Muscle physiology and its relations to the whole body in health and disease
Skeletal muscle makes up a substantial portion of the human body. Its most obvious function lies in enabling the body's posture and locomotion. It is a very dynamic and highly organized tissue and partakes in regulatory mechanisms that enable the body to maintain homoeostasis in metabolic, immune, and energy organization or to adapt these systems to intrinsic and extrinsic influences if needed (Figure 1).1
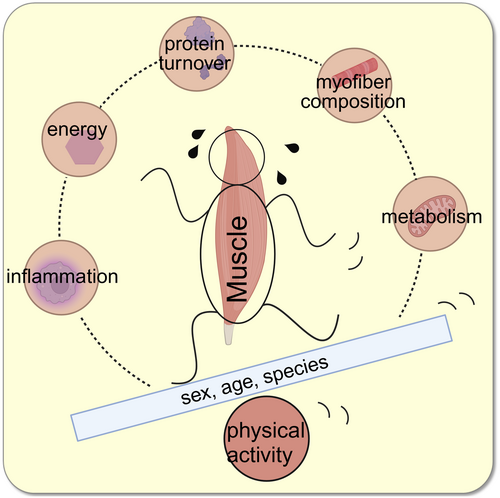
Over the past years, researchers increased awareness of the connection of muscle tissue to the rest of the human body and of the possibilities to tweak the muscles' metabolism in order to gain positive effects on whole body metabolism, energy balance, and immune system. Physiotherapists and doctors aim to find practical exercise guidelines for patients to follow for ideal treatment.2 To do so, much research is happening to understand the complexity of muscle–body balance. This article reviews the most recent research in this topic that was published by ActaPhysiologica.
1 BALANCED PROTEIN SYNTHESIS AND DEGRADATION MATTERS
Healthy muscle fibre needs a well-balanced turnover rate of fibre proteins. Shifts in this balance lead to changes in muscle composition and performance.
Many degenerative and inflammatory diseases lead to an alteration in muscle mass and strength. Alterations in muscle phenotype can be triggered by a diversity of internal or external factors. This can result in muscle hypertrophy, which is characterized by an increase in myocyte number and size, or atrophy, which is characterized by a loss of muscle mass, volume, and force.
A major pathway that regulates protein synthesis is the IFG1-Akt/PKB-mTOR signaling pathway, which promotes protein synthesis via its downstream targets mTOR or GSK3ß or FOXO. Activation of the IFG1-Akt/PKB-mTOR signaling pathway can lead to hypertrophy, inhibition can lead to atrophy.3 In a healthy person, exercise will activate satellite cells via G-protein-coupled receptor Gαi2. Gαi2 can bypass parts of the IFG1-Akt/PKB-mTOR signaling pathway and cause hypertrophy, resulting in strong and healthy muscles. Exercise is often recommended as treatment of disease-related atrophy.
Physical exercise causes small injuries in muscle fibres. This activates satellite cells, which migrate to the site of injury and proliferate and differentiate into muscle fibres. In addition, the number of pro- and anti-inflammatory macrophages increases after fibre injury. Satellite cells are adult stem cells, located in the “niche.” Patients with Marfan syndrome (MFS) show a fibrotic niche, caused by Fbn1 mutations. The myogenic potential of satellite cells is reduced and the number of macrophages is suppressed. This leads to reduced myofiber size and contractile force in regenerative muscles.4
As atrophy is a widespread pathophysiological complication, exercise is a widely recommended countermeasure. In order to build up muscle mass, the protein synthesis needs to be increased. Resistance exercise induces the stimulation of protein synthesis via mTORC1 (mechanistic target of rapamycin complex 1)- and JNK (c-Jun N-terminal kinase) signaling. But the ambitious sportsperson or patient needs to be aware that established training methods may lead to better performance, but not necessarily to muscle growth. For instance, altitude training may, among other effects, lead to an increase in haematological capacity. But a low level of oxygen inhibits resistance exercise-induced mTORC1- and JNK signaling and so slows down protein synthesis.5
Sarcopenia, age-induced loss of muscle mass and function, usually is a combination of fibre atrophy, which is caused by a shift in protein synthesis6 and fibre loss.7
2 BALANCED MYOFIBER COMPOSITION MATTERS
Myofibers of vertebrate skeletal muscles are classified into two phenotypes: So-called slow twitch (type 1) and fast twitch (type 2) fibres. The phenotypes differ in their response to neuronal input, metabolic properties, and their movement rates. Due to their different compositions, the responses of the two phenotypes to intrinsic and extrinsic influences differ as well. This can result in tissue-specific pathologies of muscle diseases. Understanding the complexity of genetics and pharmacology in fast versus slow fibre types shall help to find approaches to fight neuromuscular disorders8 and disease-related muscle dystrophies.9
The identification of fibre type compositions of skeletal muscle samples is a crucial part of (patho-)physiological studies. Lundquist and colleagues recently presented an automated deep learning-based platform that can be used to evaluate muscle tissue.10
3 BALANCED ENERGY HOMEOSTASIS MATTERS
Muscle tissue plays a key role in glucose homeostasis. In type 2 diabetes (T2D), malfunctioning tissues are resistant and unable to respond to insulin and blood glucose levels remain high. Such insulin resistance (IR) and impaired glucose homoeostasis increase the risk for cardiac, metabolic, and neurologic pathologies.
Many T2D treatments try to control blood glucose levels, but more holistic approaches are scouted. For instance, F. Bagheripour and colleagues reviewed the possibility to tweak nitric dioxide (NO) bioavailability in mice and humans with diabetes 2. They describe that glucose uptake by skeletal muscle tissue increases after administration of L-citrulline (Cit), a precursor of NO production. Furthermore, an increase of insulin secretion from the pancreatic β cells, increase lipolysis and β-oxidation, and decreased glyceroneogenesis in the adipose tissue are described effects of Cit administration.11
Another approach of T2D therapy can be to address the malfunctioning tissue in order to restore its insulin response. Several recent studies show possible ways to do so.
Prolonged exercise reduces glucose tolerance and increases insulin resistance in endurance athletes the following day. These findings are associated with an increased lipid load, a high capacity to oxidize lipids, and increased fat oxidation.12
Sedentary behavior is closely linked to muscle IR, but not to liver IR. Low levels of physical activity and behavior that is more sedentary have repeatedly been associated with (IR) and impaired glucose homeostasis. IR can develop in several tissues simultaneously, and different metabolic phenotypes differ in muscle versus liver tissue.13
Another key player that provides energy for active skeletal muscles is ATP. A steady glucose turnover rate ensures the ATP supply for contracting skeletal muscle via muscle mitochondrial ATP synthesis. A disturbed mitochondrial turnover can lead to pathologic myopathies. Human congenital myopathy for instance goes along with abnormal myosin post-translational modifications and ATP turnover time.14
After Duchenne, myotonic dystrophy type 1 (DM1) is the second most common muscular dystrophy. The patients' muscles cannot relax following a sudden contraction. In DM1 patients, the mitochondrial balance is disturbed. The transcriptional regulation of mitochondrial turnover and dynamics are impacted: DM1 mice display elevated expression of autophagy and mitophagy regulators. Physical exercise might contradict this unbalance. In a recent study, a single dose of exercise successfully enhanced canonical molecular pathways and skeletal muscle mitochondrial biogenesis despite failing to alter the cellular pathology in DM1 mice.15
4 BALANCE DIFFERS BETWEEN SEXES
Muscle composition and metabolism shifts with species, age, sex, and physical condition.8, 16 A large degree of sex- and muscle-related heterogeneity could be detected by implementing the before mentioned deep learning-based image segmentation methods, for fresh and postmortem human skeletal muscle.10
Therefore, it makes sense that beneficial associations between physical activity and whole-body insulin resistance differ between sexes.13 The physical activity spectrum of male and female participants show tissue-specific and sex-specific changes in insulin resistance. Also, a higher sport index was associated with lower liver IR in men, but not in women.
In the context of chronophysiology, it is even suggested that the timing of exercise during the day might influence the impact of exercise-induced adaptations.17 Over the course of a month, the female body is exposed to hormone fluctuations, which represents yet another variable that influences muscle physiology.18
Differences in fibre composition are accompanied by differences in neuromuscular properties. Consequently, neuromuscular recruitment differs between sexes. Guo and colleagues took a closer look at the neuromuscular recruitment of the right knee extensors of male and female participants. To recruit muscle fibres for slight contraction, the motor unit area that was involved was smaller, but showed a higher firing rate in female participants. To induce moderate contractions, neuromuscular recruitment parameters changed in similar ways, meaning the strategies to gain muscle force were similar.19 Another moderate- and high-intensity contraction study supports the idea of sex-related differences in neuromuscular patterns. They observed divergent motor unit behavior between sexes during high-intensity contraction.20
5 MUSCLE TISSUE PREFERS PHYSICAL ACTIVITY OVER PHYSICAL REST
A review by F. Sarto summarizes alterations in skeletal muscle health and metabolic functions that were induced by the step reduction model. In particular, decrements in muscle mass, muscle function, muscle protein synthesis, cardiorespiratory fitness, endothelial function, and insulin sensitivity, together with an increased fat mass and inflammation, have been observed. They also summarize validations that exercise can counteract the negative effects.21
Exercise puts muscle tissue into stress. Particularly, the endoplasmic reticulum (ER) undergoes stress-related adaptations. For instance, physical exercise increases the levels of proteins and genes related to protein folding in skeletal muscle ER, leading to misfolded proteins inside the ER and their accumulation. Such disturbance in protein organization cause acute ER stress, but in the long run, regular exercise is known to cause adaptations that dampen ER stress generally. Marafon et al. summarize these effects and predict that chronic excessive exercise—so-called over training—may cause pathologic levels of chronic ER stress.22 Rest periods between physical exercise may be just as important as non-exhaustive exercise levels in order to enable muscle ER to turn the stress-induced imbalance of protein synthesis into ER stress resilience.
NAD+/NADH+H+ is an important redox coenzyme that is essential for the continuous turnover of energy substrates in the cell. Impaired NAD+ levels are a shared property of various diseases and aging. Walzik and colleagues reviewed the hypothesis that exercise can restore NAD+ homeostasis through metabolic adaption to chronically recurring states of increased energy demand. Acute exercise leads to profound alterations of intracellular NAD+ metabolism in various investigations. Exercise modality, cell type, and investigated animal model or human population strongly influence the extent and direction of changes. Exercise training elevated NAD+ levels and NAD+ metabolism enzymes in various tissues.23
As mentioned above, physical exercise causes small injuries in muscle fibres, which induces regenerative processes. In a recent study, they tried to support the regeneration of collagen-rich extracellular matrix of tendon tissue by oral supplementation and showed that remodeling of skeletal muscle by resistance training can be supported by supplementation of bioactive collagen peptides.24
6 CONCLUSION: EXERCISE CAN TWEAK YOUR METABOLISM HOMEOSTASIS INTO THE RIGHT DIRECTION
Above-explained correlations between muscle physiology and whole body physiology indicate that obtaining healthy and active muscle tissue is a key factor in general health as well as in pathophysiological manifestations. A shift into a less physically active lifestyle can quickly lead to an abundance of negative effects. To improve patients' health, exercise is indicated to be a good therapy or therapy addition to improve the health of patients with diseases that affect their immune system, metabolism, muscle physiology, or energy homoeostasis. Exercise modalities should be individually adjusted to gain ideal positive effects.
AUTHOR CONTRIBUTIONS
Kathrin Groeneveld: Writing – original draft; investigation.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The author declares no financial or other conflicts of interest that might bias this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




