Preoperative BRAFV600E mutation detection in thyroid carcinoma by immunocytochemistry
Abstract
The BRAFV600E (BRAF) mutation is present in 40–50% of papillary thyroid carcinomas (PTC) and has been associated with more aggressive clinicopathological characteristics of PTC. The aim of this study was to evaluate different methods for preoperative identification of the BRAF mutation in PTC using cytological and histological specimens. Prospectively collected preoperative cytological clots from patients with suspected PTC were tested with BRAF immunocytochemistry (ICC) and the Cobas Test (PCR). In addition, histological specimens were tested with BRAF immunohistochemistry (IHC) and the Cobas Test. All nodules were histologically examined. Fifty-three patients were included in the study. Complete mutation testing was available in 32 patients. The main reason for exclusion was insufficient cell content in the cytological specimen. Twenty-seven nodules were histologically diagnosed as PTC, and 41% (n = 11) of PTCs were BRAF ICC positive. All non-PTC nodules were negative by BRAF ICC. In 26 nodules, all four BRAF tests were concordant, while discordant test results were found in six nodules. ICC was in accordance with the consensus BRAF status in five of these nodules, while BRAF status was undetermined in one nodule. BRAF ICC showed high concordance with the Cobas Test and a low rate of false negative stain. These results indicate that BRAF ICC may be a feasible method for preoperative detection of the BRAFV600E mutation in patients with PTC.
INTRODUCTION
Molecular markers and the detection of disease specific mutations have the potential to refine the preoperative risk-stratification of thyroid nodules in terms of diagnosis and prognostication. Several specific mutations as well as test kits assessing combinations of mutations have been investigated (1-4). As with other diagnostic tests, a substantial overlap in genetic alterations between benign thyroid lesions and cancers has been reported, which compromises the sensitivity and the specificity (5).
The BRAFV600E mutation is present in 32–90% of papillary thyroid carcinoma (PTC), in 24% of anaplastic thyroid carcinoma (ATC), and in poorly differentiated carcinoma, although with low prevalence (9%) (6-9). This mutation has never been detected in other subtypes of thyroid carcinoma, benign thyroid lesions, or normal thyroid tissue (6). The BRAFV600E mutation seems associated with more aggressive clinicopathological characteristics of PTC (6, 10), although this finding remains controversial (8, 11). Several studies, even if based on cytological specimens (12), found this mutation to be associated with lymph node metastasis, multifocality, extrathyroidal extension, advanced stage at diagnosis, and a higher recurrence rate in PTC (6, 10, 13-15). Further, the BRAFV600E mutation was found with higher frequency in the more aggressive pathological subtypes of PTC including tall-cell variant (77%), as opposed to a lower frequency in the follicular variant (12%) (16). More recent studies suggest that BRAFV600E positivity in combination with telomerase reverse transcriptase (TERT) mutation depicts a poorer prognosis of PTC (17, 18), than each mutation does alone. TERT mutations co-exist with BRAFV600E in 7–9% (19, 20). If preoperative BRAFV600E mutation status can be assessed on cytological specimens this would have important implications for the perioperative strategy as well as the postoperative management of the patient.
Different methodologies are available for BRAFV600E detection. Methods based on gene sequencing and polymerase chain reaction (PCR) can be used on histological as well as cytological specimens (6, 12, 21, 22). The Cobas Test is a real-time PCR method designed to detect the BRAFV600E mutation (22). The Cobas Test has shown excellent performance for identifying the BRAFV600E in formalin fixed paraffin embedded tissue whether it is from PTC or malignant melanoma tissue (23-26). However, the sensitivity of PCR is highly dependent on the fraction of malignant thyroid tissue contained in the sample. This complicates the procedure because isolation of tumor tissue often is necessary prior to mutation analysis (21, 27).
Immunohistochemistry (IHC), using a mutation-specific antibody (VE1), is another method for BRAFV600E detection in histological specimens of PTC. Compared with direct sequencing or PCR, BRAF IHC has a pooled sensitivity of 98–100% and a pooled specificity of 84–89%, and this method has been proposed as a reliable screening test for PTC (28). Immunocytochemistry (ICC) is based on a similar principle but can be applied to cytological specimens (i.e.), which is a major advantage as the sample can be obtained preoperatively (29).
The aim of this study was to improve preoperative thyroid nodule risk-stratification, by evaluating cytological application of BRAFV600E ICC on thyroid nodules suspected of thyroid carcinoma, and to compare the results with BRAFV600E IHC and the Cobas Test.
METHODS
Patient inclusion
The study was an open-label prospective cohort study. Patients were recruited in the period November 2014–July 2016 at the Department of Oto-Rhino-Laryngology, Aarhus University Hospital. Inclusion criteria were adult patients with thyroid nodules ≥1 cm, indication for thyroid surgery, and a preoperative fine needle aspiration biopsy (FNAB) in category V–VI, as classified by the Bethesda system for reporting thyroid cytopathology (BSRTC). Patients were excluded from analyses if cells were absent in the cytological clot or if tissue samples were missing during follow-up. Written informed consent was obtained from all participants before enrolment in the study.
Patients were examined according to national guidelines (30), including clinical examination, thyroid and neck ultrasound, FNAB, and in most patients a thyroid 99Tc-scintigraphy. All nodules were removed by surgery, and the histopathological examination, performed by a specialized endocrine pathologist, served as the diagnostic gold standard.
The thyroid nodules were assessed ultrasonographically according to a modification of the TIRADS score (31). Features of high suspicion were microcalcifications, hypoechogenicity (pooling of mild and marked hypoechogenicity), irregular margins, and taller-than-wide shape. TIRADS 3 was assigned nodules without any suspicious ultrasound feature; TIRADS 4 referred to nodules harboring 1–2 suspicious features; TIRADS 5 was assigned nodules with 3–4 features present and/or suspicious lymph nodes at neck ultrasound examination.
Thyroid specimens
All FNABs were guided by thyroid ultrasound. At least two needle passes were performed, one for air-dried smears and one for a cytological clot. Cytological clots contained aspirated cells suspended in isotonic saline. In some patients, cytological specimens were available from the pre-operative routine FNAB examination. In patients without available clots, ultrasound guided FNABs were collected perioperatively, using a 23G needle. The smears were used for a conventional cytopathological evaluation. The cytological clots were formalin fixed and paraffin embedded and sliced for BRAFV600E testing. Both smears and clots were reassessed by the study pathologists (SHM, MLJ). Histological tissue for BRAFV600E testing was collected, by a pathologist, from the formalin fixed and paraffin embedded (FFPE) post-surgical specimen.
BRAFV600E mutation testing
The Cobas 4800 BRAF V600 Mutation Test (Molecular Diagnostics, Roche Diagnostics A/S, Hvidovre, Denmark) was performed on both the cytological clot (cCoT) and the histological specimen (hCoT). The tests were performed according to the manufacturer's instructions, on 5 μm nodular tissue section of FFPE histological or cytological samples from the nodule. DNA was isolated using the Cobas DNA Sample Preparation Kit. The real-time PCR tests were performed by two complementary primers containing parts of the BRAF-gene covering Codon 600. One probe detected the wild-type BRAFV600, and the other one detected the BRAFV600E sequence. Results were binary, that is, the presence or absence of mutation.
Staining by immunochemistry was performed on histological slides (IHC) and cytological clots (ICC). The monoclonal mouse antibody VE1 (dilution 1:40, Spring Bioscience, Pleasanton, CA, USA) directed against proteins in BRAFV600E mutated cells was used. VE1 was visualized by Ultraview, employing a Horse Radish Peroxidase conjugated polymer together with the chromogen 3,3′-diaminobenzidine (Roche A/S, Hvidovre, Denmark). The intensity of the BRAFV600E protein stain was blindly and independently evaluated by two experienced pathologists (MLJ, SHM) using light microscopy. Samples were assigned a category according to the staining pattern: (0) absent (no stain), (1) weak positive (<10% stain or weak stain intensity), or (2) present / positive (≥10% stain and strong stain intensity), (3) invalid / non-conclusive sample (no cells). In case of disagreement between the two observers, consensus was reached by discussion.
Pathological examination
The pathological examination was performed by specialized thyroid pathologists (MLJ, SHM). Cytological assessment was performed using the BSRTC (32) with assignment of a category from I-VI. Histological assessment was performed according to the WHO classification (33). Nodules harboring a PTC of less than 1 cm within an otherwise benign index nodule were categorized as microPTC.
Statistical methods
Categorical variables are presented as numbers and percentages. Continuous variables are presented as mean and standard deviation (SD) or median, interquartile range (IQR), and range according to normality testing. Comparison between groups was done by Student's t-test or Mann–Whitney rank-sum test for continuous data, and chi-squared or Fischer's exact test for categorical data. Proportions of agreement assessed the correlations between observers or test modalities, respectively. A level of significance of 0.05 was applied. The statistical software used was Stata 13 (Metrika Consulting AB, Stockholm, Sweden) and Excel 2010 (Microsoft).
RESULTS
Participants
Patient characteristics are summarized in Table 1. A total of 53 patients and 53 thyroid nodules were included. BRAFV600E testing was performed in 38 nodules, whereas complete follow-up succeeded in 32 patients (Fig. 1). The main reason for exclusion (n = 18) was a cytological clot without cellular content (Fig. 1). The majority of the BRAFV600E tested nodules were TIRADS 4–5 (91.3%) by thyroid ultrasound, and almost equally distributed between BSRTC V (46%) and BSRTC VI (54%) by pathological examination (Table 1). Nodules examined for a BRAFV600E mutation included 27 PTC (71%) (including two microPTC in an otherwise benign index nodule), seven benign nodules (18%), three non-PTC malignancies (8%), and one well-differentiated tumor with unknown malignancy potential (WDT-UMP) (3%) (Table 1). The risk of malignancy in all nodules (n = 53) was 48% and 92% in the BSRTC V and BSRTC VI category, respectively. In BRAFV600E tested nodules (n = 38), the corresponding risks were 65% and 95%, respectively. The mean size of the nodules was 27.3 ± 9.1 (SD) mm (range: 10.0–49.8 mm) on preoperative ultrasound.
| All patients, n = 53 | BRAFV600E tested, n = 38 | |
|---|---|---|
| Demographics | ||
| Age, mean ± SD years | 53 ± 14 | 54 ± 15 |
| Sex, F/M, n (%) | 41 (77)/12 (23) | 28 (74)/10 (26) |
| Levothyroxine substitution, n (%) | 4 (8) | 3 (8) |
| Imaging, n (%) | ||
| TIRADS1 | ||
| TIRADS 2–3 | 4 (11) | 2 (9) |
| TIRADS 4 | 13 (36) | 9 (39) |
| TIRADS 5 | 19 (53) | 12 (52) |
| Pathological lymph nodes by ultrasound | 5 (9) | 4 (11) |
| Pathology, n (%) | ||
| Cytology (smear)2 | ||
| Suspect for malignancy (BSRTC V) | 25 (49) | 17 (46) |
| Malignant (BSRTC VI) | 25 (47) | 20 (54) |
| Histology | ||
| Benign | 17 (32) | 7 (18) |
| PTC/microPTC3 | 28 (53)/3 (6) | 25 (66)/2 (5) |
| FTC | 1 (2) | 1 (3) |
| MTC | 1 (2) | 1 (3) |
| Other | 3 (6)4 | 2 (5) |
- BSRTC, The Bethesda system for reporting thyroid cytopathology; TIRADS, thyroid imaging reporting and data system.
- 1 TIRADS score registered in 36 patients (67.9%).
- 2 Data missing in 3 patients.
- 3 MicroPTC: area of PTC <1 cm within an otherwise benign index nodule.
- 4 Well-differentiated tumor with unknown malignancy potential originated from the follicular epithelia (n = 2); Metastasis from squamosal cell carcinoma (n = 1).
BRAFV600E mutation testing
BRAFV600E test results are shown in Table 2. BRAFV600E ICC was positive in 41% (n = 11) of PTC nodules. Four PTC and one non-PTC samples were non-conclusive by BRAFV600E ICC due to low cell content. No non-PTC nodules were positive by either ICC, cCoT, or hCoT. One sample was false positive on IHC, and histologically characterized as a WDT-UMP. The resulting accuracy for identifying the BRAFV600E mutation and for the diagnosis of PTC is shown in Table 3.
| BRAF test | Test result | |||||
|---|---|---|---|---|---|---|
| Positive, n | Negative, n | Inconclusive, n | ||||
| PTC | Non- PTC | PTC | Non- PTC | PTC | Non- PTC | |
| ICC | 11 | 0 | 12 | 10 | 4 | 1 |
| IHC1 | 10 | 1 | 16 | 10 | ||
| hCoT | 9 | 0 | 17 | 11 | 1 | 0 |
| cCoT2 | 8 | 0 | 16 | 11 | 2 | 0 |
- cCoT, cytological Cobas Test; hCoT, histological Cobas Test; ICC, immunocytochemistry; IHC, immunohistochemistry; PTC, papillary thyroid carcinoma.
- 1 One sample not tested.
- 2 One sample not tested.
| BRAF test | n | Proportions of agreement, % | Sens1 | Spec1 | NPV1 | PPV1 |
|---|---|---|---|---|---|---|
| Test result compared with hCoT (identifying BRAFV600E mutation) | ||||||
| ICC vs hCoT | 33 | 93.9 | 100 | 91.7 | 100 | 81.8 |
| IHC vs hCoT | 37 | 89.2 | 88.9 | 89.3 | 96.2 | 72.7 |
| cCoT vs hCoT | 32 | 84.4 | 66.7 | 92.0 | 88.5 | 75.0 |
| Test result compared with histological diagnosis (diagnosing PTC) | ||||||
| ICC | 33 | NA | 47.8 | 100 | 45.5 | 100 |
| IHC | 37 | NA | 38.5 | 90.9 | 38.5 | 90.9 |
| hCoT | 37 | NA | 34.6 | 100 | 39.3 | 100 |
| cCoT | 35 | NA | 33.3 | 100 | 40.7 | 100 |
- n, number; NA, not applicable; PTC, papillary thyroid carcinoma.
- 1 Exclusion of non-conclusive or samples not tested.
Concordance between all four BRAFV600E mutation tests was found in 26 of 32 nodules, including six BRAFV600E positive and 20 BRAFV600E negative nodules. Discordance was found in six nodules (Table 4), of which four PTC were negative by hCoT (n = 2) or cCoT (n = 2), but BRAFV600E positive by all three remaining tests. In one case, representing a microPTC, hCoT was positive for BRAFV600E mutation and ICC was weakly positive, while IHC and cCoT both were negative. The sixth discordant result was found in the patient with a WDT-UMP. Based on all available results, consensus on the BRAFV600E status was reached among the authors (Table 4), except in one case. This resulted in a BRAFV600E mutation prevalence of 40% (10/25) in PTC and 0% in non-PTC lesions.
| ID | Histology | BSRTC | ICC | IHC | cCoT | hCoT | Consensus BRAF status | Conclusion |
|---|---|---|---|---|---|---|---|---|
| 507 | PTC/microPTC | 6 | pos | pos | neg | pos | Positive | FN cCoT |
| 515 | PTC | 6 | pos | pos | neg | pos | Positive | FN cCoT |
| 522 | PTC | 6 | pos | pos | pos | neg | Positive | FN hCoT |
| 5251 | miPTC | 5 | weak pos 1 | neg | neg | pos | Indeterminate | |
| 5291 | Other | 5 | neg | pos 1 | neg | neg | Negative | FP IHC |
| 5411 | PTC | 6 | pos | pos1 | pos | neg | Positive | FN hCoT |
- Other: well-differentiated tumor with uncertain malignancy potential.
- BSRTC, The Bethesda system for reporting thyroid cytopathology; cCoT, cytological Cobas Test; FN, false negative; FP, false positive; hCoT, histological Cobas Test; ICC, immunocytochemistry; IHC, immunohistochemistry; neg, negative; pos, positive; PTC, papillary thyroid carcinoma.
- 1 Diagnosis solved by consensus (see Table 5). Results considered as false are highlighted in the table.
The accuracy of each method for BRAFV600E testing (ICC, IHC, hCoT and cCoT) was determined, using the BRAFV600E status obtained by consensus as reference. By such an approach, ICC showed 100% concordance with the consensus BRAFV600E result (10 BRAFV600E positive, 22 BRAFV600E negative), whereas the corresponding concordance rates for IHC, hCoT, and cCoT were 97%, 94%, and 94%, respectively.
Qualitative assessment of BRAF ICC and IHC
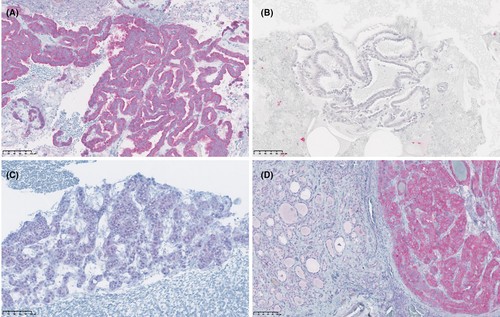
In the majority of ICC samples, the BRAFV600E stain was assessed as either unambiguous positive or negative (Fig. 2A, B). All eleven BRAFV600E ICC positive samples were diagnosed as PTC, of which ten were categorized as BSRTC VI. The last of these samples was characterized as BSRTC V and was weakly BRAFV600E positive by ICC (Fig. 2C). Histologically, this was diagnosed as microPTC. A clear demarcation between BRAFV600E positive PTC tissue and normal parenchyma was seen in all BRAFV600E positive samples, when examined by IHC (Fig. 2D).
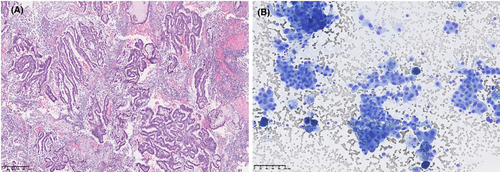
Importantly, the cytological clot provided valuable architectural information, in terms of micro-biopsies in several samples, in contrast to separated cells presented in the conventional smears (Fig. 3A, B).
BRAF IHC and ICC interrater agreement
Two study pathologists independently assessed the same ICC and IHC slides. The proportion of agreement of BRAFV600E ICC and IHC was 92.1% and 94.6%, respectively. Six specimens, three ICC and three IHC, were subject to discussion (Table 5). Three of these showed weak stain (Fig. 2C), two were non-conclusive by consensus due to low cell content, and one was judged positive by consensus after an initial disagreement between the investigators.
| ID | Pathologist A | Pathologist B | Consensus | Histology |
|---|---|---|---|---|
| ICC | ||||
| 511 | Negative | Non-conclusive | Non-conclusive | Benign |
| 525 | Weak positive | Negative | Weak positive | microPTC |
| 538 | Negative | Non-conclusive | Non-conclusive | PTC |
| IHC | ||||
| 529 | Weak positive | Positive | Positive | WDT-UMP |
| 535 | Weak positive | Negative | Negative | Benign |
| 541 | Positive | Negative | Positive | PTC |
- ICC, immunocytochemistry; IHC, immunohistochemistry; WDT-UMP, well-differentiated tumor with unknown malignancy potential.
DISCUSSION
This prospective study compares, head-to-head, four different methods for BRAFV600E detection. The BRAFV600E mutation was identified in 40% of PTC and in no non-PTC lesions, which is in line with previous findings (16, 34, 35). Our results were based on the results of all four tests used in the study and on the consensus reached by the investigators. The performance of ICC, using preoperative cytological clots, was excellent and showed high accuracy for the detection of the BRAFV600E mutation. Importantly, the ICC performed better than the Cobas Test, with a lower rate of false negative results than provided by both cCoT and hCoT. Both ICC and the Cobas Test were prone to a low cell content in the sample.
Importantly, the main advantage of ICC is the combined assessment of the mutation status and the quality of the sample in terms of cell count and tumor content. In this study, the Cobas Test was false negative in two cytological and histological samples, respectively, most likely due to a low tumor content in the specimen as assessed by ICC and IHC. This is an advantage in the area of thyroid cytopathology, because non-diagnostic tests account for approximately 12–16% of samples (36), which would be categorized as BRAF negative by other methods, for example, PCR. In case of a negative stain, the cytological appearance may tell the investigator whether this is due to a non-representative sample insufficient for diagnostic use. In addition, the cytological characteristics of the specimen can be evaluated simultaneously with the BRAF stain, thereby assessing the diagnosis of the sample and thus the representativeness of the sample in order to account for sampling error. Further, ICC is easily implemented as the method is well-established in most pathology departments.
IHC and ICC, using VE1 for the detection of the BRAFV600E mutation in PTC, have a sensitivity approximating 100% (28, 37). In a retrospective study, in which the prevalence of BRAFV600E was 67%, ICC detected the mutation with a sensitivity and a specificity of 93.8% and 93.8%, respectively (29). However, the method used in that study differed from ours in terms of tissue preparation as well as the gold standard employed for the mutation status (Sanger Sequencing) (29). A more recent study, using ICC (VE1) for the diagnosis of PTC, reported a specificity of 91% and a sensitivity of 62% of BRAFV600E positivity (38).
Different techniques exist for preparation of liquid-based cytology (LBC) and cytological cell blocks (39). In this study, we used saline as preservative prior to fixation in cellblocks, while LBC employ special preservatives (e.g., ethanol) and automatized processing of the samples (39, 40). Cell blocks provide additional information to a conventional smear, including micro-biopsies that reveal architectural and morphological features, and the possibility to apply ICC and molecular testing (39). In our study, a learning curve was evident, as most of the non-diagnostic cytological clots were seen in the initial phase of the study. Controversies exist as to whether LBC contributes to higher diagnostic yield or a higher rate of non-diagnostic samples. This may depend on the sampling method, that is., either a separate needle pass or use of remaining material after a smear. For now, LBC does not seem superior to conventional smear (39, 41-43).
The Cobas Test provides a dichotomous result and is observer independent due to the automatized set-up. The Cobas Test has been considered the gold standard for detection of BRAFV600E mutations due to a high reproducibility and an excellent analytic sensitivity, at least when applied to histological specimens (23, 24). In histological samples, the presence of tumor tissue is ensured by macro- and micro-dissection of the removed tissue, which is not applicable in cytological specimens. When applied to cytological clots, the validity of the Cobas Test may be compromised by the fact that assessment of the cellular tumor content can be challenging. This may explain why we found a higher rate of false negative results by the Cobas Test, as compared with ICC. Thus, samples with a low cellular content may be judged BRAFV600E negative by the Cobas Test, rather than being non-conclusive. Another drawback of the Cobas Test is the documented cross-reactivity with other BRAF-mutations at codon 600 including V600K and V600D (23-25). These latter mutations do not seem to be present in thyroid carcinomas (44), although V600K may be found in follicular variants of PTC and follicular carcinomas (34).
The prevalence of BRAFV600E mutation in PTC varies between 32% and 90%, depending on method, geographic area, and population (6, 34, 45). In addition, the prevalence differs between the pathological subtype of PTC, being 12% in the follicular variant of PTC (range: 0–32%), 60% in the conventional PTC (range: 38–83%), and 77% in the tall-cell variant of PTC (range: 33–100%) (16, 34). Considering its high PPV, ICC used for BRAFV600E detection may be a valuable preoperative PTC rule-in test, even in populations with a low mutation prevalence. The risk of malignancy increases with higher BSRTC category. The main challenge of thyroid cytopathology is the indeterminate BSRTC categories, accounting for approximately 20–30% of samples (32, 46), in which the malignancy rate varies between 10% and 30% (32). A diagnosis of PTC based on a cytological specimen is strongly supported by the presence of BRAFV600E, which may swift the surgical strategy towards a one-step total thyroidectomy (47). The BRAFV600E mutation has been reported also in the follicular variant of PTC (fvPTC), often cytologically classified as BSRTC IV (16, 48). Usually, the diagnosis of this subtype relies on a histopathological demonstration of invasion (32), but the diagnosis may be settled preoperatively if BRAFV600E is detected by ICC.
In tumors of follicular origin, the diagnostic threshold between benign and malignant disease is subject to variation among pathologist, as these lesions are difficult to differentiate histologically (49-51). Included in this group are other encapsulated follicular patterned thyroid tumors such as the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), well-differentiated tumor with uncertain malignancy potential (WDT-UMP), and follicular tumors of uncertain malignant potential (33). These tumors are characterized by a follicular and non-invasive pattern, and the presence of papillary nuclear features (33, 52). The detection of BRAFV600E in such tumors may prove valuable, as this would strongly support a diagnosis of PTC instead of NIFTP or WDT-UMP (53).
Molecular testing has the potential to improve diagnostics in thyroid nodules with indeterminate cytology and has been validated on a variety of specimen types, for example, LBC, smears, and cellblocks. American guidelines recommend molecular testing in BSRTC III and BSRTC IV nodules, mainly to rule out malignancy and thus reducing unnecessary diagnostic surgery (32, 54, 55). European Thyroid Association does not currently recommend molecular testing (3). The frequency of BRAFV600E mutations in BSRTC III and BARTS IV nodules is relatively low (1.8–22.9%) (48), and routine molecular testing, by applying combined panels, seems most relevant in indeterminate nodules. Several commercial test panels are available, but they are expensive and not globally accessible (53, 56). The recent third generation molecular panels (e.g., ThyroSeq v3, Gene Sequencing Classifier) perform with even higher sensitivity and specificity than previous versions (57, 58).
The diagnostic performance of molecular testing depends on the prevalence of malignancy in the investigated cohort and the reliability of other diagnostic tests used routinely. Close collaboration with specialized pathologists and high quality of the cytological evaluation are therefore crucial in order to select the most suitable patients for molecular testing (57). Most recent suggestions recommend always to compare cytological findings with the results of molecular testing and with reference to clinical features, ultrasound characteristics, and pathology results, however, such approaches need systematic evaluation (53, 57).
A few limitations need to be mentioned of this small explorative study. Due to the limited number of participants, only BSRTC V and VI nodules were included, which limits the external validity. In addition, the cell content was relatively low in some of the cytological clots due to either insufficient cell aspiration or as a result of the processing of the samples. An important strength of our study is the head-to-head comparison of different methodologies, cytological as well as histological, for the assessment of BRAFV600E mutation status. Further, the BRAFV600E status was assessed both at the protein level (VE1: ICC/IHC) and genetically (PCR: The Cobas Test). These issues are particularly important because thyroid nodules are heterogeneous of nature, which increases the risk of sampling error when performing FNAB.
CONCLUSION
Gene sequencing methods applied to histological samples are considered the gold standard for the detection of a BRAFV600E mutation (6). However, such methods applied to cytological specimens have an inherent risk of classifying acellular samples (BSRTC I) as negative rather than inconclusive. Accordingly, our study found ICC to be more accurate in the assessment of the BRAFV600E mutation status in BSRTC V-VI nodules, and with a higher specificity than found by the Cobas Test. The most likely explanation for this is the cytopathological examination embedded in ICC, whereby BSRTC I can be identified as non-diagnostic rather than BRAFV600E negative, leading to lower rates of false negative results.
If our results are confirmed in future studies including other BSRTC categories, ICC may turn out to be a feasible method for the preoperative detection of a BRAFV600E mutation in patients with PTC, and with important clinical implications. This method has the potential to personalize the treatment by directing the surgical approach and influencing the prognosis for the patient.
The study was supported financially by the Hans Skouby Foundation, Musikforlæggerne Agnes and Knut Mørks Foundation, Søster and Verner Lipperts Foundation, and Aase and Ejnar Danielsens Foundation.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare that are relevant to the content of this article.
CONSENT STATEMENT
Written informed consent was obtained from all participants before enrolment in the study.




