Loss of Na+,HCO3−-Cotransporter NBCn1 Inhibits Net Acid Extrusion in the Atria and Causes Hypertension-Associated Cardiac Hypertrophy
Funding: This work was supported by the Aarhus University Research Foundation (AUFF-E-2021-9-18 to Boedtkjer), the Novo Nordisk Foundation (NNF21OC0071822 to Boedtkjer), and the Danish Cardiovascular Academy (PD2Y-2 021 002-DCA to Espejo). The Danish Cardiovascular Academy is funded by the Novo Nordisk Foundation (NNF20SA0067242) and the Danish Heart Foundation. Exchange visits from Espejo and Orlowski to Aarhus University were supported by Coimbra scholarships and a travel stipend from the Science and Technical Secretary of Universidad Nacional de La Plata, Argentina.
ABSTRACT
Aim
Metabolic disturbances challenge pH homeostasis in cardiomyocytes. The electroneutral Na+,HCO3−-cotransporter NBCn1/Slc4a7 mediates net acid extrusion, and genetic variation in SLC4A7 contributes to human hypertension and cardiovascular risk. Nonetheless, the cardiac consequences of disrupted NBCn1 expression and function remain unclear. Here, we test the hypothesis that NBCn1, either directly or indirectly, influences cardiac structure, contractile function, and electrophysiological properties.
Methods
Based on mice with global loss of NBCn1, we measure intracellular pH in atria and ventricles of the heart (fluorescence microscopy), membrane potential responses (patch clamping), electro- and echocardiographic variables, blood pressure (telemetry), and cardiac dimensions (in vivo and postmortem analyses).
Results
We find that protein and mRNA expression of NBCn1 are more prominent in atrial than in ventricular cardiomyocytes. Disruption of NBCn1 expression lowers Na+,HCO3−-cotransport activity more than 50% in atria without significantly influencing net acid extrusion activity of ventricular cardiomyocytes. Loss of NBCn1 is associated with hypertension (blood pressure increased by ~15 mmHg), cardiac hypertrophy (heart/body weight increased by ~10%), and prolonged ventricular isovolumic relaxation time (increased by ~25%). NBCn1 knockout does not affect cardiomyocyte size, collagen content in the heart wall, overall cardiac contractile function, electrophysiological properties of ventricular cardiomyocytes, or the electrocardiogram.
Conclusion
NBCn1 is a main mechanism of Na+,HCO3−-cotransport in atrial tissue and contributes substantially to net acid extrusion during intracellular acidification. NBCn1 does not play any major direct role in ventricular cardiomyocytes of unchallenged mice, but global knockout of NBCn1 increases systemic blood pressure and results in the development of cardiac hypertrophy.
1 Introduction
Acidic waste products derived from the constant metabolic demand of the pumping heart and H+ leaks driven by the inwardly directed electrochemical H+ gradient challenge the control of intracellular pH (pHi) in cardiomyocytes. At steady-state, the concerted actions of multiple acid–base transporters balance the acid load and maintain pHi well above the transmembrane equilibrium. Net acid extrusion in the heart is largely Na+-dependent and mediated via Na+/H+-exchange and Na+,HCO3−-cotransport, with additional contributions from monocarboxylate transporters that extrude H+ ions together with carboxylate metabolites such as lactate [1].
Electroneutral and electrogenic Na+,HCO3−-cotransport have both been demonstrated in ventricular cardiomyocytes, but their relative contribution is controversial [2]. Expression of the electroneutral Na+,HCO3−-cotransporter NBCn1, encoded by the Slc4a7 gene, has been confirmed in ventricular biopsies [3]; however, conclusions from immunofluorescence imaging differ among investigators: some studies show NBCn1 expression exclusively in cardiac capillary endothelia [3] whereas others provide evidence of NBCn1 in cardiomyocytes [4]. Based on a transcriptional reporter mouse, we previously identified Slc4a7 promoter activity in atrial but not ventricular cardiomyocytes [5]. In the current study, for the first time, we directly evaluate the role and consequences of NBCn1 for the heart using a functional genomics approach.
Although Na+,HCO3−-cotransport activity is elevated in hypertrophic hearts, the respective roles of NBCn1 and the electrogenic NBCe1 in cardiac structure development and adaptation remain disputed [6, 7]. Genetic variation in SLC4A7 modifies human hypertension risk [8], and the hypertension-associated genetic variants alter NBCn1 expression and function in vascular smooth muscle cells [9]. Likewise, loss of NBCn1 expression in mice causes hypertension and interferes with the function and structural adaptations of conduit and resistance arteries [10-12].
Acid–base disturbances and altered mechanisms of net acid extrusion influence cardiomyocyte function and cardiac structure. Especially, enhanced Na+-dependent net acid extrusion or shifts from electrogenic (1 Na+ + 2 HCO3−) to electroneutral (1 Na+ + 1 HCO3− or 1 Na+ for 1 H+) transport have been reported to provoke cardiac hypertrophy by increasing the concentration of intracellular Na+ that, through modified Na+-Ca2+ exchange, enhances pro-hypertrophic pathways [13, 14]. Changes from electroneutral to electrogenic transport can hyperpolarize cardiomyocytes and reduce action potential duration, with consequent short QT syndrome [6].
NBCn1 is a promising therapeutic target, particularly within breast cancer [15-18]. However, more information on systemic drug safety is needed. Small molecule drugs with NBCn1-inhibitory potential are generally nonselective across the Slc4-family, block multiple other ion transporters, and have pharmacokinetic properties unsuited for in vivo therapy [19, 20]. Instead, we recently showed that antibodies can be used to specifically block NBCn1 activity and alter the trajectory of breast cancer disease [21].
Whereas loss of NBCn1 expression is known to result in hypertension [12], the consequences for the heart have not previously been explored. Here, we test the hypothesis that NBCn1, either directly or indirectly, influences cardiac structure, contractile function, and electrophysiological properties. We investigate the impact of global disruption of NBCn1 to mimic effects of loss-of-function genetic variants or systemic therapeutic intervention. We show that (a) protein and mRNA expression of NBCn1 are more prominent in atrial than in ventricular cardiomyocytes, (b) disrupted NBCn1 expression lowers Na+,HCO3−-cotransport activity more than 50% in atria, whereas net acid extrusion activity in ventricular cardiomyocytes remains unaffected, and (c) loss of NBCn1 is associated with hypertension, cardiac hypertrophy, and prolonged ventricular isovolumic relaxation time without electrophysiological disturbances.
2 Materials and Methods
We studied Slc4a7Gt (40G1)Cmhd transgenic mice with globally disrupted expression of NBCn1 [12]. We compared male knockout mice at an age of 12–14 weeks to corresponding wild type mice. To avoid differential genetic drift within the genotypes, the wild type and knockout mice evaluated experimentally were either littermates or from homozygous breeding where the parents were offspring from heterozygous breeders. All animal procedures were approved by the Danish Animal Experiments Inspectorate (№ 2012-15-2934-00103 and 2021-15-0201-00986).
2.1 Ventricular Cardiomyocyte Isolation
Ventricular cardiomyocytes were enzymatically isolated from hearts mounted in Langendorff systems [22]. Hearts were cannulated through the aorta and perfused for 5 min with Ca2+-free Tyrode's solution containing (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 0.33 Na2HPO4, 10 HEPES, and 10 glucose; adjusted to pH 7.4. The hearts were then perfused for 15 min at 37°C with Tyrode's solution containing 0.05 mM Ca2+ and 1 mg/mL collagenase type 2 (approximately 300 U/mg; Worthington Biochemical, USA). Then, the ventricle was mechanically separated with forceps, and ventricular cells were dissociated by gentle trituration with a Pasteur pipette. Finally, the cells were filtered through a mesh and gradually returned to normal Tyrode's solution through stepwise washes to 0.1, 0.3, 0.6, 1, 1.6, and 2 mM Ca2+. Only rod-shaped cells with distinct striations were used for subsequent functional experiments.
2.2 Intracellular pH Measurements
We measured pHi from atrial strips and isolated ventricular cardiomyocytes using the pH-sensitive fluorophore 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF). Cells and tissues were incubated with 10 μM acetoxymethyl ester of BCECF (Thermo Fisher Scientific, Denmark) for 10 (cardiomyocytes) or 20–25 (atrial strips) minutes. During alternating excitation at 485 and 440 nm, we collected epifluorescence at 510 nm using an Olympus IX70 inverted microscope connected to an EasyRatioPro fluorescence imaging system (Photon Technology International, USA) or a Nikon Diaphot 200 inverted microscope with a Q-Imaging fluorescence camera and VisiView software (Visitron, Germany). To calibrate the BCECF fluorescence signal to pH, we added combinations of NH4Cl and Na-acetate to atrial strips under CO2/HCO3−-free conditions and determined steady-state pHi by the null-point technique [23-25]. We established the dynamic relationship between changes in BCECF ratio and pH from high-K+/nigericin calibrations [26].
After loading with BCECF and following a 10-min stabilization period, we induced intracellular acidification by the NH4+-prepulse technique [27]. We added NH4Cl to the bath solution for 7 min and observed intracellular acidification after NH4Cl washout. Based on pilot experiments, the NH4+ concentration (40 mM for atrial strips, 10 mM for isolated ventricular cardiomyocytes) was chosen to reach the desired level of intracellular acidification (ΔpHi around 0.4). We fitted polynomial functions to the subsequent recovery phase where pHi in the presence of bath Na+ returned toward the resting level. From the fitted curves, we calculated the corresponding net acid extrusion activity—as dpHi/dt × buffering capacity—at pHi 6.9 (ventricular cardiomyocytes) or 6.8 (atrial strips). The intrinsic buffering capacity was calculated from the change of cardiomyocyte pHi in response to the addition and washout of NH4Cl in the absence of CO2/HCO3− [11]. Intrinsic buffering capacity values corresponding to pHi 6.9 and 6.8 were calculated using a one-phase exponential decay function fitted to the buffering capacities plotted as a function of pHi. The contribution of CO2/HCO3− to intracellular buffering was calculated as 2.3 × [HCO3−]i. Intracellular concentrations of NH4+ and HCO3− were calculated from the Henderson-Hasselbalch equation. Resting steady-state pHi was analyzed as the value before the addition of NH4Cl.
The CO2/HCO3−-containing solution used for pHi recordings contained (in mM): [28] 115.88 NaCl, 2.82 KCl, 1.60 CaCl2, 1.20 MgSO4, 22 NaHCO3, 1.18 KH2PO4, 10 HEPES, 5.50 glucose, and 0.03 EDTA. For ion substitution, Na+ and HCO3− were replaced with equimolar amounts of N-methyl-D-glucammonium (except for NaHCO3, which was replaced with choline-HCO3) and Cl−, respectively. The CO2/HCO3−-containing solutions were aerated with 5% CO2/balance air and CO2/HCO3−-free solutions with nominally CO2-free air before pH was adjusted to 7.4 at 37°C.
2.3 Electrophysiology
Membrane potentials were recorded from isolated ventricular cardiomyocytes using the Amphotericin B-perforated whole-cell current clamp technique [29]. We acquired data with an Axopatch 200B amplifier, a Digidata 1440A analog-to-digital converter, and Clampex 10 Software (Molecular Devices, UK). Borosilicate patch pipettes were pulled with a P-97 puller (Sutter Instruments, USA) to a final resistance of 2–3.5 MΩ. Pipettes were filled with solution containing (in mM): 125 K-gluconate, 20 KCl, 5 NaCl, 0.22 Amphotericin B, and 10 HEPES, adjusted to pH 7.2 with KOH. The extracellular solution consisted of (in mM): 120 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 27 NaHCO3, 5 glucose, and 13 choline-chloride, adjusted to pH 7.4 after bubbling with 5% CO2/balance O2 at room temperature. Action potentials were triggered at 1 Hz by 2-ms square depolarizing pulses. Electrophysiology experiments were performed at room temperature, and the HCO3− concentration of the physiological saline solution was adjusted to approximately 27 mM to account for the greater CO2 solubility in water (Henry's law constant of 0.0436 vs. 0.0307 mM/mmHg), the lower saturated water vapor pressure (21 vs. 47 mmHg), and the raised apparent pKa value (6.17 vs. 6.12) at 23°C compared to 37°C [30]. Absence of spontaneous rupture of the sealed membrane patch—which would convert the experiments from permeabilized to conventional whole cell configuration—is supported by the lack of sudden changes in membrane potential or injection pulse amplitudes [31].
2.4 Immunoblotting
We isolated protein from atrial and ventricular tissue stored at −80°C and lysed in a buffer containing 20 mM Tris·HCl, 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 20 mM β-glycerophosphate sodium salt, 1% Triton-X, 0.1% Tween-20, and Halt Protease and Phosphatase Inhibitor Cocktail (#78444; ThermoFisher Scientific, Denmark) at pH 7.5. Protein concentrations were determined by a bicinchoninic acid protein assay (ThermoFisher Scientific). We loaded 10 μg of total protein diluted in sample buffer in each lane of a sodium dodecyl sulfate-polyacrylamide gel for electrophoresis and subsequent transfer to polyvinylidene difluoride membranes blocked with 3% skimmed milk in Tris-buffered saline with 0.1% Tween-20 for 2 h at room temperature. The membranes were probed with rabbit anti-NH2-terminal NBCn1 antibody (kind gift from Dr. Jeppe Praetorius, Aarhus University) [3] that we affinity-purified using the corresponding immunizing peptide (21st Century Biochemicals, USA), with mouse anti-NHE1 antibody (#sc-136 239; Santa Cruz Biotechnology, USA; diluted 1:1000), or with rabbit anti-NBCe1 antibody (AB3212-I, Sigma-Aldrich; diluted 1:500). The applied antibodies have previously been used for immunoblotting on murine tissue [12, 32, 33]. The specificity of the antibodies against NBCn1 and NHE1 was previously confirmed using tissue or cells with knockout or knockdown of the transporters [12, 16, 34]. After thorough washing, membranes were incubated for 1–2 h at room temperature with matched anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies from Cell Signaling Technology (USA). We visualized total protein amounts loaded in each lane on an Azure Biosystem c600 imager using stain-free gels (Bio-Rad Laboratories, Denmark) or using pan-actin as loading control (primary antibody diluted 1:1000: #4968, secondary antibody diluted 1:2000: #7074; Cell Signaling Technology) [35].
2.5 Histology
Atria and ventricles were fixed overnight in neutral-buffered 10% formalin, paraffin-embedded, and cut to 3-μm sections, and stained with picrosirius red to study collagen in the extracellular matrix. Detection was done using fluorescence microscopy comparing collagen content (excitation: 575 nm, emission: 600 nm) to overall wall mass interpreted from auto-fluorescence (excitation: 475 nm, emission: 525 nm). The relative collagen content was analyzed semi-automatically using Fiji software [36].
2.6 Single-Cell RNA Sequencing
We obtained transcriptomic data at single-cell resolution from a previous study evaluating adult human hearts [37]. Data from cardiomyocyte populations in the left atrium and ventricle were extracted for SLC4A3, SLC4A4, SLC4A7, and SLC9A1.
2.7 In Vivo Experiments
Systemic blood pressure, heart rate, and electrocardiograms were recorded from NBCn1 knockout and wild type mice using radiotelemetry (Data Sciences International, USA) [12]. To implant transmitters (HD-X11, Data Sciences International), mice were initially injected intraperitoneally with 0.1 mg/kg buprenorphine (Temgesic, Indivior Europe Limited, Ireland), and 30 min later, anesthesia was induced with 4% isoflurane and maintained with 1.4% isoflurane in pure O2 on a mask. Surgical procedures were performed on a homeothermic blanket system (50-7222F, Harvard Apparatus, MA, USA) to maintain core temperature at 37°C. After the operation, mice were allowed to recover for at least 2 weeks before the measurements. Telemetry recordings were analyzed separately for the light (6 AM to 6 PM) and dark (6 PM to 6 AM) periods. Duration of the QRS complex, and RR, PR, and QT intervals were calculated using Ponemah software (v. 6.42; Data Sciences International). From the telemetry recordings, we also derived mouse physical activity data that are a function of both distance and speed of animal movement.
We measured cardiac function and structure by transthoracic echocardiography using a Vevo 3100 ultrasound system (FUJIFILM VisualSonics, Canada). The mice were lightly sedated using 1% isoflurane in air and immobilized in a supine position on a heating pad with integrated ECG electrodes. Body core temperature was measured with a rectal thermal probe and kept constant at 37°C. After a stabilization period of 10 min, parasternal long axis, short axis, and 4-chamber views were acquired and assessed using the Vevo LAB 5.8.2 software (FUJIFILM VisualSonics) to estimate systolic and diastolic heart function.
3 Statistics
Data are expressed as mean ± SEM; n equals the number of independent cardiomyocyte preparations or animals. If multiple cells were analyzed in parallel, we used the average of those recordings as n = 1. An unpaired, two-tailed Student's t-test was used for comparison of one variable between two groups. To evaluate the effects of two or three independent variables on the measured variable, we used two-way or three-way ANOVA, respectively, followed by Šidák's posttests or Fisher's least significant difference test. When the variable was measured multiple times for each mouse, repeated measures ANOVA was employed. To circumvent the low sensitivity of normality tests when applied to small sample sizes, we evaluated sample distributions for normality based on visual inspection of Q-Q plots. Details of the employed statistical tests are provided in the figure legends. P-values smaller than 0.05 were considered statistically significant. Statistical analyses were performed using Microsoft Excel or GraphPad Prism 10 software.
4 Results
Here, we use NBCn1 knockout and corresponding wild type mice to evaluate the role of NBCn1 for the heart and cardiovascular system.
4.1 NBCn1 Is Differentially Expressed Between Atria and Ventricles
We first evaluate expression by immunoblotting on tissue lysates and find that NBCn1 shows higher protein expression in atria than ventricles (Figure 1A,B). We also note that the estimated molecular weight of NBCn1 from wild type mice is approximately 3 kDa higher in atria than in ventricles (Figure 1C). As expected, the protein expression of NBCn1 is absent in cardiac samples from NBCn1 knockout mice (Figure 1A,B).

Heterogeneity between cell types (e.g., endothelial cells vs. cardiomyocytes) and between species complicates analyses based on tissue lysates. To address this point and provide information with human translational value and potential clinical implications, we next extend our observations using publicly available single-cell RNA sequencing data from adult human heart (Figure 1D–G). Whereas the mRNA levels for SLC9A1 (Figure 1D), SLC4A3 (Figure 1E), and SLC4A4 (Figure 1F) are very uniform between cardiomyocytes from the left atrium and ventricle, SLC4A7 mRNA is more abundantly expressed—with 92% ± 25% higher transcript levels—in cardiomyocytes from atria compared to ventricles (Figure 1G). The preferred expression of NBCn1 in atria is consistent with our previous observation that Slc4a7 transcriptional activity is substantial in murine atrial tissue but undetectable in ventricular tissue [5].
4.2 NBCn1 Facilitates Net Acid Extrusion in the Cardiac Atria
We next assess whether the preferential expression of NBCn1 in cardiac atria is reflected in the pHi regulatory function within atrial strips. In the presence of CO2/HCO3−, steady-state pHi is very similar in atrial strips from wild type (7.15 ± 0.03) and NBCn1 knockout (7.14 ± 0.04) mice (Figure 2A,B). Omission of CO2/HCO3− acidifies the atrial tissue, which indicates that atrial cardiomyocytes perform net HCO3− uptake at steady-state (Figure 2A,B). Yet, as illustrated in Figure 2B, the acidification upon removal of CO2/HCO3− in atrial tissue from NBCn1 knockout mice (ΔpHi = −0.15 ± 0.04) is not significantly different from that in wild type mice (ΔpHi = −0.23 ± 0.04).
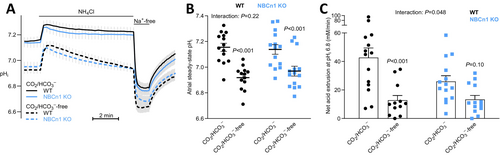
To study the mechanisms of net acid extrusion, we induce intracellular acidification by the NH4+-prepulse technique [27]. We add and subsequently wash out NH4Cl to acid load the atrial strips and evaluate their ability to recover pHi in the absence and presence of extracellular Na+ (Figure 2A). The atrial tissue from NBCn1 knockout mice shows slower pHi recovery and attenuated net acid extrusion relative to wild type mice when investigated in the presence of CO2/HCO3− (Figure 2A,C). The NBCn1-dependent recovery of pHi is Na+-dependent and abolished in the absence of CO2/HCO3− (Figure 2A,C), which is consistent with the function of NBCn1 as a plasmalemmal Na+,HCO3−-cotransporter.
4.3 NBCn1 Does Not Noticeably Contribute to Net Acid Extrusion From Ventricular Cardiomyocytes
We next turn to ventricular cardiomyocytes and explore their pHi regulatory function. Consistent with the lower expression of NBCn1 in ventricular compared to atrial cardiomyocytes (Figure 1B,G), we observe no effect of NBCn1 knockout on steady-state pHi (Figure 3A) or net acid extrusion activity after NH4+-prepulse-induced intracellular acidification (Figure 3B). The relative contribution of Na+,HCO3−-cotransport to net acid extrusion appears overall smaller in ventricles compared to atria (Figures 2C and 3B); and in accordance, omission of CO2/HCO3− does not significantly influence steady-state pHi in the ventricular cardiomyocytes (Figure 3A).
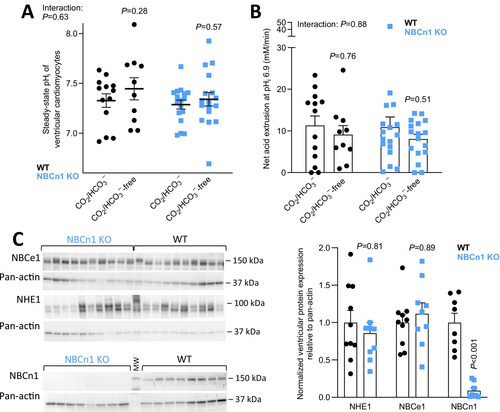
To probe the possibility that knockout of NBCn1 does not reach functional consequence due to compensation by other acid–base transporters, we investigate the expression levels for the two other major net acid extruders, NBCe1 and NHE1. We find no difference in the ventricular protein expression levels of NHE1 or NBCe1 between NBCn1 knockout and wild type mice (Figure 3C).
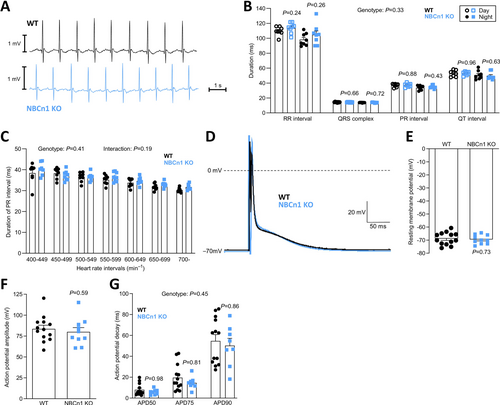
4.4 Electrophysiology of Ventricular Cardiomyocytes Is Not Markedly Affected by NBCn1 Knockout
Changes to pHi or the mechanisms of net acid extrusion—particularly shifts from electroneutral transport via NBCn1 and NHE1 to electrogenic transport via NBCe1—have potential electrophysiological consequences [6]. Therefore, we next study telemetrically recorded electrocardiograms from wild type and NBCn1 knockout mice (Figure 4A) and find no differences in RR, PR, or QT interval lengths, or in the duration of the QRS complex (Figure 4B). We report the unadjusted interval durations, because we observe no differences in heart rate between wild type and NBCn1 knockout mice, and previous studies find that heart rate correction using approaches optimized for humans can lead to errors in data interpretation from mice [39]. We speculate that subtle electrophysiological effects of NBCn1 might be revealed when pHi regulation of atrial cardiomyocytes is challenged by elevated metabolism at higher heart rates. However, even when we plot the PR interval duration as a function of heart rate, we observe no differences between the wild type and NBCn1 knockout mice (Figure 4C). When we evaluate action potentials recorded from ventricular cardiomyocytes by perforated patch clamp technique (Figure 4D), we observe no effect of NBCn1 knockout on the resting membrane potential (Figure 4E), the action potential amplitude (Figure 4F) or the rate of action potential decay (Figure 4G) between isolated ventricular cardiomyocytes from wild type and NBCn1 knockout mice. The normal electrocardiogram and electrophysiology of ventricular cardiomyocytes from NBCn1 knockout mice are consistent with the unperturbed pHi regulation in the ventricles (Figure 3A,B) and our observation that (electrogenic) NBCe1 does not compensate for the lack of (electroneutral) NBCn1 (Figure 3C).
4.5 NBCn1 Knockout Mice Are Hypertensive With Mild Cardiac Hypertrophy and Delayed Ventricular Relaxation
Using radiotelemetry, we next explore the blood pressure of freely moving NBCn1 knockout and wild type mice. We previously showed [12], and confirm here, that mice lacking NBCn1 are hypertensive with a 15–20 mmHg elevation in diastolic, systolic, and hence mean systemic blood pressure (Figure 5A,B) but no difference in heart rate (Figure 5C). As shown in Figure 5B, the blood pressure difference between NBCn1 knockout and wild type mice is observed both when the mice are awake (night) and during sleep (day). The physical activity of the mice shows the expected circadian pattern—with greater activity at night-time compared to daytime—but it does not vary between NBCn1 knockout and wild type mice (Figure 5D) and therefore cannot explain the genotype-dependent differences in blood pressure.
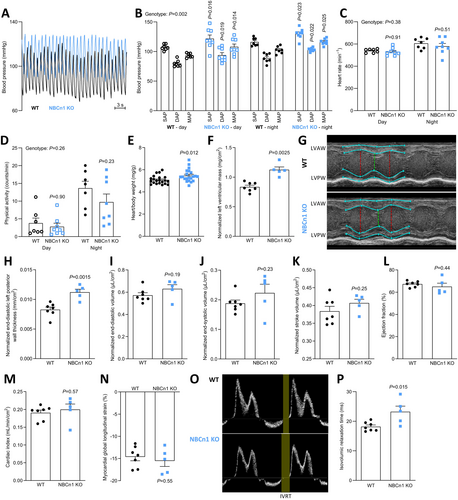
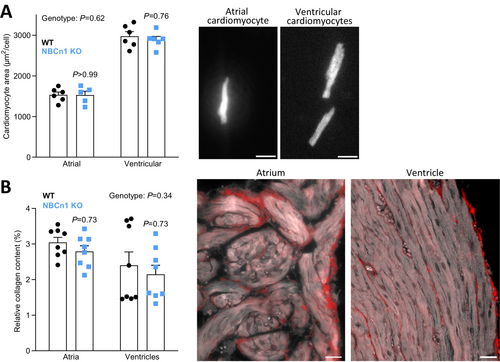
The hearts from NBCn1 knockout mice show hypertrophy evaluated by postmortem weighing (~8%; Figure 5E) and echocardiography (~20%; Figure 5F–H). Whereas the left ventricular chamber volumes (Figure 5I,J) are unaffected in NBCn1 knockout mice, the wall thickness is increased (Figure 5H). The cardiac hypertrophy appears to compensate for the elevated afterload as we observe maintained stroke volume (Figure 5K), left ventricular ejection fraction (Figure 5L), and cardiac index (Figure 5M). We also see no difference in the left ventricular myocardial global longitudinal strain (Figure 5N). The cardiac hypertrophy is not related to cardiomyocyte enlargement since the projected areas of isolated cardiomyocytes from the atria and ventricles do not reveal genotype-related differences in cell size (Figure 6A). Our finding that mouse atrial cardiomyocytes are smaller than ventricular cardiomyocytes is in line with previous reports [40].
By pulsed wave Doppler, we measure a prolonged left ventricular isovolumic relaxation time (Figure 5O,P). Impaired ventricular relaxation is consistent with the greater ventricular mass (Figure 5E,F) and thicker ventricular wall (Figure 5H). Other echocardiographic variables do not differ significantly between wild type and NBCn1 knockout mice (Table S1). The prolonged isovolumic relaxation time, but normal left ventricular early diastolic filling velocity, late diastolic filling velocity, and early diastolic transmitral flow deceleration time (Table S1) are consistent with findings from humans with hypertension and left ventricular hypertrophy [41].
Increased extracellular matrix deposition represents an alternative explanation for the greater cardiac mass. To evaluate if extracellular collagen accumulation plays a role in the structurally modified heart of the NBCn1 knockout mice, we perform picrosirius red staining, which shows no differences in collagen content between hearts from wild type and NBCn1 knockout mice (Figure 6B).
5 Discussion
In the current study, we reveal a hitherto unknown heterogeneity in pHi regulation between the ventricles and atria of the heart (Figure 1-3). Whereas NBCn1 mediates the majority of the Na+,HCO3−-cotransport in the atria during intracellular acidification (Figure 2), it does not contribute substantially to net acid extrusion in ventricular cardiomyocytes (Figure 3). Regulation of pHi in atria has received very little prior attention, but the difference in NBCn1-dependency between atria and ventricles is consistent with our prior observation that Slc4a7 transcriptional activity is prominent in atrial cardiomyocytes, yet undetectable in ventricular cardiomyocytes [5].
We reveal that NBCn1 knockout mice have cardiac hypertrophy (Figure 5E–H), which is most likely indirect, caused by the elevated blood pressure (Figure 5B). Attenuated endothelium-dependent NO-mediated vasorelaxation raises systemic vascular resistance in mice with loss of NBCn1 expression [12, 42]. Pressure-overload cardiac hypertrophy has previously been associated with overexpression and activity of NBCn1, NBCe1, and NHE1 in the rat heart [43]. In the current study, we do not observe any differences in net acid extrusion between ventricular cardiomyocytes from NBCn1 knockout and wild type mice (Figure 3B), which might suggest that upregulation of NBCn1 contributes to the increase in net acid extrusion in other models of hypertension. The role of, and mechanism behind, increased net acid extrusion in hypertrophic hearts remains unclear. Under the current experimental settings of mild to moderate hypertension, knockout of NBCn1 was insufficient to avoid cardiac hypertrophy (Figure 5E–H).
Both augmented [43] and deficient (Figure 5E–H) function and expression of NBCn1 associate with cardiac hypertrophy. In the current study, we furthermore establish that the NBCn1-related structural remodeling of the heart is linked to delayed ventricular relaxation (Figure 5O,P), which is a common consequence of hypertension and ventricular hypertrophy also in humans [41]. The complex link between NBCn1 and cardiac hypertrophy probably reflects the diverse roles exerted by NBCn1 across various cell types in the body, e.g., in the heart, resistance vasculature, kidneys, and nervous system [12, 42, 44-46]. Notably, the action of NBCn1 is required for proper pHi control but also represents a mechanism of cellular Na+ loading [47]. Changes in pHi and the intracellular concentrations of Na+ and Ca2+ (via Na+/Ca2+ exchange) can influence tissue structure and remodeling in the cardiovascular system [48, 49]. Depending on the physiological or pathophysiological conditions, it is likely that cardiomyocytes will need to prioritize either defending pHi against acidification or reducing the cellular Na+ load [50].
We observe an interesting difference of approximately 3 kDa in the apparent molecular weight of NBCn1 between atria and ventricles (Figure 1C). Possible explanations for the higher NBCn1 molecular weight in atria include differential posttranslational modifications or splice variation involving one or more of the multiple variable cassettes identified in NBCn1 transcripts [51]. Although the functional consequences of the NBCn1 molecular weight difference between the atria and ventricles are not yet clear, we previously found a similar difference between normal breast tissue and breast cancer tissue [26] and showed that one of the variable splice cassettes of NBCn1 in vascular smooth muscle cells provides a scaffold for protein–protein interaction and posttranslation regulation of transporter activity [23].
Considering the relatively mild effects of NBCn1 disruption on cardiac function—without changes in electrophysiological parameters, the electrocardiogram, or overall contractile function—our data support that NBCn1 is a safe target for pharmacological intervention, e.g., against malignant diseases [16, 17, 21]. If extrapolated from our knockout studies, the predicted influences of shorter-term anti-NBCn1 therapy on blood pressure and associated cardiac hypertrophy seem clinically manageable, for instance, using existing vasodilator therapies. Although we find little to no direct role of NBCn1 in the ventricles of unchallenged mice (Figure 3-6), it is possible that NBCn1 gets upregulated and gains greater functional importance during cardiovascular pathologies.
We observe clear differences in pHi regulation between the atria and ventricles. In the atria, Na+,HCO3−-cotransport is responsible for approximately 2/3 of overall net acid extrusion, and NBCn1 mediates around 50% of this transport (Figure 2C). Accordingly, nominal omission of CO2/HCO3− causes a prominent drop in steady-state pHi in atrial tissue (Figure 2B) suggesting that HCO3− uptake via Na+,HCO3−-cotransport is greater than HCO3− export via Cl−/HCO3−-exchange at steady-state. Changes in net acid extrusion are not always reflected in steady-state pHi [52, 53]. The latter depends also on the pH-dependent activation kinetics of the net acid and base extruders and on the rate of metabolic acid production [1, 54, 55]. It is therefore not surprising that the 50% decrease in CO2/HCO3−-dependent net acid extrusion in response to NBCn1 knockout in the atria is insufficient to markedly lower steady-state pHi (Figure 2). Although we evaluated a relatively large number of atria (n = 13–14; Figure 2B), it is possible that a further increase in sample size would reveal a slightly reduced steady-state pHi in the atria from NBCn1 knockout mice. In the ventricles, Na+,HCO3−-cotransport plays a much smaller role for net acid extrusion (Figure 3B), and we observe no significant effect on steady-state pHi when CO2/HCO3− is omitted from the experimental solutions (Figure 3A). Based on previous investigations, NBCe1 likely mediates the Na+,HCO3−-cotransport remaining after knockout of NBCn1, whereas NHE1 is the main candidate for the Na+/H+-exchange remaining after omission of CO2/HCO3− [1].
The obvious expression of NBCn1 protein in ventricular biopsies (Figures 1B and 3C) may in part originate from heart endothelia [3], but SLC4A7 mRNA is also detected in human ventricular cardiomyocytes (Figure 1G). Based on expression in the lateral sarcolemma, intercalated discs, and transverse tubules [4], it is possible that NBCn1 regulates pHi in discrete subcellular regions of ventricular cardiomyocytes without gaining importance for global pHi. Alternatively, NBCn1 may become active only after the ventricular cardiomyocytes receive specific physiological or pathophysiological inputs.
The current study addresses the consequences of systemically targeting NBCn1 and thus describes the expected response to inherited genetic variants [8, 9] or systemic pharmacological therapy [21]. To distinguish direct effects of NBCn1 in the heart from indirect effects secondary to changes in the vasculature, nervous system, kidney, or other tissues relevant to blood pressure control [56], studies of cardiomyocyte-specific knockout mice are an important future step.
We conclude that NBCn1 is a main mechanism of Na+,HCO3−-cotransport in atrial tissue and that it contributes substantially to net acid extrusion during intracellular acidification. Even though NBCn1 does not contribute measurably to net acid extrusion in ventricular cardiomyocytes from the normal murine heart, the rise in blood pressure in NBCn1 knockout mice is associated with cardiac hypertrophy and echocardiographic signs of delayed ventricular relaxation. We observe no electrophysiological abnormalities or altered collagen deposition in hearts with loss of NBCn1 expression; and the overall mild cardiac phenotype, even in mice with global constitutive knockout, supports the therapeutic promise of targeting NBCn1 in, for instance, breast cancer where it supports malignant progression.
Author Contributions
María S. Espejo: methodology, data curation, formal analysis, visualization, writing – review and editing, writing – original draft. Alejandro Orlowski: data curation, methodology, writing – review and editing, formal analysis. Trine M. Sørensen: data curation, writing – review and editing. Vladimir V. Matchkov: data curation, writing – review and editing. Ernesto A. Aiello: conceptualization, writing – review and editing. Ebbe Boedtkjer: conceptualization, visualization, writing – original draft, writing – review and editing, project administration, resources, supervision, formal analysis, funding acquisition.
Acknowledgments
The authors would like to thank Viola S. M. Larsen and Jane Rønn, Aarhus University, for expert technical assistance. The authors thank the Phenotyping Core Facility, Department of Biomedicine, Aarhus University, for technical support.
Conflicts of Interest
Boedtkjer is an inventor on a patent addressing NBCn1 inhibition in cancer (EP-3271402). The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




