The impact of microbiota and ketogenic diet interventions in the management of drug-resistant epilepsy
Laura Diaz-Marugan and Andrina Rutsch have contributed to this equally work.
Abstract
Aim
Drug-resistant epilepsy (DRE) is a neurological disorder characterized by uncontrolled seizures. It affects between 10%–40% of the patients with epilepsy worldwide. Drug-resistant patients have been reported to have a different microbiota composition compared to drug-sensitive patients and healthy controls. Importantly, fecal microbiota transplantations (FMTs), probiotic and dietary interventions have been shown to be able to reduce seizure frequency and improve the quality of life in drug-resistant patients. The classic ketogenic diet (KD) and its modifications may reduce seizures in DRE in some patients, whereas in others they do not. The mechanisms mediating the dietary effects remain elusive, although it is known that gut microbes play an important role in transmitting dietary effects to the host. Indeed, specific commensal microbes differ even between responders and non-responders to KD treatment.
Methods
In this narrative mini-review, we summarize what is known about the gut microbiota changes and ketogenic diets with special focus on patients with DRE.
Results and Conclusions
By highlighting unanswered questions and by suggesting future research directions, we map the route towards future improvement of successful DRE therapy.
1 INTRODUCTION
Epilepsy is one of the most common neurological disorders, affecting more than 50 million people worldwide and 6 million people in Europe.1, 2 Epilepsy groups heterogeneous disorders that affect people of all ages and whose common feature is epileptic seizures.2-5 Uncontrolled seizures can lead to developmental delay, cognitive deficits, memory and learning difficulties, and they can also cause sudden unexpected death (SUDEP).6
Current treatments include anti-seizure medicines (ASM), medical diets, epilepsy surgery and stimulation devices. ASM can control seizures in about two-thirds of people with epilepsy. However, about one-third of patients suffer from drug-resistant epilepsy (DRE), that is, epilepsy that is not controlled by two or more correctly selected and dosed ASM.7-10 The mechanisms underlying drug treatment failure are multifactorial,8 and a re-evaluation of the concept of DRE, including a more comprehensive definition of the different subtypes, is currently underway.11
The causes of epilepsy include structural, genetic, infectious, metabolic and immune factors.12-16 However, the cause of epilepsy remains unknown in a significant proportion of patients. Epileptogenesis may result from a combination of various factors, including lifestyle and environmental causes, such as the use of toxic substances (such as alcohol and tobacco) and certain medications during pregnancy. Meningoencephalitis can also cause inflammation of the brain and increase the risk of epilepsy. The gut-brain axis has also been implicated in this respect.17
Changes in the gut microbiota can affect brain function and lead to inflammation in the gut and brain, which may contribute to epileptogenesis (Figure 1). The gut microbiota produces a variety of compounds that can affect brain function, such as neurotransmitters and short-chain fatty acids (SCFAs).18-20 Some of these compounds have been shown to have anticonvulsant effects in rodents.21-23 However, in patients, the mechanisms by which the microbiota might cause or contribute to epileptogenesis are completely unclear.
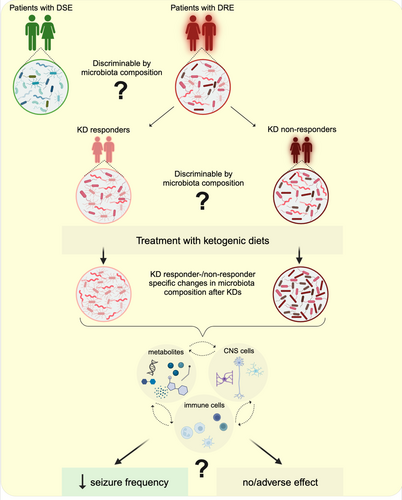
Here, we provide a timely mini-review on the impact of microbiota and diet in patients with DRE. We highlight open questions in the field with the aim of suggesting future directions. Gaining more knowledge in this area is likely to pave the way for new and more effective therapeutic approaches, such as in vivo modulation of the microbiota to ameliorate the burdens in DRE.
2 ROLE OF THE MICROBIOTA IN DRUG-RESISTANT EPILEPSY: MICROBIAL INTERVENTIONS TO TREAT DRUG-RESISTANT EPILEPSY
Evidence for a relevant role of the gut microbiota in human epilepsy comes from few and correlative studies. Descriptive studies with relatively limited sample size have suggested that gut commensals differ between individuals with drug-sensitive (DSE) and drug-resistant (DRE) forms of epilepsy (summarized in Table 1). Some of those studies showed an enrichment of various gut microbes in patients with DRE in comparison with drug-sensitive patients and healthy controls, such as Clostridium XVIII, Dorea, Coprobacillus, Ruminococcus, Akkermansia, Neisseria, and Coprococcus24 or Actinobacteria, Verrucomicrobia, Nitrospirae, Blautia, Bifidobacterium, Subdoligranulum, Dialister and Anaerostipes.25-28
| Cohort | Medication | Microbiota changes | Insights and clinical changes | Missing | Reference |
|---|---|---|---|---|---|
|
Chinese cohort of 91 patients: 42 patients with DRE, 49 patients with DSE. 65 HC (relatives of patients) Age: 5–50 years old Seizure types:
|
Oxarbazepine Levetiracetam Valproic acid Topiramate Carbamazepine Lamotrigine Clonazepam Phenobarbital Phenytoin |
In patients with DRE: Higher microbiota α-diversity ↓ relative abundance of Bacteroidetes ↑ Firmicutes and rare phyla such as Verrumicrobia ↑ abundance of rare bacteria such as Clostridium XVIII, Atopobium, Holdemania, Dorea, Saccharibacteria, Delftia, Coprobacillus, Paraprevotella, Ruminococcus, Gemmiger, Akkermansia, Neisseria, Coprococcus, Fusobacterium, Methanobrevibacter, Phascolarctobacterium and Roseburia, in comparison with HC and DSE patients Patients with <4 seizures/year showed↑ abundance in Bifidobacteria and Lactobacillus compared to patients with >4 seizures/year PICRUSt analysis-based bacterial function prediction, patients with DRE showed: ↑ metabolic pathways associated with ABC (ATP-binding cassette) transporters ↓ glucose- and lipid-associated metabolism all associated with Ruminococcus and other rare bacteria |
Gut microbiota composition of patients with DRE was different from the one of patients with DSE and HC |
No information on the diet |
Peng et al.24 |
|
2 independent western Chinese cohorts: Exploratory cohort: n = 55 patients with epilepsy (DRE and DSE) + n = 46 HC Validation cohort: n = 13 patients + n = 10 HC *HC: healthy spouses living together and sharing a similar diet for ≥10 years Age: 15–60 years old Seizure types:
|
Oxarbazepine Levetiracetam Valproic acid Topiramate Carbamazepine Lamotrigine Phenobarbital |
Patients with epilepsy had: ↓ microbial α-diversity than HC ↑ Actinobacteria and Verrumicrobia, and less abundant Proteobacteria Group with DRE showed: ↑ abundance of Actinobacteria, Verrumicrobia, Nitrospirae, Blautia, Bifidobacterium, Subdoligranulum, Dialister and Anerostipes complete depletion of Cyanobacteria and Parabacteroides in comparison to HC or DSE |
Gut microbiota in patients with epilepsy differs from HC. Gut bacteria in patients with DRE was different from the one of patients with DSE and HC Identification of 30 functional orthologs significantly different between patients and HCs by PICRUSt and KEGG pathways |
27 nutrient factors were taken into con-sideration, but no further details on the dietary regimens are available |
Gong et al.25 |
|
Korean study: 8 patients with DRE compared to HC (n = 32) Age: 1–7 years old Seizure types:
|
Oxarbazepine Levetiracetam Valproic acid Topiramate Lamotrigine Vigabatrin Clobazam Phenobarbital |
Patients with DRE had: ↓ α-diversity ↓ proportion of Bacteroidetes and Proteobacteria ↑ proportion of Actinobacteria spp and Verrucomicrobia ↑ proportion of Actinomycetaceae, Bifidobacteriaceae, Coriobacteriaceae, Enterococcaceae than in HC Enterococcus faecium, Bifidobacterium longum and Eggerthella lenta were biomarkers of patients with DRE Bacteroides vulgatus, Faecalibacterium prausnitzii were biomarkers for HC ABC transporters, quorum-sensing and starch and sucrose metabolism were pathways associated with DRE |
Patients with DRE show different microbial profile in comparison to HC |
Small cohort No detailed information of the diet composition |
Lee, K. et al.26 Journal of Clinical Medicine |
|
Probiotic treatment for 4 months 2× per day (2 × 1011/time) in a cohort of 45 Spanish patients with DRE Age: >18 years old Probiotics: Lactobacillus acidophilus DSM32241, Lactobacillus plantarum DSM32244, Lactobacillus casei DSM32243, Lactobacillus helveticus DSM32242, Lactobacillus brevis DSM11988, Bifidobacterium lactis DSM32246, B. lactis DSM32247, and Streptococcus salivarius subsp. thermophilus DSM32245 Seizure types:
|
Valproic acid Carbamazepine |
No analysis of gut microbiota | Probiotic treatment reduced more than 50% seizures frequency in ~29% of the patients and improved quality of life. GABA levels increased during probiotic treatment and decreased after treatment finished |
Analysis of microbiota missing before and after the treatment No placebo control |
Gómez-Eguílaz et al.29 |
|
6 patients with DRE suffering from variable infectious diseases (pneumonia, pertussis, otitis media), therefore treated with different types of antibiotics Age: 10–16 years old Epilepsy classification:
|
Levetiracetam Valproic acid Topiramate Lamotrigine Zonisamide Nitrazepam Clobazam |
No analysis of gut microbiota |
5 out of 6 patients achieved seizure freedom during the treatment with antibiotics, 1 patient showed a significant decrease in seizure frequency Positive effects on seizure frequency vanished within 2 weeks after cessation of antibiotic treatment |
Small cohort Duration of antibiotic treatment not stated. Analysis of microbiota missing |
Braakman and van Ingen30 |
|
23 drug-responsive and 21 patients with DRE. No statistically significant differences in age or gender comparing the groups Age: >19 years old |
NA |
Fecal microbiota analysis No significant differences in α- and β-diversity between groups ↑ relative abundance of Bacteroides finegoldii and Ruminococcus_g2 in DSE group ↑ relative abundance of Negativicutes (Firmicutes) in DRE group |
Gut microbiota composition of patients with DRE is different from patients with DSE |
No healthy controls Information about medication and seizure types is missing |
Lee, H. et al.27 Epilepsy Research |
|
20 children with epilepsy, 11 of them using a single ASM and 9 children had DRE. 7 HC in the same age group Age: 2–11 years old Seizure type:
Epilepsy classification:
|
NA |
No differences in microbial diversity between groups Ratio Bacteroidetes/Proteobacteria ↑ in the epilepsy group Presence of Megamonas exclusively in the epilepsy group Presence of Flavihumibacter, Niabella, Anoxybacillus, Brevundimonas, Devosia, and Delftia only in the control group ↑ Coriobacteriia ↑ Coriobacteriales in epilepsy group Pirellulares, Caulobacteriales only in the control group ↑ Flavobacteriaceae ↑ Hyphomicrobiaceae ↑ Comamonadaceae in the control group ↑ Coriobacteriaceae in the epilepsy group Pirellulaceae, Caulobacteraceae and Rhizobiaceae only in the control group |
There 33 distinctive taxa between epilepsy and healthy groups | Information about medication is missing | Türay, S. et al.28 Pediatric Neurology |
- Abbreviations: ASM, anti-seizure medication; DRE, drug-resistant epilepsy; DSE, drug-sensitive epilepsy; HC, healthy controls; NA, not applicable.
In fact, various microbe-based or microbiota-altering interventions have shown promising efficacy in reducing seizure frequency in patients with DRE. Fecal microbiota transplantation (FMT), initially used to treat symptoms of Crohn's disease (CD), had a positive effect on the frequency of seizures in a young woman who had also suffered from epilepsy for 17 years. Prior to the procedure, the patient underwent ASM treatment to maintain seizure control.31 Interestingly, FMT not only alleviated the symptoms associated with Crohn's disease, but also the patient remained seizure free for over two years without ASM. This suggests that the improvement in epilepsy symptoms may have been due to the effect of FMT on the patient's gut microbiota, which in turn may have altered the interaction of the microbe with central nervous system function, suggesting the potential of FMT as a treatment for epilepsy and also in reducing systemic inflammation.31 In fact, clinical trials aimed at understanding the efficacy and safety of FMT in the treatment of epilepsy began several years ago, but data are not yet available (ClinicalTrials.gov ID NCT02889627).
Other studies have confirmed that probiotics or microbiota-modifying treatments can decrease seizure frequency. In a pilot study, the use of a mixture of probiotics (eight bacterial subspecies of Lactobacillus, Bacteroides and Streptococcus) reduced seizure frequency and improved the quality of life in patients with DRE.29 Unfortunately, in this study the gut microbiota of the treated individuals was not analyzed.
Further evidence showing that the microbiota may play a role in seizures comes also from a study of six patients with DRE which were treated with antibiotics.30 While on antibiotics, five patients were seizure-free and the sixth reported a significant reduction in the seizure frequency. Here, the antibiotics were used to treat different infections, such as pneumonia otitis or pertussis and some have known inhibitory activity on cytochrome p450, enzymes which increases serum concentrations of some ASMs.30, 32 The patients were exposed to different ASM treatments, and one of them was also treated with a ketogenic diet. The positive effect of the antibiotics on seizure frequency disappeared in all the patients within 2 weeks after stopping the antibiotic treatment.30
In conclusion, human studies of microbiota modulation by FMT, probiotics or antibiotics, although few and with small numbers of patients, show improvement in terms of reduction of seizure frequency, suggesting the microbiota as a possible target for therapeutic strategies in the management of DRE.
3 KETOGENIC DIETS IN THE MANAGEMENT OF DRE
Alternative treatments for DRE include non-pharmacological approaches (e.g., vagal nerve stimulation) and dietary treatments such as ketogenic diets (KDs),33-35 which have been shown to improve conditions in not only metabolic diseases but also several neurological disorders. Restricting carbohydrate intake forces the metabolism to replace the preferred energy source, glucose, with fatty acids, which are oxidized in the liver to form ketone bodies. The latter can be used by cells that cannot metabolize fatty acids, such as the brain,36, 37 to generate energy.
Since ancient times, prolonged starvation has been widely used to treat patients with epilepsy.38 In 1921, Dr. Wilder introduced the hypothesis that fasting induces a beneficial effect related to ketonemia in patients with epilepsy and proposed that KD would have had similar effects, due to its property of inducing a metabolic state of ketosis in the host, but without the malnutrition effects induced by starvation.39 KD is a form of dietary intervention based on a high-fat intake accompanied by moderate protein, and very low or no carbohydrate. The classical ketogenic diet has been extendedly used in DRE management. Modifications of the composition in terms of fats, carbohydrates and proteins have led to less restrictive forms of the diet, such as the modified Atkins diet (MADKD), the low-glycemic index diet (LGID), and the middle-chain triglyceride ketogenic diet (MCTKD), all of them bonded with the term of (non-classical) ketogenic diets (KDs) (Table 3).
In this chapter, we will focus on what is currently known about the effect of KDs on the microbiota in DRE (Table 2). While there are now a considerable number of studies investigating the effects of classical KD on gut microbiota changes and seizure frequency, to the best of our knowledge, we have not found studies demonstrating the effects of modified ketogenic diets on the gut microbiota in patients with DRE. However, in line with the aims of our review to highlight the impact of KD on the management of DRE and to emphasize the need for personalized dietary plans according to patients' needs (e.g., food intolerances, cultural factors or food preferences), we consider it valuable to share information on the success of ketogenic therapies other than the classic ketogenic diet, even in the absence of results related to gut microbiota composition. By doing so, we also want to emphasize the need for and importance of conducting microbial studies in DRE patients following different forms of ketogenic diets. Alternatives to the classical KD, which offer the same neurological benefits, could improve dietary compliance and facilitate comparative studies on variations in gut microbiota composition.
| Cohort | Medication | Microbiota changes | Insights and clinical changes | Missing | Reference |
|---|---|---|---|---|---|
|
Chinese cohort of 20 patients with DRE treated with KD for 6 months Age: 1–10 years old Seizure types:
Epilepsy classification:
|
Oxcarbazepine Levetiracetam Valproic acid Topiramate Lamotrigine Clonazepam |
Compared to baseline, after 6 months of KD, patients with DRE showed: ↓ fecal microbial α-diversity ↓ abundance of Firmicutes, Actinobacteria ↑ abundance of Bacteroidetes KD non-responder group showed: ↑ levels of Clostridiales, Clostridia, Ruminococcaceae, Rikenellaceae, Lachnospiraceae and Alistipes |
After 6 months of KD: 10% of the patients were seizure-free 15% had ≥90% seizure reduction 25% had 50–89% seizure reduction 50% had <50% reduction KD-induced changes in gut microbiota in patients with DRE. Gut microbiota of KD non-responders was different from KD responders |
Small group of patients Control group without KD is missing |
Zhang et al.40 |
|
11 Swedish patients with DRE and one patient with pyruvate dehydrogenase deficiency treated with KD for 3 months Healthy controls (parents, n = 11) without KD Age: 2–15 years old Seizure types:
|
Oxcarbazepine Valproic acid Topiramate Carbamazepine Lamotrigine Lacosamide Vigabatrin Clobazam |
Compared to baseline, after 3 months of KD, patients with DRE showed: ↓ relative abundance of Actinobacteria (Bifidobacterium), Eubacterium rectale and Dialister ↑ relative abundance of Proteobacteria (E.coli) |
After 3 months of KD: ~41% of the patients showed >50% of seizure reduction 25% did not show seizure frequency reduction but shorter time seizures ~83% of the patients showed general improvement in cognitive and motor function ~17% of the patients were non-responders |
Small cohort HC (parents) consumed western diet and did not match in age |
Lindefeldt et al.41 |
|
14 Chinese patients with DRE treated with KD for a week Aged-matched healthy controls (n = 30) Age: 1–4 years old |
NA |
Compared to HC, patients with DRE showed: ↓ α-diversity ↑ Firmicutes, Proteobacteria ↑ Cronobacter ↓ Actinobacteria, Bacteroides ↓ Prevotella, Bifidobacterium Compared to baseline, after 1 week of KD, DRE patients showed: ↓ Proteobacteria, Cronobacter, Erysipelatoclostridium, Streptococcus, Alistipes, Ruminiclostridium, Barnesiella, Enterococcus ↑ Bacteroides, Prevotella and Bifidobacterium |
64% of the patients showed 50% decrease in seizure frequency. Of those, 3 patients were seizure-free |
Small cohort Controls did not undergo KD |
Xie, G. et al.47 World Journal of Gastroenterology |
- Abbreviations: DRE, drug-resistant epilepsy; HC, healthy controls; KD, ketogenic diet; NA, not applicable.
3.1 Classical ketogenic diet (KD)
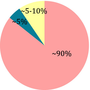
The classical KD is characterized by a 3:1 or 4:1 ratio of caloric intake from lipids (long-chain triglycerides) to both protein and carbohydrate together and with a normal overall energy intake.
Although the classical KD has been used in epilepsy for many years and its efficacy in reducing seizures has been confirmed in several studies, especially when introduced in young age,35 the mechanism behind the effects is not well understood in humans. In particular, it is not known why this dietary intervention has an anti-seizure effect in a proportion of the patients, while in others there is poor or no effect35 or even worsening of seizure frequency or appearance of adverse effects.42-44 Perna and colleagues45 showed that out of 24 patients with DRE, almost 60% benefited from the KD intervention, with a >50% reduction in seizure frequency in the first 3 months of treatment. 33% of the children were seizure-free 12 months after starting the dietary intervention. However, 41.7% of the patients were non-responders (<50% seizure reduction) and 58.3% discontinued the dietary intervention after 6 months for various reasons. Interestingly, the duration of KD was the factor that most positively correlated with treatment efficacy, highlighting the importance of compliance and adherence to the diet, but also the importance of positive responses for patients to stick to the intervention. Correlations with intestinal microbiota of the patients unfortunately were not taken into consideration in this retrospective study.45 In a different clinical trial in a small cohort of 12 patients aged between two and 17 years with DRE, KD was administered for 3 months. In terms of the efficacy of KD treatment on seizure frequency, five children were responders; instead, the responders in terms of behavior (cognition and motor function) were 10, at 3 months post-treatment. Three patients had shorter seizure duration and less post-ictal fatigue, but no change in seizure frequency. These findings highlight the potential of KD to improve not only seizure control, but also the overall condition of epilepsy.41 In addition, fecal samples were collected from the children before and after 3 months of KD treatment. The gut microbiota of the treated individuals contained a significantly reduced relative abundance of bacteria usually considered health-promoting and fiber-consuming such as Bifidobacteria, E. rectale and Dialister, 3 months post-treatment compared to pre-treatment, which was not the case in the parental control group. Instead, the relative abundance of E. coli increased with KD.41 In a study involving 20 children with DRE, 50% of them had a >50% reduction in seizures upon KD introduction, with half of this group even having >90%–100% reduction.40 Conversely, the other half of the patients were non-responders (<50% reduction in frequency) after 6 months of KD intervention. Through the analysis of the composition of the microbiota before and after 6 months of KD intervention, differences in KD responders and non-responders were found; compared to the KD responders, the KD non-responders showed increased levels of Clostridiales, Ruminococcaceae, Rikenellaceae, Lachnospiraceae and Alistipes.40 This suggests that differences in the microbiota composition may be predictive of whether a patient will respond to KD therapy and would therefore allow assigning DRE patients to KD responders and KD non-responders by microbiota analysis. Even though KD is an effective non-pharmacological therapy in the management of patients with DRE, adherence rates are in fact quite low, and many patients struggle to accept this strict dietary regimen. Therefore, adaptations of KD (with modified prescription rules, calories, fat/carbohydrate ratio, quality and quantity of macronutrients content, methods of diet implementation and maintenance) have been developed to make them less strict and more applicable, to increase patients' compliance with the diet, and possibly to achieve better and more widespread effects in different patient populations.46
In conclusion, KD is an important intervention in the management of seizures in patients with DRE, significantly reducing seizure frequency. However, a successful treatment is not guaranteed in all patients.40, 47 Failure is often related to adherence to the strict diet requiring strong commitment and perseverance.
3.2 Modifications of the classical KD
As discussed, classical KD has shown good results in the management of DRE, although adherence to the diet has also been reported as one of the main challenges due to its strongly restrictive carbohydrate allowance. More flexible diets have been established as alternative therapeutic approaches to classical KD for patients with DRE, such as the modified Atkins diet, the low-glycemic index diet and the medium-chain triglyceride ketogenic diet (Table 3).
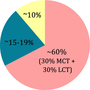
| Classical KD | MAD | LGID | MCTD | |
|---|---|---|---|---|
| Composition |
|
|
|
|
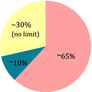
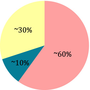
| Ratio fat: protein + CH | 4:1 | 1:1 | 1:0.6 | 2:1 |
| Restrictions | High. Food must be carefully weighted |
Moderate. No calories restriction or fluids. No need to weigh the food CH: 10-15 g/day, up to 30 g/day when seizures are improving |
Moderate. No need to weigh the food CH: 40-60 g/day, and only CH with glycemic index <50 |
Moderate. Admits more variety of food. No need to weigh the food |
| References | Kossoff et al.48; Sharma and Jain49; Verrotti et al.50; Yoon et al.51 | |||
-
Note:
 fat;
fat;  carbohydrate and
carbohydrate and  protein.
protein.
- Abbreviations: CH, carbohydrates; KD, ketogenic diet; LCT, long-chain triglycerides.LGID, low-glycemic index diet; MAD, modified Atkins diet; MCT, middle-chain triglycerides; MCTD, medium-chain triglyceride diet.
3.2.1 Modified Atkins diet
The modified Atkins diet (MAD) is a variation of the Atkins diet that is used to treat epilepsy. The classic Atkins diet is a high-fat, low-carbohydrate diet developed by Dr. Robert Atkins in the 1970s, initially intended for weight loss. Due to the restricted amount of carbohydrates (20 grams per day) the Atkins diet was reported to induce ketosis and therefore the production of ketone bodies,52 which has encouraged its use in DRE. Indeed, three out of six patients aged between seven and 52 years old, showed a >50% reduction in their seizure frequency following the introduction of the Atkins diet. Several studies then moved on to the MAD, which limits carbohydrate consumption to 10 g per day for children and 15 g for adults, and unlike the classical KD, fats account for around 65% of calories and there is no restriction on protein (which accounts for around 30% of calories).49, 53, 54 In contrast to the classical KD, where most target patients are children, MAD has been tested in adult cohorts with promising results. In one clinical trial, almost half of the patients had at least 50% reduction in seizures after one and 3 months on MAD, with improvement starting as early as the first 2 weeks of treatment, and after three and 6 months, two patients were seizure-free.54 In another study, 66% of patients had >30% reduction in seizure frequency after 6 months of MAD,55 and in an observational study, 36% of patients with DRE had a ≥50% reduction in seizure frequency and 16% were seizure-free after 3 months of MAD. Approximately one quarter of patients had <50% seizure reduction, no improvement or worsening seizures after 3 months of treatment.56
A recent clinical trial involving 160 patients between the ages of 10 and 55 showed that MAD combined with drug treatment was more effective in reducing seizure frequency than ASM therapy alone. Around 26% of the patients receiving MAD and ASM together showed a 50% reduction in seizure frequency and improved quality of life within 6 months after starting the diet.57 Similarly, a randomized controlled trial showed that the number of children with a >50% reduction in seizure frequency after the introduction of MAD was higher than the number of children in the group receiving ASM alone after 12 weeks of treatment.58
Several studies have highlighted the benefits of the MAD in patients with DRE, showing an overall improvement of >50% of seizure frequency in approximately 50% of the patients.48, 53-55, 59, 60 Interestingly, there were no significant differences between MAD and KD when comparing children with 90% seizure frequency reduction after 6 months of diet, but KD was more effective in cases with >50% seizure reduction.61
In general, these results suggest that MAD is a good alternative to KD, as patients appear to be more compliant with this diet compared to KD, with no adverse effects in both the short and long term.
3.2.2 Low-glycemic index diet
The low-glycemic index diet is used as a more flexible alternative to the classical KD, as patients are allowed to consume a higher amount of carbohydrates (approximately 40–60 g) daily,51 which are monitored and should have a low-glycemic index (<50 glycemic index). Calories from fat should account for about 60% of the total intake.51
A study in children aged between 1.5 and 17 years showed that more than 70% of patients (of a total of 42) had a ≥50% reduction in seizures after just 2 weeks on a low-glycemic index diet.62 Kim and colleagues63 showed that more than half of the children with DRE (36 patients in total) on low-glycemic index diet had ≥50% reduction in seizure frequency after 3 months of treatment, and 6% were seizure-free for 1 year, with very low incidence of adverse events. Similarly, a retrospective study in Italy64 showed a ≥50% reduction in half of the patients (n = 15) in a cohort of children and young adults (11.3–22 years old). This diet has also been used in other conditions, such as the Angelman syndrome, which is characterized by drug-resistant seizures. Here, patients on this diet showed a reduction in seizures or were even seizure-free, with very mild to low side effects.65
Although this diet is more palatable than classical KD and offers good clinical outcomes for patients with DRE, studies of low-glycemic index diets have mainly been conducted in children or young adults, so the effect of the diet in the adult population is not well established.
3.2.3 Medium-chain triglyceride ketogenic diet
The fats present in the classical KD are mainly of long-chain triglycerides (LCT). Alternatives are medium-chain triglycerides (MCT), which can be used to produce ketones more easily than long-chain triglycerides, thus reducing the total fat required and allowing more carbohydrate and protein to be included in the diet.50, 66 The medium-chain triglyceride ketogenic diet was first described in the 1970s and provided up to 60% of the calories in the form of MCTs.67 Due to tolerability problems,68, 69 the diet was modified to include 30% of calories from the MCT and 30% from LCT.70
The effects of the MCT ketogenic diet have been studied in patients with DRE. Indeed, after 3 months on the new diet, children showed a significant reduction in seizure frequency compared with the baseline. More than 60% of the patients (n = 16) showed a 50% reduction in seizure frequency and almost 30% were seizure-free.71 In addition, supplementation with the MCT oil in six adult patients with DRE showed a reduction in the seizures rate72 and a comparison between MCT and KD in 94 children with DRE showed that MCT was as effective as KD in reducing the number of seizures.66 Therefore, good management of the MCT diet could provide a good substitute for those patients who cannot maintain KD or other dietary alternatives.73
Taken together, this evidence suggests that modifications of the classical KD could provide similar success rates in reducing seizure frequency74 and this opens the possibility of using less restrictive diets to treat DRE patients. However, the reality is not as simple and unanswered questions remain. In a randomized controlled study, in pediatric patients75 the MAD and the low-glycemic diet were not as effective as classical KD, whereas LGD offered fewer adverse effects than MAD and KD with a good balance of seizure reduction.
4 CONCLUSIONS AND FUTURE PERSPECTIVES
As we discussed in the previous chapters, there is increasing evidence that the gut microbiota is an important player in drug-resistant epilepsy and an essential mediator of dietary effects on the host organism (summarized in Tables 1 and 2). Indeed, diet is one of the major forces shaping the microbiota in terms of composition and metabolism, contributing to host homeostasis and disease susceptibility. Nutrition is currently a complementary and alternative approach in the treatment of neurological disorders, including epilepsy, with beneficial effects particularly in drug-resistant patients. In this review, we aim to highlight the importance of the diet and its interactions with the host microbiota as a new and promising approach to the management of DRE. We have pointed out here that some DRE patients treated with KD respond efficiently to dietary intervention, with >50% reduction in seizures, whereas another proportion of patients do not respond, with <50% reduction in seizures frequency even after several months of KD treatment. The reasons for this variable response to KD among patients remain completely unclear. One of the major challenges in clinical studies is translating findings from small cohorts into effective approaches for more patients with DRE (and other neurological disorders). Because of the heterogeneity of DRE and the interpersonal differences in the composition of the patients' microbiome, and thus possible differences in dietary interaction effects, larger cohort studies are needed. Positive effects after dietary intervention depend on the metabolic and microbiome profile of the individual, highlighting the importance of personalized dietary treatments.76
In line with this, detailed analyses of the impact of potential confounding factors (e.g., sex, age, ethnicity, ASM use, environmental and lifestyle factors, including diet) on the microbiota composition/function in DRE patients and its impact on the modulation of seizure frequency upon KD introduction are still almost completely lacking.77 Therefore, analyzing the microbiota composition and function, such as the metabolic profile, of each patient before and after dietary intervention will pave the way to more personalized treatments and even predict the success of the intervention, similar to what has been shown in other diseases.78
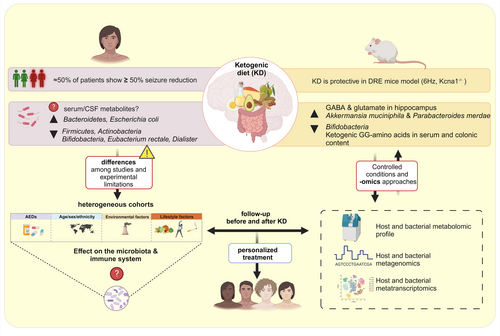
The classical ketogenic diet is perceived as a restrictive diet, and its consumption could also cause some adverse effects, such as nausea, vomiting, diarrhea, constipation and weight loss, which could contribute to lower acceptance or adherence to the diet. Modifications of classical KD, termed as ketogenic diets, have been developed to reduce the side effects and improve acceptance and compliance to the diet. However, there is a paucity of data on the effect of ketogenic diets on the composition of the patient's microbiota. It is also unclear whether ketogenic diets would improve seizure frequency and quality of life in those patients who do not respond to classical KD, as there is very little data on this topic. To address these unanswered questions, it is necessary to understand the effect of the diet on the host microbiota and on the host central nervous system. Analysis of the gut microbial composition together with bacterial metabolic functions would help to identify molecular pathways or metabolites that could be used in the clinic. It has been suggested that the gut microbiota can influence the phenotype and function of mucosal and systemic immune cell responses and central nervous system function through the dissemination of bacterial products or metabolites, and this is strongly dependent on the substrates available through dietary intake.79 Identification of the specific bacterial products and metabolites in each dietary state in different patient populations is currently needed to deepen our understanding of the pathogenesis of diseases such as DRE and to develop new and more effective therapeutic strategies. Interestingly, only bacteria such as Akkermansia muciniphila and Parabacteroides merdae mediated seizure protection in germ-free (GF) KD-fed or antibiotic-treated specific-pathogen-free (SPF) KD- or control diet-fed mice.23 The authors suggested that KD may be responsible for changes in the expression of neuroactive molecules, such as α-glutamyl-amino acids, α-aminobutyric acid (GABA) and glutamate, which may contribute to seizure protection. Despite these promising results, information on similar pathways in humans is lacking. Addressing this question through metagenomics and metatranscriptomics of the microbiota before and after KD treatment, together with metabolomics on host samples (such as serum), would provide comparative results across human populations, and ultimately lead the way to new targets for treating DRE and other neurological diseases through personalized dietary or pre- and probiotic interventions (Figure 2).
ACKNOWLEDGMENTS
FR is supported by the following funding systems: Helmut Horten Foundation Grant, Biostime Institute Nutrition & Care (BINC)-Geneva grant, FISM-Fondazione Italiana Sclerosi Multipla-Cod.:2020/R-Single/029 and financed or co-financed with the ‘5 per mille’ public funding, JPND Research AD_imprint. LDM is supported by a Margarita Salas call from the Spanish Ministry of Universities (University of Barcelona). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no commercial or financial conflict of interest.




