The effect of neuromuscular electrical stimulation on the human skeletal muscle transcriptome
Johanna Flodin and Stefan M. Reitzner contributed equally.
Abstract
Aim
The influence on acute skeletal muscle transcriptomics of neuromuscular electrical stimulation (NMES), as compared to established exercises, is poorly understood. We aimed to investigate the effects on global mRNA-expression in the quadriceps muscle early after a single NMES-session, compared to the effects of voluntary knee extension exercise (EX), and to explore the discomfort level.
Methods
Global vastus lateralis muscle gene expression was assessed (RNA-sequencing) in 30 healthy participants, before and 3 h after a 30-min session of NMES and/or EX. The NMES-treatment was applied using textile electrodes integrated in pants and set to 20% of each participant's pre-tested MVC mean (±SD) 200 (±80) Nm. Discomfort was assessed using Visual Analogue Scale (VAS, 0–10). The EX-protocol was performed at 80% of 1-repetition-maximum.
Results
NMES at 20% of MVC resulted in VAS below 4 and induced 4448 differentially expressed genes (DEGs) with 80%-overlap of the 2571 DEGs of EX. Genes well-known to be up-regulated following exercise, for example, PPARGC1A, ABRA, VEGFA, and GDNF, were also up-regulated by NMES. Gene set enrichment analysis demonstrated many common pathways after EX and NMES. Also, some pathways were exclusive to either EX, for example, muscle tissue proliferation, or to NMES, for example, neurite outgrowth and connective tissue proliferation.
Conclusion
A 30-min NMES-session at 20% of MVC with NMES-pants, which can be applied with an acceptable level of discomfort, induces over 4000 DEGs, of which 80%-overlap with DEGs of EX. NMES can induce exercise-like molecular effects, that potentially can lead to health and performance benefits in individuals who are unable to perform resistance exercise.
1 INTRODUCTION
Physical exercise of human skeletal muscle is well-known to effectively prevent the negative consequences of immobilization.1-4 However, during certain periods of immobilization, such as after surgery or injury, muscle activation may not be possible, which may lead to muscle atrophy5 and decreased blood circulation.6
Neuromuscular electrical stimulation (NMES) activates muscles during, for example, immobilization,7-9 and is used in rehabilitation to minimize atrophy and for muscle strengthening purposes together with voluntary muscle contractions.7, 10 NMES without voluntary contractions can provide positive effects in immobilized patients on muscle strength and structure,7, 8, 11-13 including preservation of muscle mass through prevention of atrophy, but regular voluntary exercise (EX) seems to have more prominent effects on muscle mass increase.14, 15 However, the differences in transcriptomic changes following an acute NMES- compared to EX-session are not clearly defined.16-19 Thus, the underlying mechanisms mediating the effects of NMES on skeletal muscle, as compared to EX, are poorly known.
The impact of a single NMES-session, compared to EX, on quadriceps muscle mRNA-expression has been examined in one previous study.20 Twenty-four hours following a 30-min NMES-session, it was shown that many genes common with EX were regulated at the mRNA level.20 However, the level of muscle activation with NMES was not defined and change in mRNA-expression was not studied in the first hours post-treatment. Thus, the early effects of a single NMES-bout with defined levels of muscle activation compared to EX are unknown.
Earlier studies have suggested that an NMES-intensity equal to at least 20% of maximal voluntary contraction (MVC) is needed for muscle strengthening effects,21, 22 and can be reached with an acceptable level of discomfort (i.e., VAS 0–10 scale values below 423).24 Yet, one limitation with current NMES is the high degree of pain and discomfort,25, 26 and time-consuming set-up of the apparatus, leading to low compliance.27, 28 To overcome such an accessibility barrier, textile NMES-electrodes have been integrated into elastic training pants (NMES-pants), which increases NMES compliance.29, 30 However, whether 20% of MVC can be achieved using NMES-pants to provoke early molecular effects of NMES in parity with EX effects is unknown.
The primary aim of this study was to investigate the effects on global mRNA-expression in the quadriceps muscle early after a single NMES-bout at 20% of MVC, compared to the effects of weight-stack knee extension EX. The secondary aim was to explore if NMES-pants results in a similar linear increase in % of MVC with increased intensity and low level of pain/discomfort at 20% of MVC as previously shown with regular self-adhesive electrodes. It was hypothesized that one session using NMES-pants at 20% of MVC could be achieved at acceptable discomfort while still eliciting an early mRNA-expression signature similar to EX.
2 METHODS
2.1 Ethical approval
Before the interventions, all participants were informed about the study outline, familiarized with the experimental procedures and potential complications, and written consent was obtained. The study was approved by the Stockholm regional ethics board (Dnr: 2020-03374) under observance of the declaration of Helsinki.
2.2 Participant recruitment and characterization
A total of 30 healthy participants (15 males, 15 females) aged 18–43 years were included in the study and completed the entire study (Table 1). The study had a drop-out of seven participants due to different reasons as described in Figure 1. Prior to inclusion in the study, potential participants completed a screening questionnaire about basic characteristics and health questions. The inclusion criteria were age between 18 and 55 years and healthy (no known acute or chronic disease). The exclusion criteria were obesity (BMI >30 kg/cm2), smoking, pregnancy, skin ulcer, pacemaker, intracardiac defibrillator, advanced heart disease, kidney failure, neuromuscular, or metabolic disease.
| Variable | All subjects mean (±SD) | NMES/EX mean (±SD) | NMES/CON mean (±SD) | EX/CON mean (±SD) |
|---|---|---|---|---|
| Sex, male/female | 15/15 | 8/8 | 3/4 | 4/3 |
| Age (years) | 28.9 (±6.88) | 26.9 (±6.14) | 31.1 (±7.73) | 31.1 (±7.24) |
| Height (cm) | 173 (±10.4) | 174 (±11.9) | 173 (±10.1) | 171 (±7.85) |
| Weight (kg) | 74.4 (±16.4) | 74.8 (±17.6) | 71.7 (±18.4) | 76.3 (±13.5) |
| BMI (kg/cm2) | 24.6 (±4.08) | 24.5 (±4.34) | 23.5 (±3.90) | 25.9 (±3.85) |
| Type 1 fiber (%) | 46.1 (±9.84) | 42.5 (±8.56) | 51.3 (±8.03) | 49.0 (±12.7) |
| Type 2 fiber (%) | 53.9 (±9.84) | 57.5 (±8.56) | 48.7 (±8.03) | 51.0 (±12.7) |
| MVC, Biodex (Nm) | 200 (±79.7) | 206 (±93.0) | 185 (±66.1) | 199 (±66.0) |
| MVC, Easyforce (N) | 419 (±131) | 406 (±137) | 421 (±145) | 403 (±205) |
| NMES 20% MVC (mA) | 48.2 (±9.01) | 47.1 (±8.66) | 50.5 (±6.11) | – |
| VAS at 20% MVC | 3.89 (±1.31) | 3.80 (±1.44) | 4.08 (±0.90) | – |
| 1RM weight, (kg) | 47.3 (±33.0) | 48.8 (±20.1) | – | 44.0 (±10.3) |
- Note: The fiber type was determined after immunohistochemical staining and the cross-sectional cuts were analyzed using ImageJ.
- Abbreviations: BMI, body mass index; mA, milliampere; MVC, maximal voluntary contraction; N, Newton; Nm, Newton meters; NMES, neuromuscular electrical stimulation; VAS, Visual Analogue Scale; 1RM, one repetition maximum.
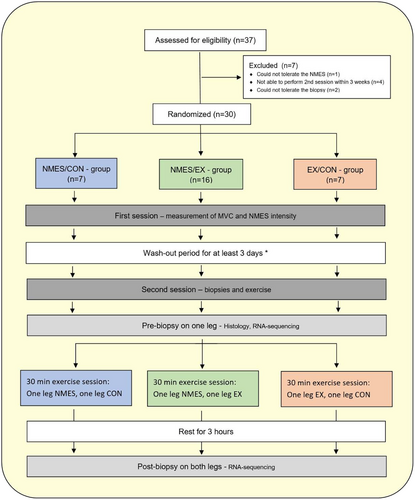
2.3 Study design
The participants were divided into three groups, conducting different combinations of unilateral quadriceps muscle exercise intervention protocols for the dominant and non-dominant leg consisting either of neuromuscular electrical stimulation (NMES), knee extension resistance exercise (EX) or no exercise, that is, control (CON): (1) NMES/EX, (2) NMES/CON, and (3) EX/CON (Figure 1). There were no statistically significant differences between groups in any of the measured characterization parameters (Table 1).
2.4 Knee extensor force measurement
The participants isometric knee extension torque was measured using an isokinetic dynamometer (Biodex System 3 Pro; Biodex Medical Systems, Shirley, New York) with the knee joint in 90 degrees of flexion and position as describe in a previous study.24 The knee extension torque was additionally measured using the EasyForce dynamometer (Meloq AB, Stockholm, Sweden) to enable a constant supervision of the produced force during the biopsy session and ensure that the targeted 20% of MVC was reached during NMES. Correlation analyses demonstrated good reliability of the measurements with the EasyForce and Biodex, for both the MVC (R2 = 0.76) and 20% of MVC (R2 = 0.70) (Figure S1).
Thereafter, the single-leg 1 repetition maximum (1RM) was measured in a knee extension machine according to a standardized protocol. The measurement was performed after a warm-up of eight repetitions of approximately 50% of MVC, followed by tree repetition at an estimated 80% of MVC. Thereafter each participant got three attempts to reach the 1RM with 1 min rest in between. The mean (SD) values for the MVC determined with the Biodex and EasyForce dynamometers for the leg randomized to NMES, and the 1RM for the leg randomized to EX are presented in Table 1.
2.5 Neuromuscular electrical stimulation pants
For the NMES-testing all participants were supplied with a pair of NMES-pants, that was designed and created as described in a previous study.31 The same pants have been used in a previous study, and have been shown to produce a muscle contraction without any pain/discomfort31 and self-adhesive electrodes placed on the same positions has been shown to produce a knee extension force corresponding to 20% of MVC with a mean VAS below 4.24
The size and placement of the electrodes in the pants were based on a previous study comparing different pre-determined electrode placement and sizes32 and are displayed in Figure 2. The placement of the electrodes corresponds to areas with a high likelihood for motor points.33 In this study only the electrodes over the quadriceps muscle were used, and only the electrodes on the NMES-leg were connected to the NMES-machine.

2.6 Pre-testing
After the participants put on the NMES-pants, it was made sure that all electrodes had contact with the skin and thereafter 10 mL conductive gel (Conductor transmission gel, Chattanooga, Eco-Med Pharmaceutical Inc., Canada) was applied on all of the electrodes over the quadriceps muscles (Figure 2C). Thereafter, the NMES-testing began with the participants seated in the dynamometer as described for the MVC measurement. The NMES was delivered with a Chattanooga Physio (DJO Nordic, Malmoe, Sweden) with the same settings and intensity-increase as used in a previous study.24 When the 20% of MVC was reached, the participants were asked to evaluate the general discomfort of the stimulation according to the Visual Analogue Scale (VAS) on a 100-step scale (0–10, with one decimal), with 0 indicating no discomfort or pain and 10 indicating the worst imaginable discomfort or pain.34 All participants were informed that they could end the stimulation whenever they wanted but were encouraged to reach 20% of MVC. The mean (SD) intensity required to reach 20% of MVC and the pain perceived at 20% of MVC are presented in Table 1.
2.7 Intervention
Prior to the second session participants were asked not to take any medications containing acetylsalicylic acid within 7 days before, perform physical activities within 72 h before, drink alcohol within 48 h before, and use tobacco products or caffeine on the same day as the session. In addition, all participants were instructed to eat a proper meal at least 3 h before the session and thereafter they were not allowed to eat and drink other than water.
At the intervention day, the participants were randomized to one of three different intervention layouts (Figure 1). The two sessions were performed with a mean (SD) of 9.5 (3.8) days apart, and to be included in the study the two sessions had to be performed between 3 to 21 days apart in order to let the participants rest enough before the second session, while the measurements from the second session still would be valid.
The EX-protocol consisted of one warm-up session of one set with 8 repetitions at 50% of 1RM, followed by nine sets of eight repetitions at 80% of 1RM, separated by 2.5 min rest in between. The NMES-protocol consisted of a warm-up session, that started with two minutes of stimulation at low-intensity (1 Hz, 15 mA) followed by one set of eight stimulations (frequency of 36 Hz, pulse duration 800 μs, on-time 8 s (1 s ramp-up/down), and 8 s off-time) during which the NMES-intensity was increased step-wise up to the intensity equal to 20% of MVC. Thereafter, nine sets of eight repetitions at the intensity equal to 20% of MVC was performed, with 1 min of rest in between.
2.8 Skeletal muscle tissue biopsies
Skeletal muscle biopsies were taken from left and right M. vastus lateralis before and 3 h after one single bout of NMES and/or EX using the Bergström needle technique.35 To prevent possible dominant/non-dominant leg effects, the first muscle biopsy was taken from either the dominant or the non-dominant leg by randomization. The post biopsies were taken from both legs, 3 h after the NMES, EX or control session was finished for that leg respectively. The skeletal muscle biopsies were then snap-frozen in liquid nitrogen using 2-methylbutane as a secondary coolant.
2.9 Immunohistochemical fiber typing
Skeletal muscle samples were cut on a cryostat (CryoStar NX70, Thermo Scientific, Waltham, MA, USA) at 7 μm and immunohistochemically stained for myosin heavy chain I (DSHB, Cat# BA-F8, RRID:AB_10572253) and laminin (Sigma-Aldrich, Cat# L9393, RRID:AB_477163). In brief, unfixed slides were incubated with in a blocking buffer (1% bovine serum albumin [BSA] and 1% normal goat serum in phosphate-buffered saline [PBS]) overnight at 4 degrees. On the next day, following PBS-wash, slides were incubated with secondary antibodies (Thermo Fisher Scientific Cat# A-21141, RRID:AB_2535778 and Thermo Fisher Scientific Cat# A-11011, RRID:AB_143157) for 60 min in room temperature, and mounted with cover glasses using Prolong Gold Antifade Reagent (Invitrogen). An Olympus IX-73 wide-field fluorescence microscope (Olympus Corp., Shinjuku, Tokyo, Japan) was used for image acquisition. Analysis of immunohistochemically stained cross-sectional cuts was performed blinded using ImageJ. The histological fiber type was included as a baseline characteristics of the participants (Table 1) and representative images of the staining's are provided in Figure S2. We stained for type 1 fibers, and presented the rest as type 2 fibers without distinguishing between type 2A and 2X as the proportion of type 2X is generally very low in young active adults.36
2.10 RNA sequencing
RNA extraction from skeletal muscle tissue was performed using the phenol-based TRIzol method (Invitrogen #15596018, Thermo Fisher Scientific, Waltham, MA, USA), quantified and quality checked using the 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA, USA). Libraries were prepared by poly-A selection (TruSeq mRNA, Illumina, San Diego, CA, USA) and multiplexed at the National Genomics Infrastructure Sweden. Clustering was done by “cBot” and samples were sequenced on NovaSeq6000 (NovaSeq Control Software 1.6.0/RTA v3.4.4) with two lanes of a 2 × 151 setup “NovaSeqXp” workflow in “S4” mode flow cell. The Bcl‘to FastQ conversion was performed using bcl2fastq_v2.20.0.422 from the CASAVA software suite. The quality scale used is Sanger/phred33/Illumina 1.8+. QC and processing were performed using the nfcore/rnaseq analysis pipeline publicly available at github (https://github.com/nf-core/rnaseq).
3 QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed with R version 3.6.0. For baseline analyses, ANOVA and t-test were used. Gene expression was measured by differential gene expression determined by edgeR package analysis.37 Alpha was set to 0.01, unless stated otherwise, error bars represent standard error of the mean.
4 RESULTS
4.1 Discomfort and characteristics of textile electrodes in NMES-pants
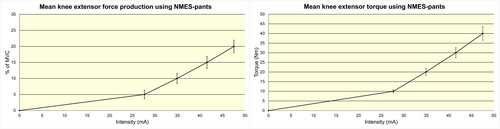
The experiments demonstrated that the current intensity applied through the NMES-pants had a linear curve behavior with the amount of % of MVC as well as torque production (Nm) between 5% and 20% of MVC (Figure 3). Moreover, we demonstrated that it was possible to achieve 20% of MVC using the NMES-pants with average VAS below 4 (Table 1).
4.2 Differential gene expression analysis and functional annotation
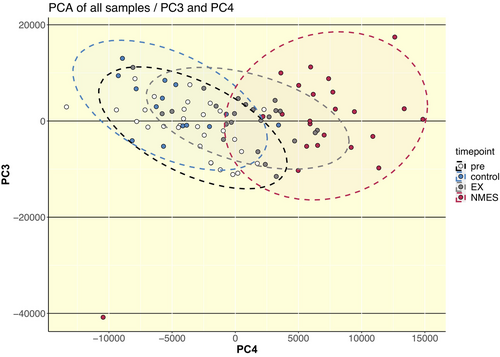
Unsupervised principal component analysis revealed large overlaps between pre timepoints and untrained control leg, while the overlap of NMES with EX was larger than the overlap of NMES with both pre (baseline)- and control conditions (Figure 4). Subsequent differential gene expression analysis showed large numbers of differentially expressed genes (DEGs) following both regimes. A larger number of DEGs was shown following NMES in the NMES-leg in both the NMES/CON (3682 DEGs) and NMES/EX (4452 DEGs) protocols, as compared to EX in the EX-leg EX/CON (1188 DEGs) and NMES/EX (2571 DEGs) (Figure 5A).
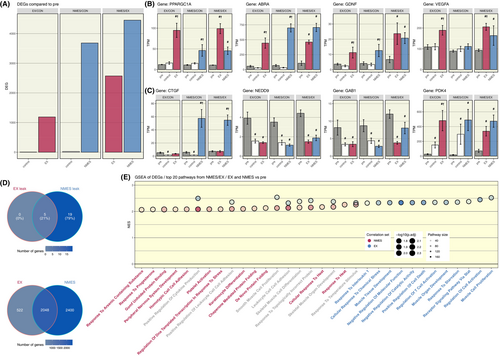
In this analysis we found several particularly interesting DEGs which are highly relevant for the acute exercise response, that were stimulated partly in different ways by NMES and EX (Figure 5B). PPARGC1A, a master regulator of energy metabolism, and VEGFA, an inducer of proliferation and migration of vascular endothelial cells, were significantly stimulated by both NMES and EX, but significantly more by EX. However, ABRA, a positive regulator of transcription and protein trafficking, and GDNF, a survival and differentiation factor for neurons, were significantly stimulated in similar extent by both NMES and EX.
Additionally, we found significant effects of both interventions on genes important for the development and differentiation of neurons (Figure 5C). Hence, the gene expression of both NEDD9, regulating cell attachment, migration and apoptosis in the cell cycle, and GAB1, a factor of the cellular growth response, transformation and apoptosis, was significantly decreased following both NMES and EX, while that of PDK4, a contributor to the regulation of glucose metabolism was significantly increased. CTGF, a growth factor of connective tissue, gene expression was significantly increased only following NMES, but not after EX.
Furthermore, we found a large overlap of genes between the acute effect of NMES and EX in the NMES/EX protocol. Of all DEGs in the NMES/EX protocol as compared to the pre-timepoint, 2048 DEGs (41%) were shared between both the NMES and EX legs (Figure 5D, lower). NMES exhibited 2400 DEGs (48%) that were only expressed in the leg that received NMES, while EX exhibited only 522 DEGs (11%) only in the leg that performed EX.
The methodological design also made it possible to compare the “cross-over” effect into the contralateral control leg, in both the EX/CON and NMES/CON protocols. In response to EX, we found five leaking DEGs, all of which were also differentially expressed in the CON leg in response to the contralateral leg performing NMES (Figure 5D, upper). Additionally, we found 19 additional DEGs leaking only from a response to NMES.
Using the DEG results from the NMES/EX protocol arm to directly compare both exercise regimens we performed gene set enrichment analysis (GSEA) and found EX to generally result in higher normalized pathway enrichment scores compared to NMES (Figure 5E). GSEA demonstrated pathways exclusive to EX, such as muscle tissue proliferation, development, cell activation and immune system activation. Similarly, there were pathways exclusive to NMES, including heat response, stress responses and peripheral nervous system development. Pathways that exhibited significant activation by both intervention types revolved around immune system activation and skeletal muscle development. Interestingly, a key pathway that was significantly enriched in both the EX- and NMES-leg of the EX-NMES cohort comparing post- and pre-training was the GO biological process pathway connective tissue development.
4.2.1 Connective tissue development
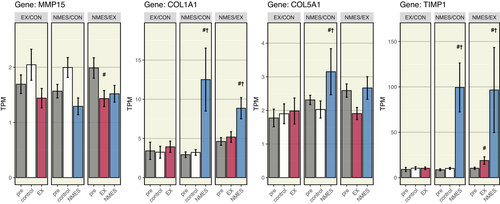
Furthermore, in the control-NMES cohort, the NMES-leg enriched the connective tissue development pathway to significantly higher levels from the control leg at the post-timepoint. Thus, our further investigation took a closer look at connective tissue genes that were significantly affected by EX and NMES and part of the aforementioned pathway (Figure 6). Hence, the gene expression of COL1A1 and COL5A1, which form collagen molecules that strengthens many supportive tissues, including cartilage, bone, and tendon, was significantly increased following NMES, but not after EX. MMP15, a matrix metalloproteinase involved in breakdown of extracellular matrix, was not significantly regulated by NMES. However, TIMP1, a tissue inhibitor of metalloproteinases, was significantly increased only following NMES, but not after EX. While NMES resulted in a higher total number of DEGs, the molecular signature of NMES and EX showed to be largely overlapping (Figures 5D and 7).
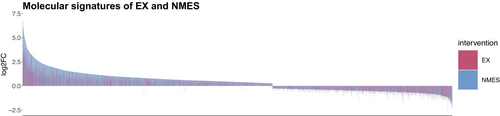
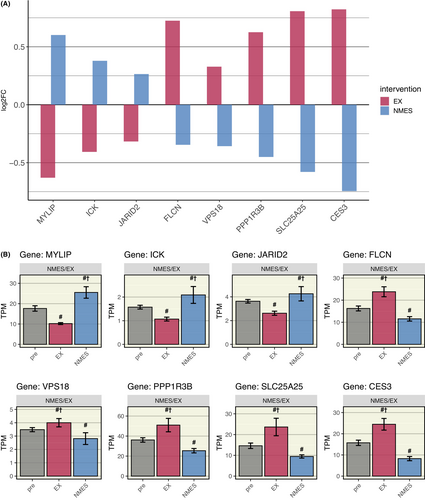
4.2.2 Oppositely regulated genes
However, further analysis of the molecular signature revealed eight genes to be oppositely regulated comparing NMES and EX (Figure 8). Of these, three were upregulated following NMES (and downregulated following EX), amongst them MYLIP (Myosin Regulatory Light Chain Interacting Protein), a regulator of neurite outgrowth in skeletal muscle. Five oppositely regulated genes were upregulated following EX (and downregulated following NMES), such as FLCN (Folliculin), which itself is a regulator of the two master regulators mTOR (mechanistic target of rapamycin kinase), and PPARGC1A, both highly important for the regulatory response to acute exercise.
5 DISCUSSION
The present study offers novel insights into the acute transcriptomic effects of a NMES-session at 20% MVC on skeletal muscle compared to traditional voluntary resistance exercise (EX). NMES and EX trigger a predominant fraction of overlapping DEGs. GSEA demonstrated common pathways as well as pathways exclusive to either EX or NMES. The molecular signature revealed only eight genes to be oppositely regulated comparing NMES and EX. These findings provide insights into NMES-stimulated transcriptomic activation of exercise-related genes, which can control exercise-induced health and performance benefits in individuals not able to perform EX. In addition, we demonstrated that NMES-pants can transduce the electricity to muscle energy in a linear fashion and that 20% of MVC could be produced without pain, which reflects that wearable NMES could improve compliance.
Most strikingly, we showed that 80% of the 2570 DEGs induced by a single bout of EX overlap with the 4448 NMES-induced DEGs. These findings display that the acute transcriptomic effects of a NMES-session at 20% MVC largely exhibit a similar gene expression response to that of EX. The observation also suggests that the NMES-intensity used in this study was adequate to examine similarities/dissimilarities between the two models. These results provide, on a molecular level, support to earlier findings that propose NMES as an interventional strategy to alleviate loss in muscle mass and function in a variety of clinical conditions.9, 38-42 However, it should be noted that NMES activates a more limited fraction of muscle fibers than EX, and maybe the biopsy-site might have been relatively more highly activated of NMES than by EX.
The acute effects of a single-bout NMES compared to EX, especially in the hours following the muscle activation, has to the best of our knowledge only been examined in one study. At 24 h following 30 min of quadriceps NMES 384 pre-selected genes were assessed by RT-PCR in patients with chronic obstructive pulmonary disease.20 However, only 18 genes were altered by NMES, whereof 14 genes in common with the 68 genes altered by EX.20 The discrepancy in results compared to our study could depend on several vital differences in the study protocols. We used RNA-sequencing which does not limit the number of genes, and also is more specific and sensitive compared to RT-PCR.43, 44 The timepoint for the post biopsy also differed, 3 vs. 24 h, and substantially fewer genes can be expected to be differentially regulated at the 24-h timepoint.45
Further pathway analysis of the molecular signature of NMES and EX showed them to be largely overlapping (40% of all significant pathways each), suggesting NMES as a potential alternative in patients unable to perform EX. Additionally, the observation that individual, well-established exercise-responsive genes, such as PPARGC1A and ABRA, were significantly upregulated corroborate the molecular similarities of NMES and EX. ABRA was upregulated to the same magnitude with NMES and EX, suggesting similar activation of ABRA's mechano-transductive effects using the two regimes. ABRA link the mechanical process of muscle contraction with the molecular pathways involved in skeletal muscle regulation, that is, cell proliferation, differentiation and growth,46-49 amongst others via its connection to PPARCG1A.46, 50 The observation of a higher upregulation of PPARGC1A with EX versus NMES suggest that the known effects of PPARGC1A on muscle hypertrophy,51-54 oxidative metabolism, and differentiation into slow oxidative type 1 fibers52 may be less pronounced with our NMES-protocol. A previous study on the effect of NMES on PPARGC1A and ABRA expression seem to strengthen our findings by showing that gene expression changes contribute to skeletal muscles adaptations, such as a more oxidative phenotype.55
We further found that growth factors, VEGFA and GDNF, were upregulated in response to both regimens. This strengthens the notion that NMES at 20% MVC can be adequate to stimulate several essential growth factors to a similar degree as EX. GDNF, one of the most potent survival factors for motor neurons56 and known to play a central role in exercise-induced changes in the peripheral nervous system (PNS),57 were found to be equally stimulated by NMES and EX. VEGFA, which stimulates angiogenesis in skeletal muscle58, 59 and is upregulated by a single EX-session,60 has also been shown to be elevated in the context of wound healing after electrical stimulation.61
Another interesting finding was that only eight genes were regulated in opposite directions by the two regimes. The three genes upregulated by NMES and downregulated by EX were involved in neurite outgrowth in skeletal muscle (MYLIP),61 intestinal epithelial cell proliferation and differentiation, and regulation of mTORC1 signaling (ICK),62 and negative regulation of cell proliferation signaling (JARID2).63 Furthermore, CTGF, COL1A1, COL5A1, and TIMP1, which all play a role in connective tissue proliferation and differentiation, build-up and inhibition of break-down, were upregulated only by NMES. Overall, the effects limited to NMES seem to upregulate proteins involved in different cell proliferative actions, such as neurite outgrowth and connective tissue proliferation, which was also strengthened by the findings in the GSEA. This indicates that NMES can induce development of supportive tissues such as bone, cartilage, and tendon, which are substantial parts of the movement apparatus and essential underlying mechanisms in repair after injury.64-66 Hypothetically, NMES could speed recovery after connective tissue injury, but this conclusion necessitates further studies.
The GSEA showed patterns of a more unspecific and broad gene activation with NMES, which is one reflection of the fact that a substantially higher number of genes were upregulated by NMES, but that EX generally resulted in higher normalized pathway enrichment scores. The involuntary NMES activated less pathways related to specific exercise effects, which may be explained by the different recruitment order of muscle units22, 26, 67 and the synchronous activation of muscle fibers during NMES compared to the asynchronous activation of voluntary muscle contractions.68
Intriguingly, the combination of NMES on one leg and EX on the other leg resulted in a higher gene activation on each of the legs as compared to the groups that had unilateral intervention, suggesting synergetic effects. Previous studies have shown that NMES and EX at the same time, in the same leg with the same muscle, results in a greater muscle strengthening effect than EX alone.7, 10, 26, 69, 70 Interestingly, this study demonstrated that the NMES and EX can potentiate each other even when performed in opposite extremities. These observations suggest involvement of either systemic hormonal mechanisms and/or effects mediated through the central nervous system, for example, recruitment and activation of additional motor units. Such mechanisms could be beneficial for patients, for example, after an injury or during partial immobilization, when they are not able to recruit and activate motor units fully during EX.7, 70
Our observation that both NMES and EX resulted in similar DEGs in the control leg, that is, a “cross-over” effect in the untrained limb, suggest similar underlying mechanism that could be explored in immobilized patients using NMES. Our findings are supported by previous studies showing that unilateral training can increase contralateral strength, both during regular exercise71-73 and NMES.74-76 Increased neural drive from the motor cortex of the untrained limb is a key explaining factor here.73, 75, 76
An important clinical aspect of this study was that the NMES-session at 20% of MVC resulted in acceptable level of discomfort,23 with a mean VAS score below 4. Contributing factors to high comfort include optimized electrode placement and appropriate pressure by integration into custom made elastic pants, which made it possible to reduce otherwise painful side effects of NMES.28-30, 32 In addition, the results indicated that the textile electrodes transduce the electricity similar to self-adhesive electrodes, since an approximately linear increase in % of MVC and torque with increased intensity between 5% and 20% of MVC was demonstrated using the NMES-pants, as previously shown with self-adhesive electrodes.24 This suggest that the NMES-pants and our stimulation protocol can be used with high patient compliance and similar effects as commercial electrodes. However, further studies are needed to investigate if the protocol can be used with similar comfort in other patient groups.
A potential limitation of this study was that the knee extension force was only measured with the isokinetic dynamometer, Biodex, during the pre-testing. It is therefore possible that the force produced by the NMES, as well as the EX weight did not correspond to exactly 20% of MVC respectively 80% of 1RM during the intervention session. However, it was logistically unfeasible to use the Biodex during the interventional application, so this study reflects a possible clinical situation with similar restraints. Instead, in order to control for the NMES quality, an inexpensive, hand-held dynamometer, Easyforce, was used both during the pre-testing and intervention session and was shown to correspond well to the Biodex measurements. Additionally, the participants were divided into three intervention groups and it is possible that some of the differences found were due to differences between the participants, rather than EX or NMES. However, there were no significant differences in participant characteristics between the groups, and this study design resulted in several additional research benefits such as the possibility to investigate the “cross-over” effect and the effects of combined NMES and EX.
6 CONCLUSION
In summary, this study demonstrates that the acute transcriptomic effects on skeletal muscles of a 30-min NMES-session at 20% of MVC using the NMES-pants, which can be applied with an acceptable level of comfort, to a predominant degree overlap that of resistance exercise at 80% of 1RM. These observations provide insights into the extent of NMES-triggered activation of exercise-related genes such as PPARGC1A, ABRA, VEGFA, and GDNF. The effects limited to NMES, such as neurite outgrowth and connective tissue proliferation, indicate that NMES may induce healing effects on supportive tissues, but however warrants further studies. These findings can translate into clinical used of wearable NMES that can provide positive, exercise-induced health benefits in individuals not able to perform resistance exercise.
AUTHOR CONTRIBUTIONS
Johanna Flodin: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing – original draft; resources; writing – review and editing. Stefan M. Reitzner: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; resources; writing – original draft; writing – review and editing. Eric B. Emanuelsson: Conceptualization; formal analysis; investigation; resources; writing – review and editing. Carl Johan Sundberg: Methodology; investigation; supervision; writing – review and editing. Paul Ackermann: Writing – review and editing; project administration; supervision; funding acquisition; methodology; conceptualization.
ACKNOWLEDGMENTS
The authors would like to thank the Division of Clinical Physiology at Karolinska Universitetssjukhuset Huddinge for providing the clinical research environment to conduct the intervention and sample collection required for this study. Furthermore, the authors acknowledge support from the National Genomics Infrastructure in Genomics Production Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation and the Swedish Research Council, and SNIC/Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. The authors would also like to thank the Swedish Research Council (2017-00202) and The Swedish Research Council for Sport Science for their financial support as well as the research participants volunteering to be part of this study.
CONFLICT OF INTEREST STATEMENT
Paul W. Ackermann declares a potential conflict of interest. He has a granted patent related to neuromuscular electrical stimulation. UK Patent, publication number GB2601757. A system comprising a controller and an electrical stimulation system. Otherwise, the authors declare no other conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




