Left superior cervical ganglia lymph node mimicry and its role in rat ventricular arrhythmias following myocardial infarction
Qingxia Yu and Yan Li contributed equally to this work.
Abstract
Aim
Sympathetic overactivation may lead to severe ventricular arrhythmias (VAs) post-myocardial infarction (MI). The superior cervical ganglion (SCG) is an extracardiac sympathetic ganglion which regulates cardiac autonomic tone. We aimed to investigate the characteristics and functional significance of SCG on neuro-cardiac communication post-MI.
Methods
Constructed MI rat model by left anterior descending coronary artery ligation, and electrophysiological, SCG sympathetic nerve activity testing, echocardiography and histology study were performed. The proteins and gene expression were detected using RNA-seq, spatial transcriptomics, quantitative PCR, and western blotting.
Results
The SCG neuronal remodeling was recognized by significant increase in adrenergic tyrosine hydroxylase (TH) (+) neurons and decrease in neuronal size. Top differentially expressed genes enriched in pro-inflammatory profile and nerve regulatory factor in left SCG (LSCG) post-MI. Interleukin (IL)-1β and IL-6 increased significantly at Day 3, ahead of nerve growth factor (NGF) which peaked at Day 7 post-MI. Spatial transcriptomics further identified the relativity of TH enrichment with macrophages and cytokines. Therapeutic LSCG-ectomy successfully triggered cardiac denervation and improved VA vulnerability. Eventually, cardiac denervation attenuated macrophage/mast cell infiltration at para-infarct regions, thus improved cardiac dysfunction. Mechanism study revealed that genetic knockdown of NGF receptor trkA in LSCG reversed sympathetic remodeling and cardiac inflammation, which may be partially mediated by substance P and calcitonin gene-related peptide (CGRP).
Conclusion
Extracardiac sympathetic LSCG remodeling participated in arrhythmogenesis and cardiac inflammation/function post-MI. NGF bridged neuro-immune crosstalk between pro-inflammatory shifting and sympathetic overdrive. Targeting LSCG modification facilitated cardiac protection and prevented VAs post-MI.
1 INTRODUCTION
Globally, ventricular arrhythmias (VAs) post-myocardial infarction (MI) remains a major cause of sudden cardiac death.1 The pathophysiological mechanism of VAs following acute MI (AMI) involves sympathetic dysfunction within the heart as well as the global autonomic nervous system disruption, including augmented sympathetic drive and parasympathetic withdrawal.2
Cardiac sympathetic denervation provides clear efficacy in sympathectomy for preventing arrhythmias.3 For instance, the left cardiac sympathetic denervation (LCSD) presents a viable therapeutic option for channelopathy-like catecholaminergic polymorphic ventricular tachycardia (CPVT) and long QT syndrome (LQTS).4, 5 Furthermore, targeted extracardiac neuromodulation therapies, including ganglionated plexi ablation, renal sympathetic denervation, spinal cord stimulation, vagus nerve stimulation, and carotid baroreceptor stimulation exert an antiarrhythmic effect for refractory VA caused by LQTS, CPVT, and structural heart disease.6 Therefore, ganglion innervating to the cardiac sympathetic nerves present notable potential for arrhythmia therapy. The superior cervical ganglia (SCG), located behind the carotid bifurcation and primarily innervating the anterior myocardium, has been recently discovered in regulating cardiac sympathetic activity.7, 8 In MI animal models, the left anterior descending artery (LAD) nourished the SCG-charging area, which in turn facilitated SCG as a learning objective to exert sympathetic control over myocardium.9 It is still not well elucidated whether the sympathetic nerves control the immune response or whether the cardiac immune response/cells regulate sympathetic remodeling in the process of MI.
Macrophage depletion of stellate ganglia (SG) alleviated cardiac sympathetic activation and ventricular arrhythmogenesis via attenuating SG neuroinflammation in a heart failure animal model.10 Furthermore, T-cell mediated cytotoxicity toward SG from LQTS/CVPT patients was raised to interpret the enhanced electrical instability.11 These findings indicate that sympathetic ganglia undergo a neuroinflammatory process cardiac diseases, and targeting the ganglia could present a new strategy to prevent arrhythmias. Meanwhile, a clinical analysis on SG from cardiomyopathy and refractory VAs demonstrated inflammation, neurochemical remodeling, oxidative stress, and satellite glial cell activation,12 which strengthened the therapeutical significance of targeting sympathetic ganglia. Cardiac sympathetic denervation by bilateral SCG-ectomy identified the critical role of SCG in cardiac inflammation,13 and indirectly demonstrated the neuroimmune crosstalk in MI model. However, the characteristics of sympathetic ganglia remodeling, especially the inflammatory state in sympathetic ganglia, and to what extent the sympathetic ganglia is involved in VAs post-MI remain unexplored. This study was designed to quantify the inflammatory response of SCG post-MI and address the role and mechanism of left SCG (LSCG) functions in cardiac control, especially in ventricular arrhythmogenesis after MI.
2 RESULTS
2.1 Enhanced LSCG inflammatory response following acute MI
SCG underwent morphological changes after acute MI as shown in Figure 1A, where the SCG nuclei numbers were 37 ± 1.793/mm2 in the control SCG group versus 43.43 ± 1.798/mm2 in the MI SCG group (Figure 1A, the bar graph) at Day 7. The number of adrenergic phenotype marker tyrosine hydroxylase (TH)-positive neurons showed a significant increase between SCG from control (1.12 ± 0.24) and MI groups (1.58 ± 0.36). On the contrary, TH-positive cell size decreased significantly in SCG from the MI group (93.13 ± 4.86) compared with that from the control group (106.4 ± 3.65) (Figure 1B). The increased nuclei numbers and decreased TH positive cell size implying the corresponding morphological changes of SCG in response to myocardial ischemia challenge which is consisted with previous reports.7, 14 The structure changes indicated adrenergic shifting and potentially provide a significant anatomical contribution to cardiac innervation since postganglionic cardiac sympathetic axons synapse projected from pre-ganglion.
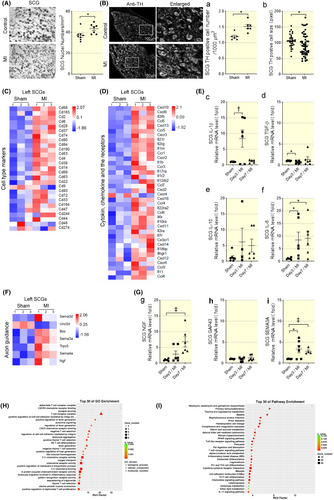
By sequencing the overall mRNA transcriptions of SCG from control and MI rats (Day 7 post-MI), an increase in mRNA transcription of inflammatory cell markers with cytokines and chemokines, cytokine and chemokine receptors in LSCG from MI group, such as CD68, CD14 and IL-1β (Figure 1C,D). By performing the GO and pathway (KEGG) enrichment analysis on differential mRNA from control and AMI SCG, 18 clusters out of the top 30 enrichment were related to inflammatory process (T cells, cytokine-cytokine receptor interaction, chemokine binding, and cell–cell adhesion). The KEGG pathway enrichment clustered at least four inflammatory signaling pathways involved in AMI SCG, which included natural killer (NK) cell-mediated cytotoxicity, toll-like receptor signaling pathway, T cell receptor signaling pathway, and IL-17 signaling pathway (Figure 1H,I). As for the mRNA transcriptions of the RSCG, neither inflammatory pathway nor nerve regulatory factor showed a significant difference (Figure S2), which is in accordance with the fact that Somata of cardiac afferent neurons are assumed to be located primarily in LSCG and cranial thoracic dorsal root ganglia.15
We also determined the mRNA levels of cytokines that mediated inflammatory response which showed increased IL-1β and IL-6 in SCG at Day 3 post-MI (Figure 1E-c, d, and f). IL-6 maintained a high level in SCG through Days 3 and 7 post-MI, whereas TGF-β was lower at Day 3 (Figure 1Ef). The data above suggested the inflammatory response of SCG following AMI. Meanwhile, by analyzing the axon guidance genes that differed in SCG from control and MI groups, we found nerve growth factor (NGF) highly expressed in MI SCGs and peaked at Day 7, which lagged behind proinflammatory factor increase (Figure 1F). NGF mRNA levels increased in SCG on Day 7 post-MI (Figure 1Gg). Sema3a mRNA in SCG maintained high expression through Day 3–7 post-MI (Figure 1Gi). The increased NGF and Sema3a indicated neuronal growth in SCG. While GAP43, the index for newly grown axons, did not change in SCG (Figure 1Gh) indicating that there might be no axon regeneration in SCG.
2.2 SCG temporal immune composition by spatial transcriptomics post-MI
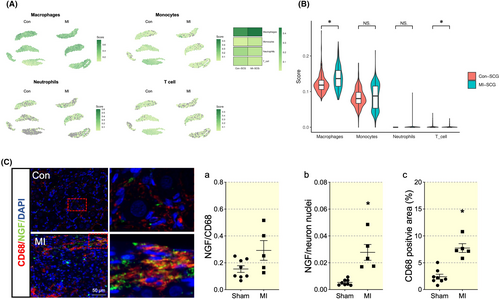
To identify diverse immune cell types in SCG and comprehensively characterize their dynamic alterations during the pathological progression of MI, we performed transcriptomics analysis at Day 7 post-MI. Consistent with previous data, the TH population from LSCG increased significantly in MI groups compared with the control group (Figure 2A,B). Uniform manifold approximation and projection (UMAP) plot of the Sham and MI groups was presented. The heatmap showed that Cluster 3 transcriptomics exhibited the most significant differential expression of TH (Figure 2C,D). Cluster 3 was significantly enriched in pathways and gene ontology (GO) terms related to inflammatory pathway (Figure S3; Figure 2E). Similarly, TH enrichment was positively related to pro-inflammatory cytokines IL-1β and IL-6; instead, negatively related to anti-inflammatory cytokines IL-10 and TGF-β (Figure 2F).

Four major immune cell populations, T cells, monocytes, macrophages and neutrophil were identified based on the expression of highly variable genes (gene markers presented in Table S1). Significant increase in macrophages were observed between the Sham and MI group. A slight increase in monocytes was also observed but with no significant difference (Figure 3A,B). We further confirmed macrophage infiltration by positive staining for the macrophage marker CD68. Then we assess NGF, a secretory protein that links macrophage and sympathetic innervation. Following MI, both NGF expression and the CD68-positive macrophages expressed NGF increased, suggesting a potential link of neuron-immune crosstalk (Figure 3C,D).
2.3 LSCG-ectomy inhibited inflammatory cell infiltration in myocardial tissues post-MI
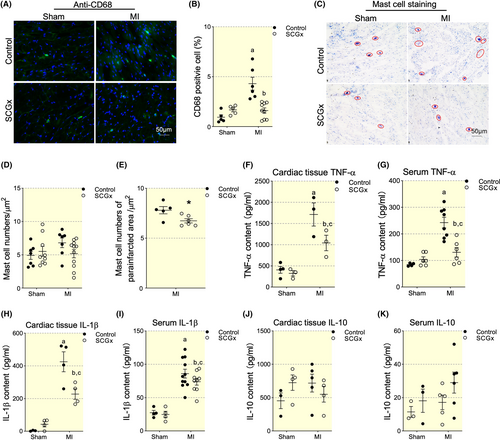
To investigate if LSCG and the inside inflammation were involved in cardiac inflammatory response, we performed LSCG-ectomy (SCGx). The protocol for animal study was indicated in Figure S1. The results showed a significant increase in CD68+ cells in the MI group compared with the Sham group, while SCGx abolished MI-induced increase of CD68+ cells (Figure 4A,B). Meanwhile, we tested mast cell distribution and numbers which was shown increased in MI rats as observed in our previous studies. The total mast cell number showed no difference among the Sham, MI, SCGx/Sham, and SCGx/MI groups (Figure 4D). However, SCGx decreased mast cells in the parainfarct zone significantly in MI group (Figure 4E). As expected, SCGx alerted inflammatory state post-MI indicated by decreased expression profile of pro-inflammatory cytokines (Figure 4F–K), confirming that SCG affected macrophage dominated cardiac inflammation post infarction.
2.4 LSCG-ectomy attenuated cardiac sympathetic nerve regeneration and improved cardiac function
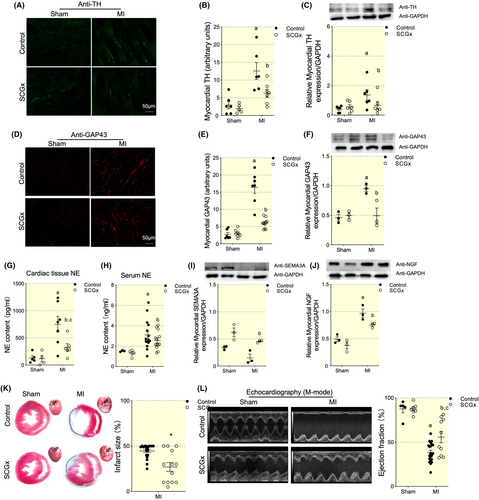
Next, we observed the functional implications of SCG on cardiac sympathetic nerve regeneration. All the tissues used for immunofluorescent staining and western blot were collected at Day 7 post-MI. TH positive nerve fibers were enhanced in infarct myocardial tissues while LSCG-ectomy abolished the significantly increased TH staining (Figure 5A,B). The TH protein level increased significantly in myocardial tissues following MI and decreased by 249.5 ± 97.78% by SCGx (Figure 5C). The regeneration of sympathetic nerve fibers was evaluated by GAP43, which was generally used in nervous and myocardial ischemia models. GAP43 was highly positive stained in MI myocardial tissues while it was reversed by LSCG removal (Figure 5D,E). The GAP43 protein level tendency was consistent with those of staining data (Figure 5H). Then, we measured cardiac and circulating norepinephrine concentration. Interestingly, the SCGx procedure diminished the decrease in the release of norepinephrine in the myocardium to a degree less in that of circulation compared with the MI vehicle (Figure 5G,H). The above data indicated the beneficial effect of LSCG removal in cardiac denervation and sympathetic tone amelioration. Next, we explored the role of SCGx on cardiac neural growth factors expression. Sema3a is the axonal guidance and sympathetic denervation index in the heart. There was no statistical significance of Sema3a expression among Sham and SCGx groups. However, SCGx significantly increased Sema3a expression in MI myocardial tissues (Figure 5I). NGF, which facilitated cardiac sympathetic nerve growth, increased significantly in MI myocardial tissues. SCGx reduced NGF production in MI group by 22.5% (Figure 5J). The data indirectly demonstrated the anterograde axonal transport of neural regulatory factor may be the potential mechanism of LSCG promoting the overwhelmed cardiac sympathetic nerve regeneration post-MI.
To investigate if SCG removal leads to functional alteration of the heart, we performed Masson staining and echocardiography on rats at Day 7 post-MI. Ejection fraction (EF) was significantly decreased in MI rats (47.75 ± 2.52%) comparing with that from Sham group (87.46 ± 4.33%) (Figure 5K). SCGx did not alter EF values in SCGx/sham group (85.98 ± 2.01%), but partially rescued EF to 61.56 ± 8.12% by in MI rats, as well as a progressive decrease in LV internal dimension diastole (LVIDD) and internal dimension systole (LVIDS) (Table 1). Masson's trichrome staining revealed a smaller infarcted area in the SCGx/MI group than those in the MI group (p < 0.05), coinciding with deteriorating cardiac dysfunction (Figure 5L). These data indicated the protective function of SCGx by improving the cardiac pump in the early stage of MI.
| shCtrl/Sham | MI | SCGx/Sham | SCGx/MI | |
|---|---|---|---|---|
| Protocol 1 | ||||
| Rat no. | 28 | 20 | 30 | 20 |
| Body weight (g) | 309.5 ± 8.0 | 306.6 ± 6.2 | 282.3 ± 6.7 | 279.2 ± 24.96 |
| Heart rate (bpm) | 354.6 ± 5.3 | 379.6 ± 9.2 | 381.1 ± 14.1 | 346.3 ± 7.7 |
| LVESV (μL) | 28.31 ± 12.7 | 231.4 ± 104.7a | 36.84 ± 21.97 | 169.9 ± 118a, b |
| LVEDV (μL) | 211.5 ± 42.54 | 365.6 ± 109.5a | 227 ± 61.71 | 315.5 ± 119.9 |
| EF (%) | 87.5 ± 4.3 | 47.8 ± 2.5a | 86.0 ± 2.0 | 61.6 ± 8.1a, b |
| SBP (mmHg) | 114.0 ± 3.6 | 112.7 ± 3.1 | 118.7 ± 1.9 | 109.3 ± 6.5 |
| DBP (mmHg) | 88.5 ± 3.9 | 85.7 ± 3.5 | 82.5 ± 2.6 | 79.5 ± 5.3 |
| shCtrl/Sham | shCtrl/MI | shTrkA/Sham | shTrkA/MI | |
|---|---|---|---|---|
| Protocol 2 | ||||
| Rat no. | 16 | 20 | 16 | 20 |
| Body weight | 296.5 ± 6.8 | 284.6 ± 5.2 | 292.6 ± 5.4 | 285.2 ± 7.6 |
| Heart rate (bpm) | 346 ± 4.7 | 360.6 ± 8.2 | 351.4 ± 8.7 | 356.3 ± 9.5 |
| LVESV | 29.4 ± 16.1 | 204.5 ± 87.9a | 32.9 ± 8.5 | 149.7 ± 98a, c |
| LVEDV | 215.7 ± 54.37 | 327.6 ± 112.6a | 219.1 ± 71.86 | 319.1 ± 121.9 |
| EF (%) | 86.3 ± 5.2 | 45.2 ± 3.1a | 90.1 ± 3.2 | 69.6 ± 12.1a, c |
| SBP (mmHg) | 116.0 ± 2.9 | 115.7 ± 5.6 | 118.9 ± 3.4 | 112.3 ± 5 |
| DBP (mmHg) | 85.3 ± 4.5 | 89.5 ± 6.2 | 84.7 ± 6.5 | 80.1 ± 3.9 |
- Note: Values are means ± SEM.
- Abbreviations: EF, left ventricular ejection fraction; LVESV and LVEDV, left ventricular end-systolic volume and end-diastolic volume; SBP and DBP, systolic and diastolic blood pressure.
- a p < 0.05 comparing with Sham group.
- b p < 0.05 comparing with MI group.
- c p < 0.05 comparing with shCtrl/MI group.
2.5 Arrhythmia susceptibility of MI rats following LSCG-ectomy
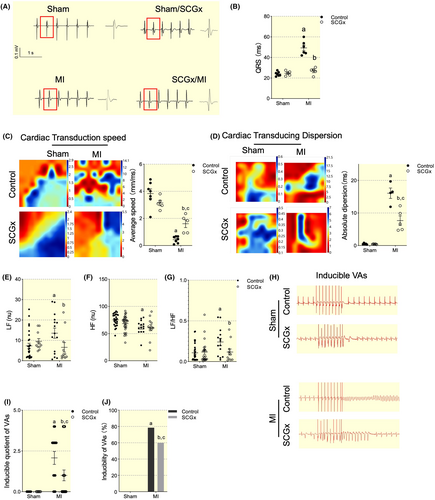
To evaluate the electrical propagation and autonomic nervous control of the heart in MI and SCGx/MI rats, we analyzed QRS interval, electrical mapping, and heart rate variability (HRV) with HF, LF, and LF/HF indexes using the frequency-domain methods. Baseline ECGs were recorded prior to cryoinjury to ensure all rats had normal cardiac electrical activity. At 7 days post-infarction, all rats exhibited significantly prolonged QRS intervals compared to both the healthy sham group, indicating that infarction disrupted normal electrical signal propagation. However, at 7 days after MI, ratsunderwent SCGx demonstrated much shorter QRS intervals compared to MI-treated animals (Figure 6A,B). SCGx can improve the propagation of electrical pulses through scar tissue (Figure 6C,D). In MI rats, both LF and LF/HF ratios increased significantly compared with data from Sham rats, while HF decreased notably, indicating an unbalance between sympathetic and parasympathetic nerve shift toward sympathetic tone. However, HRV in SCGx showed a diminished increase in LF and LF/HF ratio caused by MI (Figure 6E–G). The inducibility and inducible quotient of VAs were reduced significantly in SCGx/MI group (Figure 6H–J) indicating SCGx protected the heart from VAs attack after MI.
2.6 Targeting inhibition of NGF signaling of LSCG attenuated ganglion-cardiac sympathetic spouting and ameliorated VAs
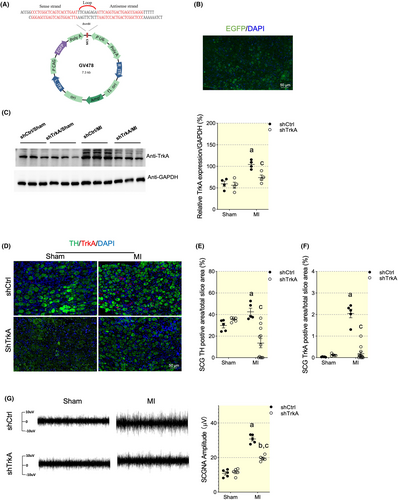
Next, we investigate the neuroimmune crosstalk link. NGF, a neuroimmune crosstalk mediator,16 exerting neurotic growth responding to immune reaction. To test whether the upregulating NGF is accountable for sympathetic spouting, we applied LSCG targeting administration of TrkA shRNA (Figure 7A–C). TrkA silencing effectively reversed the LSCG adrenergic phenotype innervation and activation (Figure 7D–F). The integrated SCG nerve activity (SCGNA) is low amplitude burst discharge activity, whereas MI induced progressively increased amplitude of SCGNA. while NGF signaling blockage abolished the significantly increase in LSCG electrical activity (Figure 7G,H). A pathophysiologic implication is that MI may induce LSCG nerve sprouting, leading to increased sympathetic outflow and VA.
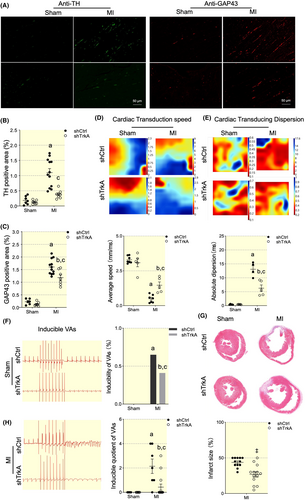
The regeneration of sympathetic nerve fibers was evaluated by TH and GAP43, which was generally used in nervous and myocardial ischemia models. GAP43 was highly positive stained in MI myocardial tissues while it was reversed by TrkA silencing (Figure 8A–C). NGF signaling blockage can improve the propagation of electrical pulses through scar tissue as well (Figure 8D,E). Consequently, VAs susceptibility was ameliorated (Figure 8F). Interestingly, NGF blockage in LSCG reduced infarct size as detected by Masson staining on rats at Day 7 post-MI (Figure 8J).
2.7 NGF played a key role in LSCG dominated cardiac inflammation
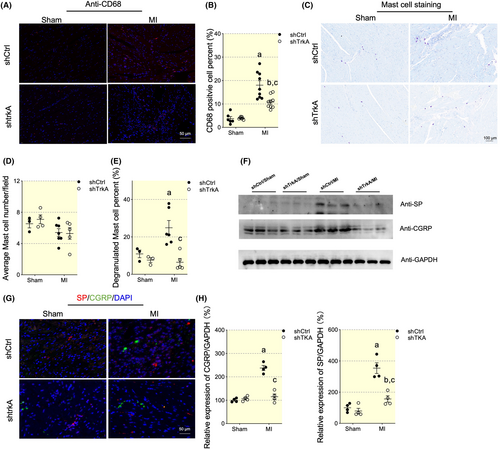
At last, we tested whether NGF signaling was responsible for cardiac inflammation modulation. TrkA shRNA reduced MI induced macrophages infiltration, as indicated by CD68 staining (Figure 9A,B). Though the mast cell number remained unaffected, the TrkA silencing effectively reduced the mast cell degranulation (Figure 9C–E). Next, we explored the potential mechanism of NGF signaling regulation on cardiac neuroinflammation. NGF signaling was reported to play an essential role in linking neuron-immune crosstalk by regulating the expression of neuropeptides. Among the neuropeptides, CGRP and SP exerted chemotactic and immune-modulating function.17 Then, we tested protein level of CGRP and SP. We found significant increase of CGRP and SP expression by immunofluorescence and western blot, while NGF signaling blockage by TrkA knockdown effectively decreased the CGRP and SP expression post-MI (Figure 9F–H).
3 DISCUSSION
This study provides crucial insights into essential extracardiac ganglion LSCG remodeling and its function in cardiac control, especially in ventricular arrhythmogenesis post-MI. In addition, NGF signaling bridged cardiac-neuroaxis and presented to be a novel therapeutic target in cardiac pathological process.
Cardiac autonomic imbalance by high sympathetic output in myocardium connects tightly with arrhythmogenesis post-MI.18 Cardiac sympathetic denervation is a well explored therapeutic methods for arrhythmias in experimental and clinical studies,19 whereas knowledge on extracardiac sympathetic ganglia modulation remains limited. Aanatomically speaking, the extrinsic cardiac sympathetic fibers that modulate the heart mainly derived from the SCG, the SG, and upper thoracic ganglia.19, 20 Wherein, SG primarily projects to the dorsal myocardium and the SCG preferentially innervates the anterior myocardium.12 Therefore, we firstly focused on the neuronal adaption or remodeling inside SCGs following MI. Structure changes with increased adrenergic phenotype TH-positive cell numbers were observed in SCG neurons, accompanied by sympathetic outflow elevation presented with increased neuronal activity, providing a significant anatomical and functional contribution to cardiac sympathoexcitation. The decreased body size could be the result of postganglionic sympathetic axon degeneration within the left ventricle at early stage post-MI.14 RNA-seq data revealed pathologic remodeling with excessive inflammation and neural regulatory factors limited within LSCG instead of RSCG, which could be explained by anatomical base that LV anterior wall innervated afferent limbs of most cardio-cardiac sympathetic reflexes are preferentially derived from left-sided gangalions.14, 21 Then, quantitative PCR confirmed significant increase of IL-1β and IL-6, ahead of NGF which peaked at Day 7 post-MI. Further spatial transcriptomics revealed that ganglia TH cluster in ganglia was enriched in immune process. Among traditional immune cells clustered in LSCG, macrophage was upregulated, in consistent with previous report that macrophage in SG participated in cardiac sympathetic overactivation in heart failure model.10 How does SCG sense innate inflammation in response to the ischemic injury in myocardium? Do cardiac sympathetic neurons signal to LSCG neurons to induce proinflammatory state in LSCG? Future studies should include analyses of the relative roles of cardiac afferent and preganglionic projections and CXCL2, IL1α, and TNFα, recruitment and activation factors previously reported for neuron-associated macrophages.22 Immunofluorescence staining found increased colocalization of macrophage with NGF, hinting suggesting immune-neuron interaction. Then we explored whether the LSCG remodeling was responsible for cardiac sympathetic hyperinnervation.
We innovatively conducted unilateral SCGx and uncovered LSCG-ectomy permit a simple, fast and selective intervention for cardiac denervation in anterior ventricular post-MI, confirming dominate role on local sympathetic control. Eventually, imbalance of the autonomic nervous control as detected by HRV diminished and cardiac VAs vulnerability was ameliorated at the early phase of MI. Interestingly, the heart rate remained stable with or without SCGx, providing indirect evidence that LSCG innervated ventricle without affecting pacemaker cells of the heart. Interestingly, downregulated NGF expression were observed in myocardium by SCGx which could partially led by decreased anterograde axonal transport, whereas augmented Sema3a expression was found with the exact source cell types remain to be determined. The data above hinted that of neural regulatory factor may be the potential mechanism of LSCG promoting the overwhelmed cardiac sympathetic nerve regeneration post-MI.
Sympathetic nerves promote inflammation in the initial phase of immune reactions.13, 23 Nerve terminals contain various neuropeptides, such as substance P (SP), neurokinin A, vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), and neuropeptide Y (NPY) exerted immune regulation and chemotactic functions.24, 25 Consistently, we found significantly decreased level of macrophage infiltration, as well as the corresponding cytokines in para-infarcted myocardium by SCGx, indicating the role of LSCG in cardiac immune modulation. Interestingly, the distribution of which in peri-infarcted zone decreased significantly though total mast cells number did not vary. It is possible that the lowered mast cell was due to loss of neuro-mast cell interaction and chemotactic neuropeptides. Eventually, SCGx improved cardiac structure and function, potentially caused by ameliorated inflammatory response, the key master for structural remodeling.26
Then we uncovered the key link in LSCG that bridged the cardiac-neuroaxis. The peaking time of inflammatory cytokines in LSCG were ahead of the uprising of NGF and Sema3a post-MI. These data made it feasible that neuroinflammation within SCG may result in neuron remodeling. NGF, supported the neuronal survival, differentiation, axonal outgrowth and elongation.27 In contrast, Sema3a, the secreted chemo-repulsive glycoprotein during axonal guidance, initiated sympathetic neuron apoptosis.28 Previous study found LSG delivery of Sema3a inhibits both ganglion and myocardial nerve sprouting following MI.29 Interestingly, in the current study, despite of self-remodeling with increased Sema3a, the feedback negative modulatory mechanism of Sema3a cannot compensate sympathetic spouting in pathological response to ischemia challenge. NGF is synthesized by a range of cell types including immune lineage and considered as classical targets for innervation by NGF-dependent neurons. We found the increasing distribution of NGF clustering around macrophage in LSCG,16 indicating that NGF neuro-immune crosstalk mediator. Whether decreased NGF played a dominate role in sympathetic sprouting under the circumstances? To answer the question, we further blocked the NGF binding receptor tyrosine kinase (TrkA)30 by targeting delivery of trkA shRNA to LSCG. Consequently, NGF signaling blockage reversed LSCG-cardiac sympathetic sprouting and reduced VAs incidence, providing evidence that alterations in the cardiac-neuroaxis are linked to increased arrhythmias following MI. In addition, evidences have shown that NGF could act as immune modulator by enhancing the expression of neuropeptide CGRP and SP.17, 31 Consequently, diminishing NGF signaling in LSCG ameliorated cardiac inflammatory infiltration, accompanied by downregulation of GCRP and SP. Considering the data above and appearance of lymph nodes mimic,32 as well as the function of the neuro-immune circuit responding to and dampened on cardiac inflammation, we innovatively raised the concept of LSCG as lymph node-mimicry.33
3.1 Clinical implications
The pathophysiologic changes in the SCG support the rationale for cardiac sympathetic denervation in the treatment of VAs refractory to conventional therapies. Pharmacological sympathetic modification targeting LSCG focusing on NGF blockage developed an approach to interfere with the sympathetic spouting and neuroinflammation post-MI. The early discoveries could be brought into the potential clinical use.
3.2 Limitation
(i) The spatial RNA sequencing technique used, the 10× Visium platform, have limitations in accurately capturing and distinguishing individual cells or cellular features within the spatial context of the tissue in terms of resolution. (ii) Besides the cardiac sympathetic nerve remodeling, fibroblast activation necessary for scar formation and endothelial cell activation necessary for angiogenesis are also key factors that are responsible for structure and functional remodeling post-MI. This study only provided several hints of the cardiac sympathetic innervation post-MI without assessing the overall consequences post LSCGx. (iii) Functional characteristics of right SCG and comparison between unilateral SCG and bilateral SCG intervention were not evaluated and should be studied in the future. (iv) More research work is eagerly needed in exploring the long-term effects regarding SCG remodeling during and post-MI.
4 METHODS
4.1 Animals
Male Sprague–Dawley rats (230–280 g, 8–12 weeks old) were purchased from Vital River Laboratory (Beijing, China). All experimental procedures were conducted following the institutional guidelines for animal care and research established by the Shandong University (Jinan, China) Institutional Animal Care and Use Committee. All the animals were housed in the specific pathogen-free (SPF) environment under a 12 h light/dark cycle during the experiment with free access to normal rat chow and water. At the end of the experiment, all animals were euthanized by an overdose of pentobarbital sodium.
4.2 Animal model of LSCG-ectomy and MI surgery
4.2.1 Protocol 1
For LSCG-ectomy, anesthetize the rats with 3% pentobarbital sodium (30 mg/kg intraperitoneally), then identify the left SCG by anatomic location which was marked with the carotid bifurcation with LSCG behind as described in detail previously.34 30 min post LSCG-ectomy, the rats were subjected to myocardial infraction surgery as we previously described.7 MI was confirmed success with elevated ST segment on a surface ECG, and stiff movement of the left ventricle (LV). The Sham group underwent all the surgery protocol without ligation on the heart. The rats were allowed to recover on a heated pad to maintain a body temperature of 37°C after surgery. Finally, then they were returned to their individual cages. The rats were closely monitored at the first 24–72 h after surgery.
4.2.2 Protocol 2
To explore the role of NGF signaling pathway in SCG remodeling, we applied trkA shRNA by injection into SCGs to block NGF receptor while GV478 with a green fluorescent protein reporter and scrambled RNA served as the blank control for the shRNA virus (shCtrl). At least 3 weeks before MI surgery, rats were subjected to LSCG injection and randomized to four groups (8 each): (i) sham + shCtrl group, (ii) sham + shTrkA group, (iii) MI + shCtrl group and (iv) MI + shTrkA group. Briefly, 1 μL of shTrkA or shCtrl was directly injected into the LSCG using a 33- G microsyringe. RNAi sequences targeting TrkA 403 position was previously designed35 and validated in our laboratory. The adeno-associated virus GV478 was constructed titer was 4.5 × 1013 genomic particles/ml. Inhibition of TrkA expression in the LSCG was confirmed by immunofluorescence localization of GFP expression and TrkA expression. MI surgery was performed 4 weeks after AAV microinjection in the LSCG. For intraganglionic injections, the SCGs were exposed as described in Section 4.2.1.
4.3 Electrophysiological study
Rats were anesthetized and mechanically ventilated as described. The protocol used for the programmed electrical stimulation was performed as previously reported.7, 36, 37 A programmed stimulation protocol was performed using electrodes implanted on the epicardial surface of the LV after surface ECG monitoring. The programmed electrical stimulation, VAs determination, and VA scores were performed as previously described.7 The experimental protocols were typically completed within 10 min.
4.4 Electrical mapping
In vivo epicardial electrocardiogram (ECG) was recorded using the electrophysiological mapping system with 64 + 64 channels (MappingLab). Expose the rat heart after ventilation, then place the 64-channel electrode (8 × 8 grids, 0.45 mm spacing) at the infarction border area of the heart. Mapping lab EMapScope 5 (version 5.8.1) software was used to record and analyze the epicardial ECG and QRS durations. High-pass (Frequency cut 100) and low-pass (Frequency cut 20) filters were applied to avoid frequency noises.38
4.5 Echocardiography
At Day 7 after MI surgery, all rats were anesthetized with isoflurane. Two-dimensional echocardiography was performed using a 21 MHz transducer and the data were analyzed with Vevo770 system. The typical B and M mode image parameters were used to determine the LV ejection fraction (LVEF). All measurements were conducted by an experienced technician blinded to study grouping and averaged for three consecutive cardiac cycles.
4.6 HRV analysis
All rats were subjected to ECG monitoring for at least 30 min before surgery, which were considered the baseline activity. For SCG removed animals, the rats received ECG monitoring for 30 min before and after SCG removal, and then MI surgery was applied. All the rats received another ECG monitoring for 30–60 min after surgery. All monitoring was in the presence of pentobarbital sodium anesthesia without supporting by ventilator. The ECG data were recorded and analyzed with Lab-Chart Pro software (AD Instruments, Sydney, Australia). Frequency-domain method was used to analyze the heart rate variance (HRV). The high frequency (HF, 0.75–2.5 Hz) and low frequency (LF, 0.2–0.75 Hz) and LF/HF indexes were used to evaluate autonomic nervous regulation in this study.
4.7 Tissue collection
The SCGs from the Sham and MI groups were collected right after rats were sacrificed, and SCGs were stored in liquid nitrogen for further experiments. SCGs protein and mRNA preparation were showed in details in Sections 4.8, 4.11 and 4.14.
4.8 RNA sequencing
SCGs RNA sequencing was performed by following the protocols provided by Shanghai Biotechnology Corporation. In brief, extract total RNA using RNeasy Micro Kit (74 004, Qiagen) following the instructions. The RIN number was obtained by an Agilent 2100 Bioanalyzer (Agilent technologies, Santa Clara, CA, US) to inspect RNA integrity. Then purify the qualified total RNA with RNAClean XP Kit (A63987, Beckman Coulter, Inc. Kraemer Boulevard Brea, CA, USA) and RNase-Free DNase Set (79 254, QIAGEN, GmBH, Germany). Paired-end sequencing technology was performed with the purified total RNA. Construct the RNA-seq library with VAHTS Universal V6 RNA-seq Library Prep Kit for Illumina® (NR604-02, Vazyme) after rRNA depletion, segmentation, etc. Quantify the library with Qubit® 2.0 Fluorometer and Agilent4200 for RNA concentration and size detection. The 2 × 150 bp paired-end sequencing (PE150) on an Illumina NovaSeq6000 sequencing system was performed. Data analyses were performed as previously described.39
4.9 Spatial transcriptome analysis
10× Genomics spatial sequencing (Gene Denovo) was performed for SCGs from control and MI rats. In brief, 3 SCGs of each side from each group were collected, frozen in isopentane, and embedded in OCT in compound (Sakura Finetek, Torrance, CA), which were then cryosectioned at −14°C (Leica CM1860). Sections were placed on the Visium Gateway gene expression slide (2 000 233, 10× Genomics). Tissue sections were then fixed and stained according to the Visium Spatial Gene Expression User Guide (CG000239 Rev. D, 10× Genomics). For gene expression samples, permeabilization time for spatial transcriptomics assay 18 min using the Visium Spatial Tissue Optimization slide by GENE DENOVO. Brightfield histology images were taken using a Nikon microscope. Raw images were stitched together and exported using BZ-X analyzer software (Keyence Corporation).
The Visium Spatial protocol produces Illumina-ready sequencing libraries. A Visium Spatial library contained standard Illumina paired-end constructs beginning and end with P5 and P7. The Visium Spatial 16 bp spatial barcode and 10 bp UMI are encoded in Read 1, while Read 2 is used to sequence the cDNA fragment. Read 1 and Read 2 are standard Illumina® sequencing primer sites used in paired-end sequencing. Images were processed with the Space Ranger software (ver 1.1.0, 10× Genomics), against the Cell Ranger mm10 reference genome “refdata-gex-mm10-2020-A”. Downstream analysis was performed by Seurat v4.40 We employed a normalization algorithm called “SCTransform” to separate for each dataset and merge them. The merged data were used for dimensionality reduction and cluster detection. We used MAST (Model-based Analysis of Single cell Transcriptomics) to find differential expression for a single cluster.41 Differentially expressed genes were identified as the following criteria: (1) p_value ≤ 0.01; (2) log2FC ≥ 0.360674; (3) the percentage of cells where the gene is detected in specific cluster >25%. Predict.score was calculated by using the ‘Find Transfer Anchors’ and ‘Transfer Data’ functions in Seurat to predict the proportion of cell types of each spot.
4.10 Enrichment analyses
Biotechnology information GO/KEGG pathway analysis was performed by Metascape3 and cluster Profiler Package in R on the Xiantao website. The Gene Set Enrichment Analysis (GSEA) analysis was performed using WebGestalt. The calculated p-value was gone through FDR Correction, taking False Discovery Rate (FDR) ≤0.05 as a threshold.
4.11 Western blotting
Rat myocardial tissues were obtained from MI hearts on Day 7 post-surgery. The para-infarction area (~3 mm zone adjacent to the infarction area) of the left ventricular was selected for protein extraction in the MI heart. Myocardial tissues (~2 mm) around the pericardiotomy region were selected for protein extraction in the Sham group. A total protein of 20 μg each sample was loaded onto a sodium dodecyl sulfate (SDS) gel for protein analysis. Stacking (5%) and separating (12%) SDS-polyacrylamide gel electrophoresis (PAGE) gels were used in this study. Proteins were transferred on to polyvinylidene fluoride (PVDF) membranes with 0.45 μM pores. Then, block the PVDF membranes with 5% non-fat milk in tris-buffered saline (TBST) for 1 h at room temperature (RT) followed by washing thrice. The PVDF membranes were incubated with rabbit anti-NGF (1:200, ab6199, Abcam), rabbit anti-Sema3a (1:1000, ab199475, Abcam), rabbit anti-trkA (1:1000, YT4741, Immunoway), rabbit anti-SP (1:2000, DF7522, Affinity), rabbit anti-GAP43 (1:5000, ab128005, Abcam), or rabbit anti-CGRP (1:1000, ab283568, Abcam) antibodies at 4°C overnight. The next day, PVDF membranes were washed in TBST thrice. Anti-rabbit secondary antibody (1:5000 in TBST) was used to incubate PVDF membranes for 1 h at RT. After rinsing thrice with TBST, the membranes were imaged with FluorChem E Imager (Protein-Simple, Santa Clara, CA, USA) in the presence of an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA).
4.12 Immunofluorescent and mast cell staining
Rat hearts were perfused and rinsed with cold phosphate-buffered saline (PBS) after removal from the thoracic cavity. Areas of interest were cut and embedded with optimal cutting temperature (OCT) compound and freeze at −80°C. For cardiac sympathetic nerve staining, the para-infarction area was selected and was cut into 1 × 1 × 0.5 (width × length × thickness in cm) pieces with the epicardium facing outside. For macrophages and mast cell staining, the hearts were cross-sectioned.
Myocardial tissues were cut into 10 μm thick slices followed by fixing with cold acetone for 10 min. The sections were then rinsed with PBS thrice. The sections were blocked with 1% bovine serum albumin (BSA) in PBS for 45 min at RT. The blocking buffer was removed, the sections incubated with rabbit anti-TH (1:200 in 1% BSA, AB152, Millipore), rabbit anti-CD68 (1:150, ab283654, Abcam), rabbit anti-NGF (1:200, ab52987, Abcam), rabbit anti-trkA (1:200, YT4741, Immunoway), rabbit anti-SP (1:200, DF7522, Affinity), rabbit anti-CGRP (1:100, ab283568, Abcam), or rabbit anti-GAP43 (1:500, ab16053, Abcam) antibodies for 2 h, followed by a 1-h incubation with a secondary antibody. They were treated with a signal amplification solution (Absin Bioscience Inc., abs50012) for 10 min. The sections were coated with DAPI. Mast cells were stained with 0.1% Toluidine blue (G2543, Solarbio), and the nuclei were stained with hematoxylin (C0107, Beyotime). Sections were examined under a confocal laser scanning microscope (Olympus LCX100 Imaging System) and images were acquired for GAP43 and TH quantification (mean fluorescence intensity) as previously described.42
4.13 ELISA
Peripheral blood was collected by subclavian vein puncture at Days 3 and 7 after MI surgery. The blood was placed into blood collection tubes with gel/clot activator (G30811, Orsin). Myocardial tissues were similarly collected after MI surgery and stored at −80°C. The following commercial ELISA kits were used in this study: Norepinephrine (NE), IL-1β, TNF-α, and Acetylcholinesterase (AChE) (CSB-E07022r, CSB-ET011614RA, CSB-E11987r, CSB-E11304rUSCN Life Science, Wuhan, China).
4.14 Real-time PCR
Total RNAs were extracted from SCG and myocardial tissues using Trizol reagent (Vazyme, Nanjing, China). Following RNA was quantification by OD260/280, complementary DNA (cDNA) was synthesized by reverse transcription as detailed described before (Promega, Madison, WI). The cDNAs were used for quantifying IL-13, IL-1β, TNF-α, IL-6, IL-10, TGF- β, Sema3A, GAP43, and NGF mRNAs by real-time PCR (run in triplicate) with gene-specific primers (Supplemental data). RT-qPCR assay was performed using a SYBR qPCR Master Mix (SABiosciences) in a CFX96 Real-Time System (Life Technologies, Grand Island, NY). For quantification, comparative CT method (∆∆CT method) was used to calculate relative each mRNA level with GAPDH as utilized as a reference for normalizing each sample.
4.15 Statistical analysis
Statistical analysis was performed with SPSS 17.0. Quantitative data are presented as the mean ± SE; t test, analysis of variance (ANOVA) with post hoc tests were used to analyze the differences among groups. p < 0.05 was considered statistically significant.
5 CONCLUSION
LSCG exhibited high pro-inflammatory characteristics and cardiac immunomodulation function that mimics lymph node post-MI. LSCG intervention may be a feasible strategy to alleviate excessive sympathoexcitation through attenuation of neural remodeling within cardiac-neuraxis, representing a promising neuromodulation option for preventing ischemia-provoked VAs and myocardial damage.
AUTHOR CONTRIBUTIONS
Qingxia Yu: Methodology; formal analysis; investigation; writing – original draft. Yan Li: Methodology; formal analysis; investigation; writing – original draft. Wenju Yan: Validation; methodology. Weizhong Han: Methodology; validation. Qian Liu: Investigation; validation. Junyi Zhang: Supervision. Xiaolu Li: Visualization; writing – review and editing; software. Yugen Shi: Writing – original draft; visualization. Yu Wang: Visualization; software. Jie Yin: Conceptualization. Suhua Yan: Conceptualization.
ACKNOWLEDGMENTS
The original items of graphic abstract were generated from Biorender website. And the authors drew the picture.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (82300354, 81900296, 82200362, and 32400994), Science Foundation of Shandong Province (2017MH067), Medical and Health Technology Development Program in Shandong Province (2017WS088), China Postdoctoral Science Foundation (2016M602154), Shandong Provincial Natural Science Foundation (ZR2023MH182, ZR2020MH023, ZR2021QH096, and ZR2021QH041), and Shandong Provincial Key Discipline Construction Project of Traditional Chinese Medicine (Clinical Discipline of Integrated Traditional Chinese and Western Medicine).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supporting Information.




