF patients can be ascribed to specific mutations in the V region of 23S rRNA [11-13] or in L4 protein [14]. This implies that AZM overcomes the penetration barrier through the cell wall of P. aeruginosa represented by low outer membrane permeability and efflux pumps. Emergence of resistance in CF P. aeruginosa isolates add to the available evidence [15, 16] suggesting that macrolides are active in vivo against P. aeruginosa.
The in vitro testing of susceptibility to macrolides shows a high minimum inhibitory concentration (MIC) against P. aeruginosa in the conventional prokaryotic media ((cation-adjusted Mueller-Hinton broth [CA-MHB] and Luria Bertani [LB]) but when the susceptibility test was performed in Roswell Park Memorial Institute (RPMI) 1640 medium, an eukaryotic cell culture medium and theoretically more similar to the nutritional conditions present in the biological fluids, the MIC was considerably reduced [17]. Buyck et al. [17] showed that the AZM MICs in CA-MHB were ≥128 μg/mL, while in RPMI 1640 medium was between 1 and 16 μg/mL. The explanation for the antimicrobial activity of macrolides in eukaryotic media against P. aeruginosa is a reduced efflux of the antibiotic and an increased uptake inside the bacteria, through a more permeable membrane [17]. These data were obtained in planktonic cultures; however, the chronic lung infections are caused by P. aeruginosa biofilms and bacteria in biofilms have a different physiology compared to planktonic cultures [18]. To get a better understanding of the clinical efficacy of AZM in patients with chronic pulmonary infections, we decided to investigate in vitro the effect of AZM on P. aeruginosa biofilms in RPMI 1640 medium compared with the LB medium which is a standard medium for experimental microbiology, the hypothesis being that AZM has a better effect on P. aeruginosa biofilms in RPMI 1640 medium than in LB medium. For comparison, the effect of the fluoroquinolone ciprofloxacin was also tested.
We used in this study P. aeruginosa reference strain (PAO1) and two mutants, a hypermutable strain (ΔmutS) as well as an antibiotic-resistant mutant due to the expression of MexCD-OprJ efflux pump, which has been shown to pump AZM out of the bacterial cell (ΔnfxB) [19]. The phenotypes of these mutants are frequently encountered in clinical P. aeruginosa isolates from patients with chronic biofilm lung infections [20, 21].
We have systematically investigated the in vitro effect of AZM on P. aeruginosa biofilms in the two different media using different AZM exposure times and concentrations for biofilms of different ages. In parallel, experiments on planktonic cultures have been performed. We show an increased susceptibility to AZM of P. aeruginosa biofilms when RPMI 1640 is used as testing media compared to LB. A concentration-dependent effect of AZM on P. aeruginosa planktonic cultures and a dose-dependent effect (concentration and time-dependent) in biofilms is demonstrated.
MATERIAL AND METHODS
Bacterial strains
The bacterial strains used in this study are presented in Table 1. The wild-type PAO1 wt1 is the background wild-type strain of the mismatch repair deficient isogenic (hypermutable) strain PAOΔmutS; PAO1 wt2 is the background wild-type strain of the overproducer of the MexCD-OprJ efflux pump (PAOΔnfxB). The use of the two background strains was necessary, as different sublineages of PAO1 are used in the different laboratories [22].
| P. aeruginosa strains | Characteristics | Reference |
|---|---|---|
| PAO1 wt 1 | ||
| PAOΔmutS constructed in PAO1 wt 1 background | PAOΔmutS deficient mismatch repair system (MMR), mutator strain | [26] |
| PAO1 wt 2 | ||
| PAOΔnfxB constructed in PAO1 wt 2 background | PAOΔnfxB deficient nfxB gene, overexpression of MexCD-OprJ efflux pump | Provided from A. Oliver lab [19]. |
Growth conditions
Prior to all experiments, bacterial overnight cultures were grown in Luria Bertani (LB) medium at 37 °C under aerobic conditions, with shaking at 200 rpm. LB broth was used for biofilm formation, and the AZM treatment was conducted in either LB broth or RPMI 1640 medium (Sigma-Aldrich; St. Louis, Missouri, USA). LB broth was chosen for biofilm formation, to insure ideal nutritional conditions for bacterial growth. LB broth and LB and RPMI 1640 agar plates were supplied by the Panum Institute Substrate Department (Copenhagen, Denmark).
Antibiotics used: ciprofloxacin (Ciprofloxacin hydrochloride—Cipro®, Bayer; Leverkusen, Germany) and azithromycin (azithromycin dihydrate—Zithromax® i.v., Pfizer; New York City, New York, USA) were added to media when needed.
Planktonic experiments
MIC determination in broth microdilution
MIC of ciprofloxacin (CIP) or azithromycin (AZM) were determined by broth microdilution in either RPMI or LB media. 100 μL of the medium was added to each well of a Nunc™ MicroWell™ 96-Well Microplates (Thermo Fisher Scientific) and 2-fold serial dilutions of the antibiotics were performed. The AZM concentrations that have been tested were 256, 128, 64, 32, 8, 4, 2, 1, and 0.5 μg/mL. The CIP concentrations that have been tested were 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.065, 0.031, and 0.015 μg/mL.
The overnight cultures of each strain were diluted (106) in the specific medium, and 100 μL was added to each well of the 96 wells, except for the blank. The microtiter plates were incubated at 37 °C, for 24 h, and the absorbance (OD 660 nm) was measured in the microplate reader Victor Nivo (Perkin Elmer, St. Louis, MO, USA). Background OD was subtracted from all values, and the MIC was defined as the lowest concentration of antibiotics that inhibit totally bacterial growth.
MIC determination by E-test
The overnight cultures of each strain were diluted (106 CFU/mL) and plated on LB or RPMI 1640 agar plates. e-test strips (Liofilchem; Waltham, Massachusetts, USA) were placed in the inoculated agar surface, and the plates were incubated at 37 °C for 24 h. The MIC was read in accordance with the manufacturer's instructions.
Growth curves in LB and RPMI 1640
In short, planktonic overnight cultures of the strains in LB broth were diluted to 106 CFU/mL in either LB or RPMI 1640, and 100 μL was added to each well of the 96 wells of the microtiter plate which was incubated in the Infinite F200 Pro plate reader (TECAN; Tecan Trading AG, Switzerland) on at 37 °C and shaken at 225 rpm for 24 h. The absorbance (OD 595 nm) was measured using the Magellan software (V2 program), every 20 min during the 24 h of incubation. Background OD was subtracted from all values.
For experiments testing the effect of the different concentration of antibiotics, CIP and AZM in different concentrations were added.
Biofilm experiments
Biofilm formation determination
Biofilms were formed on plastic pegs in the modified Calgary biofilm device (CBD) [23]. The pegs were incubated at 37 °C with bacterial cultures in LB for 24 h for formation of young biofilms and for 72 h for formation of mature biofilms. To determine the dynamic of biofilm formation, incubation periods of 2, 3, 4, 6, 8, 12, 18, 24, 48, and 72 were performed.
The biofilm biomass was quantified with crystal violet (CV) staining. In short, the biofilm formed on Nunc-TSP was rinsed three times in saline and transferred to a microtiter plate with 120 μL of 0.1% of CV in each well. In the CV, the pegs were incubated for 15 min at room temperature. The Nunc-TSP was rinsed three times with distilled water and was turned to dry. The Nunc-TSP was photographed to document the amount of biofilm that was adhered to the surface of the pegs. After the CV was dry, the Nunc-TSP was transferred to a 96-well plate with 120 μL of 30% acetic acid in each well and incubated for 15 min at room temperature. After the incubation, the absorbance (OD 590 nm) was measured in the microplate reader Victor Nivo (Perkin Elmer). Background OD was subtracted from all values.
MBIC determination (minimal biofilm inhibitory concentration)
The strains were grown in LB broth overnight. The overnight cultures of each strain were diluted (10−6 CFU/mL) in LB broth, and 120 μL was added to each well of the Nunc™.
MicroWell™ 96-Well Microplates (Thermo Fisher Scientific). The plates were incubated with the Nunc—Transferable Solid Phase screening system (Nunc-TSP; Thermo Fisher Scientific) on at 37 °C and incubated for 24 h forming young biofilms or 72 h forming mature biofilms.
After washing it three times in saline, the Nunc-TSP was transferred to the new plate with two-fold serial dilutions of antibiotics (in concentrations indicated before) and incubated for 24 h or 72 h at 37 °C.
After each 24 h of incubation, the medium with or without antibiotics was replaced.
After the treatment, the Nunc-TSP was rinsed three times in saline and transferred to a LB broth microtiter plate. The plate was sonicated in the Branson 1510 (Sigma-Aldrich) and incubated for 8 h at 37 °C. After the 8 h of incubation, the absorbance (OD 660 nm) was measured in the microplate reader Victor Nivo (Perkin Elmer). Background OD was subtracted from all values. MBIC90 was considered the lowest antibiotic concentration that shows a 90% reduction compared to the biofilm formed in the recovery plate control column after 8 h at 37 °C.
CFU determination
To determine the number of bacteria in the biofilms after treatment, CFU/mL of the sonicated biofilms were counted.
For CFU counts, cells were diluted serially in saline and plated on LB agar plates which were incubated for 24 h at 37 °C. The number of colonies was determined with a Stuart Scientific digital colony counter (Bibby Sterlin Ltd; UK).
Data processing
The excel sheets, obtained from the machines as the Victor Nivo plate reader or the TECAN with the raw data, were transferred to the program Graphpad Prism (GraphPad Software, Boston, MA, USA) to do the statistical analysis and the graphs. Before the data were introduced in the software, the blank mean obtained in each reading was deducted from each data point and the results were normalized with the control mean. The control means contained bacteria growing freely in the medium without antibiotics. All the graphs were represented with the SEM.
Statistics
All statistical tests and graphs were performed using Graphpad Prism (Graphpad Software Inc.). This was followed by a post hoc Dunnett's multiple comparison test, to test for significantly different groups within the datasets. Biofilm data were tested for statistical significance using two-way ANOVA and subsequently post hoc tests, Sidak's and Tukey's, for multiple comparisons. Six replicates for each condition were performed.
Significance representation: no significant (ns) meant p > 0.05. For significant results—* represented p ≤ 0.05; ** meant p ≤ 0.01; and *** meant p ≤ 0.001.
RESULTS
The effect of LB and RPMI on planktonic cultures
Growth curves
Analysis of the growth curves of the four P. aeruginosa strains in LB and RPMI 1640 showed no significant difference between the growth rates of the different strains in the two media. The results indicate the availability of nutrients in the growth media as a determinant factor. In the rich nutrient medium (LB), the bacterial yield was higher than in the low nutrients medium (RPMI 1640; Fig. 1). Once the maximum OD was reached, both strains had a prolonged stationary phase for the rest of the experiment.
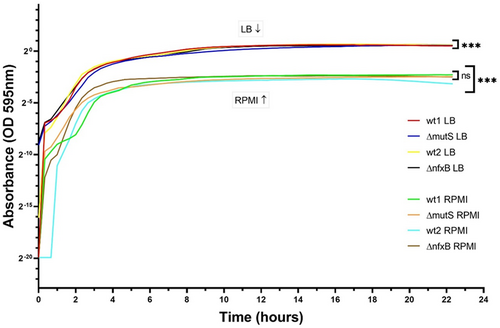
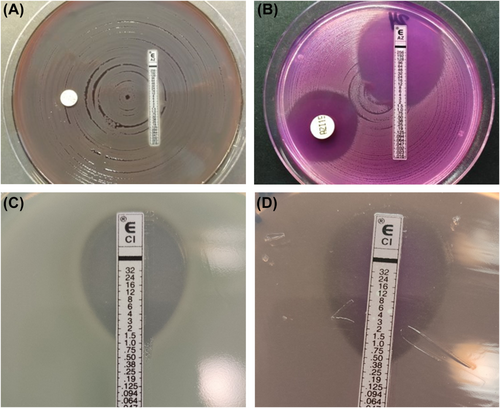
MIC of AZM and CIP on planktonic Pseudomonas aeruginosa
Minimum inhibitory concentration (MIC) determinations of CIP and AZM toward the P. aeruginosa strains by the e-test and the microdilution broth were performed. (Fig. 2A–D) and Table 2.
| Strain | E-test MIC (μg/mL) LB | E-test MIC (μg/mL) RPMI | ||
|---|---|---|---|---|
| AZM | CIP | AZM | CIP | |
| PAO1 (wt 1) | +256 | 0.125 | 3 | 0.125 |
| PAO1ΔmutS | +256 | 0.064 | 2 | 0.125 |
| PAO1 (wt 2) | +256 | 0.125 | 24 | 0.125 |
| PAO1ΔnfxB | +256 | 1.25 | +256 | 0.5 |
The MIC values determined by e-test of the four strains are shown in Table 2. Lower MICs of AZM were found on RPMI 1640 compared to LB for all the studied strains, except the PAO1ΔnfxB mutant overexpressing the MexCD-OprJ efflux pump, supporting its role in the efflux of AZM [19].
The MIC of ciprofloxacin was similar in the two growth media for PAO1 wt 1, PAO1ΔmutS, and PAO1 wt2. The PAO1ΔnfxB mutant had a lower MIC of ciprofloxacin (0.5 μg/mL) on RPMI 1640 compared to LB (1.25 μg/mL; Fig. 2C,D).
The MICs of AZM and CIP determined by broth microdilutions in LB and RPMI 1640 of the four strains are shown in Table 3. As expected, the inhibitory properties of azithromycin were higher in the RPMI 1640 medium than in LB medium for all the tested strains (Table 3).
| Strain | MIC (μg/mL) LB | MIC (μg/mL) RPMI | ||
|---|---|---|---|---|
| AZM | CIP | AZM | CIP | |
| PAO1 (wt 1) | 128 | 2 | 32 | 1 |
| PAO1ΔmutS | 128 | 2 | 32 | 2 |
| PAO1 (wt 2) | 128 | 0.25 | 8 | 0.5 |
| PAO1ΔnfxB | +256 | 4 | 128 | 4 |
The PAO1ΔnfxB mutant strain had, as expected, a higher CIP MIC (4μg/ml) compared to the background strain PAO1 wt2 (0.25μg/ml in LB and 0.5μg/ml in RPMI 1640). The difference between the MIC of AZM in LB and RPMI 1640 was though smaller when tested by broth microdilution (Table 3) compared to e-test (Table 2).
Pharmacokinetic of azithromycin in planktonic Pseudomonas aeruginosa
As the MIC determination in broth gives the antibiotic concentration which inhibits growth after 24 h incubation, we were interested in following the dynamic in time of growth inhibition by different AZM concentration. Growth curves with different AZM concentration were measured during AZM exposure for 24 h (Fig. 3). The experiments showed that AZM has a concentration-dependent effect of the planktonic cultures in both media. AZM showed a better effect in RPMI 1640 medium.
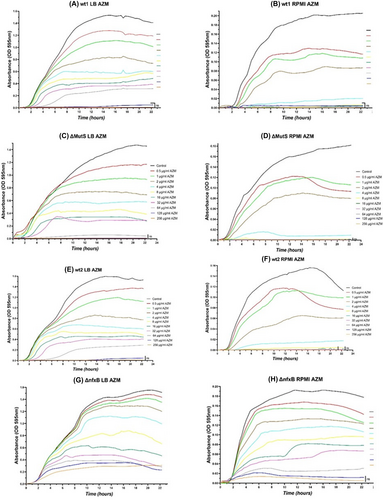
Effect of azithromycin (AZM) in the treatment of Pseudomonas aeruginosa biofilms
Dynamic of biofilm formation
Before testing the effect of the two growth media on AST on biofilms, we investigated the dynamic of biofilm formation in Modified Calgary device of the four strains during 72 h incubation in LB by measuring the biomass at 2, 3, 4, 6, 8, 12, 24, 48, and 72 h by CV staining and CFU measurements (Fig. 4).
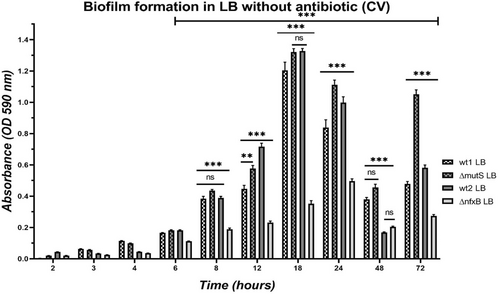
The two PAO1 wild types and ΔmutS had a similar pattern of biofilm formation (Fig. 4). After the initial attachment, the biomass was increasing with time. The biofilm formation of these three strains followed a cyclic model with a maximum growth after 18 h. The biomass decreased at 24 h, probably due to dispersion with a minimum around 48 h after the initial attachment, and the start of a next biofilm formation cycle (Fig. 4). At 72 h, there was a biomass peak again representing the maximum of a second biofilm formation cycle.
ΔnfxB had significantly lower biomass compared to its wild type (wt2), as measured by the CV absorbance (Fig. 4). The biofilm formation of ΔnfxB followed a similar cyclic model. The biomass of the biofilm formed by ΔnfxB reached a maximum peak at 24 h instead of at 18 h (as experienced for the wt2). Lower biomass was measured at 48 h when the second biofilm formation cycle started.
We investigated if the biofilm age correlated to the biomass of the biofilm, measured by OD absorbance of the crystal violet bound to the biofilm and to the number of P. aeruginosa cells in the biofilms formed on the plastic pegs, measured by determination of the colony-forming units (CFU/mL) of sonicated biofilms.
We found that there was a correlation between the age of the biofilm and the amount of living bacterial cells in the biofilm (Table 4), recapitulating the correlation between biofilm age and the biomass (CV; Fig. 4).
| Time | PAO1 (wt 1) (CFU/mL) | PAO1ΔmutS (CFU/mL) |
|---|---|---|
| 2 h | 0 (too few to be detected) | 0 (too few to be detected) |
| 4 h | 1.68 × 103 | 1.332 × 103 |
| 6 h | 2.14 × 103 | 3.54 × 103 |
| 8 h | 1.676 × 105 | 3.26 × 104 |
| 12 h | 1.04 × 106 | 2.24125 × 105 CFU/mL |
| 18 h | 8.16 × 106 | 8.74 × 105 |
| 24 h | 5.6625 × 106 | 2.225 × 106 |
| 48 h | 2.349 × 106 | 9.34 × 105 |
| 72 h | 4.44 × 105 | 4.22 × 106 |
The number of bacteria cells was increasing with time. The maximum number of cells was around 106 CFU/mL, obtained between 18 and 24 h. Once the number of live cells reached the maximum amount, the level was maintained at 48 and 72 h.
The effect of azithromycin on Pseudomonas aeruginosa biofilms of different ages (24 and 72 h)
To test the effect of the biofilm age and of the time of exposure to the antibiotic for the effect of AZM in biofilms, young (24 h) and mature biofilms (72 h) were formed. Exposure to AZM for 24 and 72 h was performed. The results for P. aeruginosa PAO1 wt1 and the ΔmutS are shown in Table 5 and for P. aeruginosa PAO1 wt2 and ΔnfxB in Table 6.
| Conditions | MBIC90 LB (μg/mL) | MBIC90RPMI (μg/mL) |
|---|---|---|
| PAO1 (wt1)–young biofilms—24 h AZM | 16 | 4 |
| PAOΔmutS–young biofilms—24 h AZM | 32 | 8 |
| PAO1 (wt1)–young biofilms—72 h AZM | 8 | 4 |
| PAOΔmutS–young biofilms—72 h AZM | 8 | 1 |
| PAO1 (wt1)–mature biofilms—24 h AZM | + 256 | 2 |
| PAOΔmutS–mature biofilms—24 h AZM | + 256 | 2 |
| PAO1 (wt1)–mature biofilms—72 h AZM | 4 | 4 |
| PAOΔmutS–mature biofilms—72 h AZM | 8 | 1 |
| Conditions | MBIC90 LB (μg/mL) | MBIC90RPMI (μg/mL) |
|---|---|---|
| PAO1 (wt2)–young biofilms—24 h AZM | 256 | 64 |
| PAOΔnfxB–young biofilms—24 h AZM | 256 | 16 |
| PAO1 (wt2)–young biofilms—72 h AZM | 4 | 1 |
| PAOΔnfxB–young biofilms—72 h AZM | 32 | 4 |
| PAO1 (wt2)–mature biofilms—24 h AZM | +256 | 8 |
| PAOΔnfxB–mature biofilms—24 h AZM | 16 | 1 |
| PAO1 (wt2)–mature biofilms—72 h AZM | 8 | +256 |
| PAOΔnfxB–mature biofilms—72 h AZM | 32 | 4 |
The MBICs90 of young biofilms treated for 24 h with AZM were lower in RPMI 1640 medium than LB broth of all the four strains (Tables 5 and 6). Prolonged AZM treatment (72 h) improved the effect of AZM on young biofilms of the four strains (Tables 5 and 6). The effect of the prolonged treatment was also observed in LB medium, although the effect was more pronounced in RPMI 1640 medium.
In general, the mature biofilms exhibit a higher MBICs90 to AZM compared to young biofilms. The MBICs90 of mature biofilms treated for 24 h with AZM were lower in RPMI 1640 medium than in LB. Prolonged AZM treatment (72 h) improved the effect of AZM on mature biofilms.
The MBIC90 in LB for mature biofilms of ΔnfxB after 24 h AZM treatment was lower (16 μg/mL) compared with the PAO1 wt (256 μg/mL) probably due to the poor biofilm formation ability of this mutant (Fig. 4). However, this was not observed in RPMI 1640.
In conclusion, despite the variation between the different bacterial populations, the biofilm experiments results showed that AZM had generally a better effect in RPMI 1640 than in LB media and that AZM has a dose-dependent (concentration and time-dependent) effect on biofilms.
DISCUSSIONS
We demonstrate in this study that AZM has a better inhibitory effect on biofilm-growing P. aeruginosa in the eukaryotic cell culture medium (RPMI 1640) than in the prokaryotic broth (LB), as earlier demonstrated for planktonic cultures. In accordance, in our study the planktonic MICs in RPMI 1640 medium were significantly lower than in LB broth for all the strains tested. Buyck et al. [17] explained the mechanism by the ability of RPMI 1640 medium to block the efflux pumps (disruption of oprM) and increase the permeability of the outer membrane of P. aeruginosa, allowing the antibiotic to enter the cell and carry out their effect. According to Buyck et al. [17], only macrolides and ketolides have a better effect in the eukaryotic cell culture (RPMI 1640) medium. In accordance, we show that the CIP MICs were similar in RPMI 1640 medium or in LB broth. This can be explained by the better penetration of the fluoroquinolone compared to AZM through the outer membrane of P. aeruginosa.
One of the adaptive strategies of P. aeruginosa for survival during chronic biofilm infections is the selection of mutator strains, with high mutation rates exhibiting increased adaptability during changing conditions. In our study, we observed similar effects of AZM on the hypermutable strain (ΔmutS) and its wild type (wt1). However, it has been shown in P. aeruginosa isolates from patients with cystic fibrosis and chronic P. aeruginosa lung infection treated with AZM, that resistance to AZM do occur due to mutations in the target gene (23S RNA and L4) [11, 12, 14]. As the hypermutable phenotype promotes occurrence of antimicrobial resistance [21, 24], evolution experiments in biofilms treated with AZM will reveal if development of resistance to AZM occur faster in mutator P. aeruginosa strains.
The importance of the efflux pumps in the resistance to AZM [19] and CIP [25, 26] in planktonic P. aeruginosa populations was confirmed by our results. We found that the overproducer of efflux pumps (ΔnfxB) showed increased MICs comparing to the background strain. So, the efflux pump, regulated by the repressor nfxB, accommodate different antibiotics and pump out macrolides and quinolones. The high AZM MIC in RPMI medium of ΔnfxB showed that a higher expression of efflux pumps compared to the wild-type can overcome the specific blocking ability of the RPMI medium, allowing AZM to be expelled out of the cell and the bacterial survival to high concentrations of the antibiotic. The interpretation of the effect of AZM on ΔnfxB biofilms compared to the wild-type strain (PAO1 wt2) is challenging, due to the poor biofilm formation of ΔnfxB. However, we observed a higher MBIC90 of young ΔnfxB after 72 h AZM treatment of biofilms compared to PAO1 wt2.
We showed that mature biofilms were more difficult to treat with AZM than young biofilms probably because of the thickness and the reduced diffusion and permeability, confirming previous results with other anti-pseudomonal drugs [18]. We also showed the superiority of long-term (prolonged treatment with AZM) in both media. This might be explained by the increased accumulation of AZM during the 3 days of treatment in the biofilm biomass.
These results suggest that in biofilms, the intracellular accumulation of AZM might take longer time than in planktonic cultures, due to the low metabolism experienced by the bacterial cells embedded in biofilms but an impaired diffusion of AZM through the biofilm matrix cannot be excluded. Our results showed that the longer the treatment, the better the effect of the drug on P. aeruginosa biofilms. Additionally, the higher the AZM concentration, the higher the antibiotic effect. It has been shown that, during the long exposure to the antibiotic, AZM accumulates inside the bacteria and in the PMNs [16]. The consequence of longer exposure period is a higher concentration of the antibiotic at the active site and, with time, more molecules will block the protein synthesis.
According to Wilms et al. [27], after a dose of 500 mg AZM, the maximum clinical achievable concentration in sputum was 53 μg/mL, while in the lung tissue was 8 μg/mL. The tissue concentration resulted from the AZM concentration inside immune cells, where most of the drug was accumulated and released. Particularly, polymorphonuclear neutrophils (PMNs) were found to have high intracellular concentrations, 305 ± 82 μg/mL [28]. Overall, tissue and intracellular concentrations were found to be considerably higher at infected sites, than in plasma. However, there are no data about the actual concentration of AZM present in P. aeruginosa biofilms in the lungs. Overall, the concentrations reached in the lungs, blood, and sputum are generally above the MBICs measured in RPMI 1640 medium. This means that the in vivo achievable AZM concentrations have the potential to inhibit P. aeruginosa biofilms, especially if the antibiotic is accumulating in the biofilm cells.
Our results confirm that besides the anti-virulence and anti-inflammatory properties, AZM also has an anti-pseudomonal effect that explains its beneficial effect in chronic P. aeruginosa lung infections [2-6].
We showed a changed pharmacokinetic profile of AZM in biofilms, compared to planktonic cultures, underlining the importance of testing the efficacy of antibiotics that are used for the treatment of chronic infections, in biofilms. The improved effect observed in prolonged treatment of biofilms support the long-term treatment with AZM of chronic biofilm infections. The main drawback of the long-term treatments is the risk of the development of resistance to the AZM [11, 12, 14].
We also showed that the production of efflux pumps is one of the main resistance mechanisms recruited by P. aeruginosa decreasing the in vitro efficacy of AZM.
In conclusion, our results support the hypothesis that AZM has a better effect on P. aeruginosa biofilms in RPMI 1640 medium than in LB medium, as shown in planktonic cultures, underlining the importance of choosing a relevant bacterial growth media when testing antimicrobial susceptibility. Future studies in more clinically relevant growth media, such artificial sputum media are warranted.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




