Moderate variations in the human diet impact the gut microbiota in humanized mice
Abstract
Aim
Drastic diet interventions have been shown to promote rapid and significant compositional changes of the gut microbiota, but the impact of moderate diet variations is less clear. Here, we aimed to clarify the impact of moderate diet variations that remain within the spectrum of the habitual human diet on gut microbiota composition.
Methods
We performed a pilot diet intervention where five healthy volunteers consumed a vegetarian ready-made meal for three days to standardize dietary intake before switching to a meat-based ready-made western-style meal and high sugar drink for two days. We performed 16S rRNA sequencing from daily fecal sampling to assess gut microbiota changes caused by the intervention diet. Furthermore, we used the volunteers' fecal samples to colonize germ-free mice that were fed the same sterilized diets to study the effect of a moderate diet intervention on the gut microbiota in a setting of reduced interindividual variation.
Results
In the human intervention, we found that fecal microbiota composition varied between and within individuals regardless of diet. However, when we fed the same diets to mice colonized with the study participants' feces, we observed significant, often donor-specific, changes in the mouse microbiota following this moderate diet intervention.
Conclusion
Moderate variations in the habitual human diet have the potential to alter the gut microbiota. Feeding humanized mice human diets may facilitate our understanding of individual human gut microbiota responses to moderate dietary changes and help improve individualized interventions.
1 INTRODUCTION
During the past two decades, our understanding of gut microbiota has transcended from predominantly passive commensals to a dynamic and metabolically active microbial community that can have profound influence on virtually every facet of human physiology.1 A large body of evidence now links the gut microbiota to cardiometabolic diseases such as obesity, type 2 diabetes, and ischemic heart disease.2, 3 Given the escalating socio-economic burden imposed by cardiometabolic diseases,4 there has been a surge of interest in unraveling the involvement of the gut microbiota. In this context, recognizing the significance of diet has emerged as a key aspect. Diet, a well-established determinant of cardiometabolic health,5 is also a prominent modulator of gut microbiota composition (Figure 1).6-8 This understanding is supported by interventional studies that implemented dramatic variations in macronutrient composition, demonstrating swift and discernable effects on the gut microbiota in humans.9-11 Similarly, comparisons between populations with large differences in dietary patterns have revealed substantial discrepancies in their respective gut microbiota profiles.12, 13
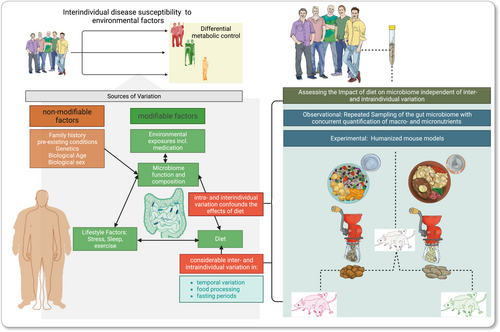
In contrast, dietary interventions frequently elicit more pronounced alterations in the microbiota in mice compared to those observed in humans.6, 14, 15 This may, in part, be due to more extreme interventions employed in mice, as well as their low interindividual variability (e.g., inbred, housed in the same consistent environment, compared at the same age and sex). Importantly, mice usually consume a consistent diet throughout a given intervention, while the human diet varies considerably over time, even throughout the day. Accordingly, cross-sectional studies conducted in populations from industrialized nations have shown less conclusive results,16-21 suggesting that long-term dietary patterns are linked with stable gut microbiome configurations over time.16 However, findings from a longitudinal study incorporating daily sampling of the microbiota suggest that the relatively limited resolution of information on the complexity and variability of the human diet as well as low frequency of fecal sampling may have hindered unraveling the true magnitude of diet-induced effects on the human gut microbiota.22 Taken together, these observations imply the necessity for controlled dietary intervention necessary to model potential individual responses and help design personalized gut-microbiota-targeted interventions.
The significance of the interplay between diet, gut microbiota, and cardiometabolic health is exemplified by the Mediterranean diet, which have evidenced efficacy in primary and secondary prevention of cardiovascular disease in large randomized, controlled trials.23, 24 This diet has been associated with profound modifications in gut microbiota composition, further underscoring its relevance in shaping cardiometabolic health.18, 25, 26 Moreover, the effectiveness of the Mediterranean diet has been demonstrated to depend on the individual's baseline microbiota composition,27 highlighting the importance of personalized microbiota-targeted dietary approaches. Additional evidence supporting the power of individualized dietary interventions comes from a study revealing that incorporating knowledge of an individual's microbial composition can enhance personalized dietary recommendations aimed at managing blood glucose levels.28 However, in addition to interindividual variation, the human gut microbiota also exhibits significant intraindividual variation, even when eliminating all variation in dietary intake (Figure 1),22, 29 adding another layer of complexity to the pursuit of gut microbiota-targeted dietary recommendations. Additionally, future microbiota-targeted diet strategies should ideally align with the spectrum of the habitual human diet to ensure long-term compliance. This seems to be even more relevant, considering that most evidence supporting the impact of diet on the gut microbiota is based on relatively extreme diet interventions and the impact of more moderate diet variations on the human gut microbiota remain less well understood.
To both address the impact of moderate diet variations on host physiology, while circumventing the large inter- and intraindividual variation of the human gut microbiome, humanized mouse models can be used (Figure 1). These models involve transplanting human fecal microbiota into germ-free mice and subjecting them to specific dietary interventions and have revealed that diet significantly influences the composition and function of the gut microbiota.30 Different dietary regimens lead to distinct shifts in microbial taxa, changes in diversity, and alterations in functional pathways within the gut microbiota, that are specific to the intervention given.10, 31 Moreover, this experimental model has provided understanding of how diet-induced changes in the gut microbiota can affect host metabolism, immune responses, and susceptibility to diseases.32, 33 These findings demonstrate the potential of humanized mouse models to understand how diet and dietary patterns influence the gut microbiota and its relevance to human health.
Hypothesizing that a reduction of inter- and intraindividual dietary variation is necessary to observe the effect, if any, of more moderate dietary variations in humans, we performed a pilot diet intervention in humans with standardized homogenous diet over the course of 5 days to study the impact of moderate variations in the habitual human diet on the gut microbiota. Herein, we standardized diet intake to the exclusive consumption of the same vegetarian ready-made meal with a balanced macronutrient composition for three days followed by exclusive consumption of a meat-based ready-made meal and a high-sugar drink representative of a modern western-style diet for two days. To further reduce interindividual variation and in order to assess individual and cohort-wide effects more closely, we repeated this diet intervention using the same diets in mice colonized with the study participants' gut microbiota. We performed 16S rRNA sequencing to analyze the impact of the dietary change on the gut microbiota composition in both humans and humanized mice.
2 RESULTS
2.1 Impact of a moderate variation in the human diet on the gut microbiota in humans
To gain insights into how much a moderate variation in the habitual dietary habits can affect the gut microbiota in humans, we performed a controlled diet intervention on five healthy male volunteers. To standardize dietary intake, all participants exclusively ate the same vegetarian ready-made meal for three days (“standardization diet”, SD). All participants then exclusively ate a meat-based ready-made meal consumed together with a high-sugar soft drink for two days (“intervention diet,” ID) (Figure 2A). The ID was designed to mimic a western-style diet composition with higher amounts of total and saturated fat and simple sugars and thus represents a moderate diet variation, and an “unhealthier” diet, compared to the SD (Table S1). Fecal samples were collected from the individuals before the diet intervention (baseline) and daily (days 1 to 5) when on the diets (Figure 2A).
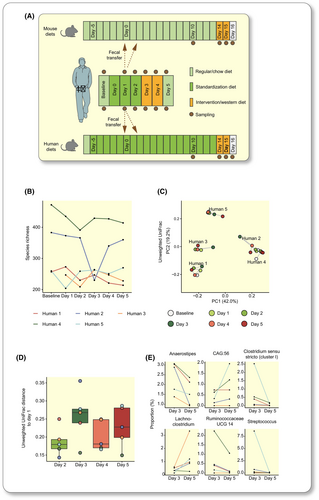
We performed 16S rRNA sequencing to assess the fecal microbiota composition and observed that the microbiota differed between the individuals before and during the diet intervention. Two individuals had higher alpha diversity than the others (Figures 2B and S1A) and clustered separately in ordination analysis based on unweighted UniFrac (Figure 2C). Most of the variation between samples was explained by the individual themself (PERMANOVA, unweighted UniFrac 79.5%, p < 0.0001; weighted UniFrac 64.4%, p < 0.0001), while diet and sampling days did not contribute significantly to the variation. Alpha diversity varied over time for each individual but to different degrees (Figures 2B and S1A), and we did not observe an overall change in alpha diversity in response to the switch to the ID (paired Wilcoxon rank sum test day 3 vs 5; p = 0.81 and p = 0.19 for species richness and Shannon index, respectively). Similarly, we did not observe a significant change in beta diversity between any of the days 2 to 5 and day 1 (paired Wilcoxon rank sum test, Figures 2D and S1C). We observed variations in higher taxonomies within individuals over time but no significant overall differences in response to the ID (Figure S1D–F). On genus level, paired DESeq2 analysis of day 3 vs day 5 identified six genera whose proportion changed significantly following the switch from SD to ID (Figure 2E).
Thus, our results are consistent with earlier studies showing that the gut microbiota varies to a large extent between individuals19 and also varies within an individual between days.22, 34 In addition, our results show that these inter- and intraindividual variations (1) cannot be overcome by short-term elimination of dietary variation and (2) make it difficult to observe any potential effect of variations in the human diet on the microbiota in a small number of humans, which motivated us to further investigate the effect of moderate diet variations in humanized mice.
2.2 Transfer of gut microbiota from humans to germ-free mice fed a human diet or chow
Our results suggest that individuality of the human microbiota precluded detection of microbiota composition changes in response to a short-term moderate diet intervention. Because there is less interindividual variation between mice than between humans and we can colonize mice with the same gut microbiota, we hypothesized that effects of moderate variations in the human diet on an individual human's gut microbiota may be observed in humanized mice. These mice provide a model where environmental influences are more controlled and where long-term exposure to diverse diets or individual habits with putative long-lasting effect on physiology are absent. We therefore used feces from the study participants on the SD to colonize germ-free mice and performed a similar diet intervention in these humanized mice. Mice colonized with the same fecal sample represent technical replicates35 and we therefore colonized groups of germ-free mice with fecal samples from each individual to assess both personalized and more generalizable effects on the microbiota.
As a first step, we determined whether transfer of human fecal microbiota to mice was improved if the recipient mice were fed the same diet as the human donors rather than chow. Germ-free mice were fed a homogenized and irradiated SD or continued on a standard rodent chow diet (Figure 2A). After five days, we colonized these mice (by oral gavage on day 0 and day 2) with fecal microbiota collected from each individual after one day on SD (n = 7–9 mice on SD and n = 3–5 mice on chow per individual) (Figure 2A).
Fecal samples were collected from mice on days 10 (see next section) and 14 (Figure 2A) and analyzed by 16S rRNA sequencing. Samples from day 14 were used to determine transfer success as the gut microbiota has been shown to stabilize by that time.36 Alpha diversity was lower in the inocula than in the donor fecal samples, and mice on either diet colonized with a highly diverse microbiota showed higher alpha diversity than mice colonized with a less diverse microbiota (Figures 3A and S2A). Samples from recipient mice grouped together with samples from their donors in unweighted UniFrac analyses (Figure 3B) but not in weighted UniFrac analyses (Figure S2B), indicating that transfer of taxa was generally successful, but proportion varied between the human donor and the recipient mice. In agreement with this observation, overlap of sequencing reads between the human fecal sample, inoculum, and mice fecal samples was generally high (Table S2) but correlation of proportions was more varied (Table S3). Of note, correlation of proportion tended to be higher in mice fed human diets (Table S3). We calculated the phylogenetic distance between samples obtained from mice and their corresponding human donor sample and showed that the overall composition was more similar to the donor in mice fed the SD than in mice fed chow in two out of the five groups when using unweighted UniFrac (Figure 3C) but not weighted UniFrac (Figure S2C).
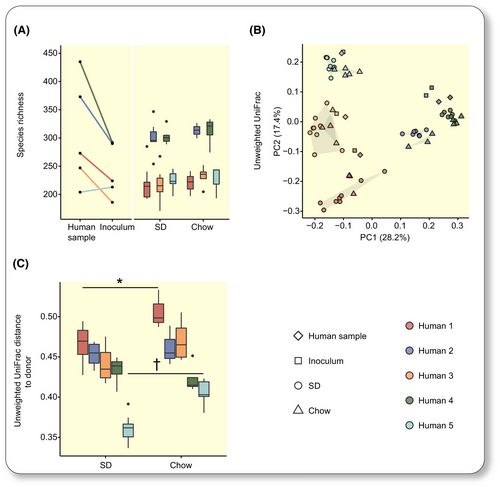
Mice fed the SD lost on average 14 (9–18) genera compared to inocula, and mice fed chow lost 16 (12–18) genera (Table S4). Genera that did not transfer were generally of low proportion in the human feces and inoculum and most of the lost genera belonged to the Clostridiales order (an average of 50.6% of all genera lost in mice fed SD and 53.6% in mice fed chow). Many genera transferred in an individual-specific fashion and seven genera did not transfer in at least three mouse groups on either diet, and could thus be considered non-transferable (Table S4). By contrast, some genera were not detected in the inoculum but bloomed in the mice (Table S4). The lower overlap of reads between inoculum and mice for individual 2 (Table S2) could be explained by the high proportion of a genus of the Prevotellacae family in the inoculum, which was not transferred to the mice (Table S4).
Taken together, our results show that microbiota transfer from two of the five donors to mice was more successful in the mouse groups fed the human diet rather than chow.
2.3 Impact of a moderate variation in the human diet on the gut microbiota in humanized mice
To evaluate the impact of the ID on gut microbiota composition in the humanized mice used above, diets were switched 14 days after colonization (to allow for stabilization of the microbiota)36: mice on the SD were switched to the ID (i.e., a variation in diet corresponding to that used in humans above) and mice on chow were switched to a rodent western-style diet (a diet change frequently used in cardiometabolic research and that is known to modulate the microbiota6, 14) (Figure 2A). Macronutrient composition of the four diets fed to the mice is shown in Table S1. As in the human intervention, the mice remained on their altered diet for two days, and additional fecal samples were collected on days 15 and 16 (Figure 2A). We only observed minor differences in biometric data between mice fed human and mouse diets (Table S5), suggesting sufficient nourishment of the mice fed human diets.
Switching mice to the ID or western-style diet did not lead to a consistent change in alpha diversity in all mouse groups (paired Wilcoxon rank sum test comparing day 14 and day 16, Figures 4A and S3A). However, ordination analysis of beta diversity demonstrated that samples in each mouse group clustered strongly by diet group (human vs mouse diet) as well as diet type (days 10 and 14 for SD or chow vs days 15 and 16 for ID or western-style diet) (Figures 4B and S3B). In contrast to the human intervention, PERMANOVA from unweighted UniFrac revealed that an average of 31.3% (24.1% based on weighted UniFrac) of microbial variation could be attributed to diet type in the different mouse groups fed human diets (Table S6). The average UniFrac distance between the two human diets (day 14 to day 16) was larger than between days on the same human diet (day 10 to day 14) (paired Wilcoxon rank sum test, Figures 4C and S3C). The change in mouse diets had an even stronger influence on gut microbiota composition (PERMANOVA, 42.0% and 55.8% of variation for unweighted and weighted UniFrac, respectively, Table S6). Similarly, distance between the two diets was larger in mice fed the mouse diets, especially when accounting for taxonomic proportion using weighted UniFrac (paired Wilcoxon rank sum test, Figures 4C and S3C), likely reflecting the larger differences in macronutrient composition in experimental mouse diets.
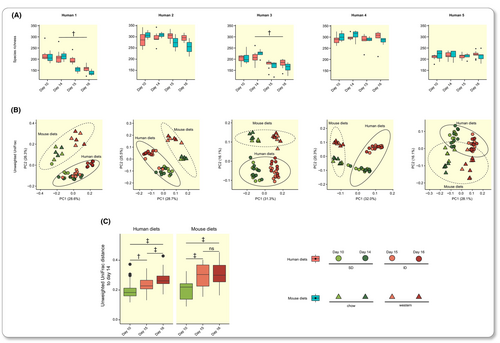
The overall compositional changes caused by the switch in diets were only partly reflected in proportional changes at higher taxonomic levels in mice fed human diets, but these were more pronounced in mice fed mouse diets (Figure S4). At the genus level, an average of 38 (28 to 55) genera changed significantly in mouse groups fed human diets and 45 (37 to 54) genera changed in mouse groups fed mouse diets (paired DESeq2, Table S7). Consistent with the results in humans, many taxonomic changes in response to the diet switch were dependent on the human donor microbiota composition. To allow for generalizable conclusions about the effects of the diet switch, we analyzed genera whose proportion changed significantly in three or more mouse groups (Table S7). As shown in Figure 2E, only six genera had changed significantly in proportion after the diet switch in humans. By contrast, the proportion of 18 genera changed significantly in at least three groups of mice fed human diets. Bifidobacterium, Bacteroides, Blautia, and Butyricicoccus diminished in all donor groups of mice in response to the ID (Figure 5); we did not observe significant reductions of these genera in the human part of the study (Figure 2E). These genera showed a similar pattern in mice fed mouse diets (Figure 5) but were not significant in all mouse groups (Table S7). Of the six genera with significantly altered proportion in the human cohort in response to the diet change (Figure 2E), several were lost during microbiota transfer and only genus CAG-56 showed a consistent increase in mice fed human diets (Tables S4 and S7).
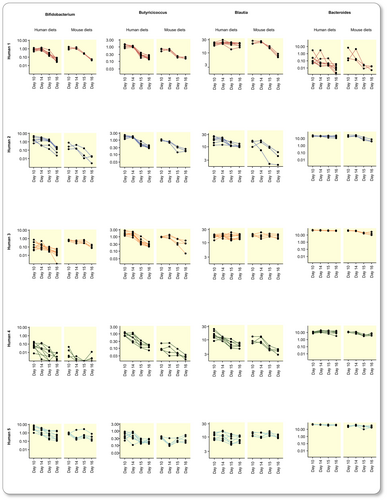
Taken together, we show that (1) moderate variations in the human diet promote changes in the gut microbiota composition in humanized mice and (2) this model can be used to study the impact of the human diet at an individual level.
3 DISCUSSION
3.1 Variations in gut microbiota and dietary interventions
The gut microbiome engages in a complex bidirectional relationship with the host diet. On one hand, the gut microbiome profoundly influences the overall health of the host and mediates some of the health effects associated with dietary factors. On the other hand, the composition of the gut microbiota is responsive to the host diet, leading to diverse downstream effects. Recognizing and appreciating this dynamic interplay between diet and the gut microbiome is essential for comprehending the intricate relationships and potential implications for human health.
Profound alterations in food intake have been demonstrated to significantly influence the composition of the gut microbiome and impact host physiology, even during short-term interventions.10, 37 However, these studies often fail to accurately reflect the reality of most western countries, where food availability and accessibility overcome the limitations of seasonality and the consequences of food scarcity observed in agrarian settings, resulting in relatively modest variations in food intake. This sustained dietary stability, although challenging to quantify, is manifested in stable configurations of the microbiome known as enterotypes.16 Understanding this concept is essential when investigating the intricate connections between diet, microbiome, and metabolism. Nevertheless, the gut microbiome exhibits a remarkable degree of individualization and may play a significant role in shaping the individual's response to metabolic interventions. Therefore, it is crucial to comprehend the implications of real-life scalable interventions while considering the inherent inter- and intraindividual variability of the microbiome. Despite the awareness of these factors within the scientific community, the majority of associations between the microbiome, diet, and health have been examined through observational studies. Interventional studies, on the other hand, have primarily focused on investigating microbiome and health changes within the context of extreme interventions. Such an approach is often employed to enhance study power and feasibility but is hardly representative of reality. In this study, our aim was to investigate the impact of moderate variations in the habitual human diet on gut microbiota composition. Despite implementing short-term elimination of dietary variation, we found that microbial variations between and within individuals were not easily overcome, and only a few consistent changes in microbiota composition were observed in response to the pilot diet intervention. To further assess individualized and cohort-wise responses of the microbiota to diet changes, we transferred microbiota from humans following the standard diet (SD) to germ-free mice, which were then switched to the intervention diet (ID). Notably, significant and often donor-specific changes in the microbiota were observed.
3.2 Challenges in assessing diet–microbiota interactions
Diet interventions using drastic diet variations have established diet as a major determinant of gut microbiota composition,9-11 and we hypothesized that reducing complexity of diet intake would facilitate assessing the impact of a more moderate diet variation in humans. In our study, despite eliminating all variations in participants' diets before introducing a moderate dietary variation, major reproducible changes in gut microbiota composition were not observed in the small cohort of five individuals. This finding is consistent with recent studies, which have shown compositional and metabolite variations despite stringent diet homogenization for 7 days,29 as well as substantial microbiota variation even when exclusively consuming a meal replacement drink.22 In contrast, in our humanized mouse model, distinct gut microbial changes were observed at both the individual and cohort levels when repeating the same diet intervention, confirming the influence of inter- and intraindividual microbial variation as a bias when assessing microbiota-targeted strategies, particularly in small cohorts and/or short intervention periods.
3.3 Humanized mice as a model for diet–microbiota interactions
The combination of germ-free animal models and human studies to elucidate potential early mediators of disease within the intricate meta-organismal framework has been widely used the past two decades.38 Humanized (germ-free) mice, which are colonized with human microbiota and fed human diets, have similarly been utilized to assess the impact of the gut microbiota in the context of malnourishment39, 40 and several studies have demonstrated that metabolic phenotypes can be transferred from humans to mice using fecal microbiota transfer.30, 39, 41, 42 However, the capacity of the habitual human diet to modulate the gut microbiota in this model has not been investigated. Additionally, we examined whether fecal microbiota transfer could be improved by matching the recipient mice's diet to the donors' diet rather than using standard mouse diets. The results indicated a largely successful transfer of genera for both diets, with recipient mice fed the human diet showing better success for two of the human donors. Moreover, the transfer of microbiota from donors with high microbial diversity resulted in recipient mice harboring microbiota with high diversity, regardless of diet. Interestingly, our results indicate that a previously described loss of members of the Clostridiales order during transfer of human microbiota to germ-free mice43 is linked to host specific differences rather than to diet differences. While humanized mice offer insights into how human diets shape the gut microbiota and allow for a closer replication of the individual human environment, these results also highlight that careful analysis of microbiota transfer success is warranted with regard to translation of results as taxa carrying out key metabolic functions such as bile acid modification may fail to transfer. This finding underlines the potential of humanized mice as a predictive tool to assess the potential outcomes of specific interventions. Given the high cost and potential risks associated with conducting such interventions directly in humans, employing humanized mice as an in vivo model may serve as a valuable pilot approach before embarking on large-scale studies.44
3.4 Distinct microbiota changes in humanized mice
Transferring the microbiota of each of the five individuals into several germ-free mice, either on SD or chow, and subsequently switching their diet to the ID diet or a rodent western-style diet, respectively, allowed for the identification of both individual-specific and more generalizable changes in the microbiota. The change in microbiota composition was more pronounced in mice switched from chow to the rodent western-style diet compared to those switched from SD to ID. This can be attributed to a larger difference in fiber content between the mouse diets compared to the human diets, which is known to exert substantial effects on the gut microbiota in both mice and humans.10, 11, 45-47 Interestingly, fibers also produce individualized responses in the gut microbiota that may be related to baseline parameters such as previous fiber intake and gut microbiota profile.48-50
3.5 Importance of moderate diet variations and individual responses
By using an experimental setup that reduced interindividual variation, we demonstrated that even moderate variations in the human diet caused distinct changes in the gut microbiota of humanized mice. This is important since the success of any future microbiota-targeted dietary strategy will be limited by its acceptance by the targeted population, and moderate dietary changes are more likely to lead to long-term compliance. Furthermore, consistent changes were observed in genera with known implications for metabolic health. One such example is reduced proportion of Bifidobacterium in mice fed a western-style mouse research diet compared to chow.47 Here, we demonstrated that a human western-style diet is also inadequate in sustaining the proportion of Bifidobacterium following a switch from a more balanced human diet. Similarly, the genus Butyricicoccus and other butyrate-producing bacteria known to be sensitive to diet, especially fiber intake51, 52 exhibited decreased proportions despite only moderate differences in dietary fiber content between the human diets. In addition to these cohort-wide changes, numerous taxonomical shifts in mice fed either human or mouse diets were specific for the donor gut microbiota underlining the relevance of personally tailored diet interventions.
3.6 The potential of microbiota-targeted dietary strategies
A growing body of evidence suggests that microbiota-targeted diets hold the potential for improving cardiometabolic health. However, the interrelation between diet, microbiota, and host health is highly complex and we are only beginning to understand the true impact of specific diet interventions. While certain microbiota-targeted nutrition strategies, such as increased intake of fermented foods and fiber, may be more generalizable and suitable for dietary guidelines,53 studies have also highlighted the individual variability in response to specific interventions and identified gut microbiota traits as a key factor in this process.27, 54-57 This suggests that more targeted, individual- and context-specific approaches could be more effective. Notably, gut microbiota profiling has facilitated to predict postprandial glucose and lipid response58, 59 and a pioneering precision nutrition study has demonstrated promising clinical outcomes.28 However, gaining a better understanding of the individual dynamics of response and nonresponse will be crucial for advancing precision nutrition. Thus, our approach of analyzing the effect of a human diet intervention on an individual's gut microbiota using humanized mice provides a valuable platform for understanding individual microbial responses to diet.
4 CONCLUSION
In conclusion, our study underscores the challenges in observing consistent microbiota changes in response to moderate variations in the human diet within a small human cohort. Nevertheless, using a humanized mouse model allowed us to overcome some of these limitations and reveal distinct changes in the gut microbiota influenced by the intervention diet. These findings emphasize the importance of personalized diet interventions, highlight the potential of microbiota-targeted strategies for metabolic health improvement, and underscore the need for further research to unravel the intricate connections between diet, microbiota, and host health.
5 MATERIALS AND METHODS
5.1 Human diet intervention
5.1.1 Study cohort
Five healthy male volunteers aged 24 to 45 years (average age 31.4 years, SD 8.2 years) with a body mass index within the normal range (average 23.3 kg/m2, SD 2.5 kg/m2) were recruited to participate in the short-term diet intervention. Participants reported stable weight over the last 3 months as well as a bowel movement frequency of 1-2/day, which did not change throughout the study. Before inclusion a dietary assessment was performed by an experienced clinical nutritionist based on 24-h recalls in combination with frequency of specific food groups to confirm that all five individuals had a habitual varied diet not excluding any specific food groups. None of the participants followed a specific or restrictive diet.
5.1.2 Study design
The study design is shown in Figure 2A. On day 0, participants began eating the diet we refer to as “standardization diet” (SD): a ready-made vegetarian meal of falafel, wheat berry, vegetables and Ajvar sauce (Falafel med matvete, Findus®, Sweden, see Supplementary Material for ingredients). During days 0, 1, and 2, this meal was the only food consumed for breakfast, lunch and dinner. The purpose of the SD was to eliminate dietary variation before the diet intervention and therefore macronutrient composition of the SD was chosen to approximate the previous macronutrient intake of the study participants so it would allow to bring participants diet to the same intake without constituting an intervention in itself. While processed and ready-made, its macronutrient composition does match common public health recommendations for a healthy diet.
On day 3, the participants' diet was switched to the “intervention diet” (ID), whose impact on the gut microbiota we aimed to assess: a meat-based ready-made meal consisting of hamburger steak, croquettes, green beans, tomatoes, and herb butter (Lövtunn bit med potatiskroketter, Findus®, Sweden, see Supplementary Material for ingredients), which was consumed for breakfast, lunch, and dinner together with 300 mL of a high sugar drink (Sprite®, The Coca-Cola Company) for each meal for two days. On day 5, participants went back to their regular diet after providing a fecal sample in the morning. Hence, fecal samples from days 1, 2, and 3 reflect exposure to the SD, whereas samples from days 4 and 5 reflect exposure to the ID. The intervention diet was chosen in order to be a clear shift in dietary intake from the SD and to reflect a western-style dietary pattern. As such, this western-style diet reflects a clear change from the standardization diet while remaining within the dietary spectrum of the western population. Assessing the impact of such a moderate dietary variation was a major goal of this study.
Food intake was restricted to study meals throughout the intervention with water, black coffee, or tea as the only additional beverages. Macronutrient composition of the meals is described in Table S1. The SD was chosen based on the recommendations on macronutrient intake provided in the Nordic Nutrition Recommendations60 and was intended to continue the participants' balanced pre-study dietary intake in a standardized fashion that would allow to eliminate dietary variation between the participants before introducing the ID. The meat dish along with a high-sugar soft drink was chosen to mimic a western-style diet with high amounts of total and saturated fat and sugar as a realistic variation in the habitual human diet and also reflecting “healthy” vs. “unhealthy” dietary pattern.
Throughout the study, participants were free to eat the respective diets ad libitum at each meal. If they did not wish to eat a whole meal at one sitting, they were instructed to eat all components proportionally. Hence, they could adapt their diet (and hence, caloric intake) to their personal need. We believe that this measure avoided the intervention being more or less severe on the different participants due to overeating.
5.2 Mouse diet intervention
5.2.1 Study design
Swiss Webster male mice at 9–13 weeks of age were maintained under germ-free conditions in a maximum of five mice per cage under a 12-h light cycle and a room temperature of 21°C. Food and water were provided ad libitum. The mice colonized by each individual were divided 2:1 into an experimental group (n = 7–9) receiving a pelleted and irradiated version of the human diets (see below) and a control group (n = 3–5) receiving standard rodent diets (irradiated chow, Teklad 2918 Global 18% Protein Rodent Diet, Envigo, USA, and western-style diet TD.09683, Envigo, USA Table S1). The mice on human diets were switched from chow to SD five days before colonization. Germ-free status was confirmed via PCR of the 16S rRNA gene before colonization.
Fecal microbiota transfer was performed on day 0 by gavage of 200 μL inoculum and was repeated once on day 2 of the mouse intervention.
For each human donor, recipient experimental and control mice were housed in separate isolators. Based on previous work,36 mice were kept on SD or chow for 14 days to allow for stabilization of the gut microbiota before switching to ID or western-style diet for two days. Fecal samples from each mouse were collected for analysis on days 10, 14, 15, and 16. Hence, fecal samples from days 10 and 14 reflect exposure to SD or chow whereas samples from days 15 and 16 reflect exposure to ID or western-style diet. Food consumption was measured cage-wise and calculated to consumption per mouse and day.
5.2.2 Processing of human diets for use in mouse diet intervention
To mimic the human diet intervention in a mouse model, the two human ready-made meals were made available as germ-free versions. The ready-made meals were purchased at local grocery stores, freeze-dried (European Freeze Dry, Kirke Hyllinge, Denmark and Department of Food and Nutrition Sciences, Chalmers University of Technology, Gothenburg, Sweden), ground with a food processor (Bosch MultiTalent 3, Bosch, Germany), and homogenized (Electrolux EKM3000, Elextrolux, Sweden). For the ID, 30 g of sugar were added to each processed meat-based ready-made meal, corresponding to 300 mL of the high-sugar drink the human participants consumed with every ID meal. Aliquots of 300 g and 500 g were vacuum-packed and irradiated at 50 kGy (Steris, Radeberg, Germany). The sterilized bags were introduced into germ-free isolators after sterilization of the surface (Clidox-S, Pharmacal Research Laboratories, Naugatuck, USA). Once inside the isolator, bags were opened, sterile water was added to the powdered food, and pellets were formed manually which were dried for at least 24 h before feeding. The composition of the processed human diets was not reanalyzed after freeze-drying and irradiation, and we cannot exclude minor modifications of macro- and micronutrient composition.
5.3 Handling of human fecal samples and preparation of inoculum
Fecal samples from each individual were collected and de-identified on the morning of days 1 to 5 and (with the exception of individual 3) before the diet intervention. The fecal samples were immediately frozen at −80°C until use. Fecal samples from day 1 were used to prepare mouse gavage solutions.
Gavage solutions were prepared 1–3 days before the fecal microbiota transfer. Fecal material was aliquoted from the fecal samples on dry ice, transferred into an anaerobic chamber and suspended 1:10 in sterile LYBHI medium containing 20% of glycerol. The gavage solution was aliquoted into 1.5 mL tubes, frozen at −80°C and thawed immediately before introduction into experimental isolators to gavage the mice.
5.4 Extraction of genomic DNA and profiling of fecal microbiota composition by sequencing of the 16S rRNA gene
Total genomic DNA was isolated from 50–100 mg of feces using repeated bead-beating and the Nucleospin® Soil kit (Macherey-Nagel). Samples were placed in SL2 buffer with SX enhancer, incubated at 90°C for 10 min, and sheared with 6 rounds of bead beating at 5.5 m/s for 60 s in a FastPrep®-24 Instrument (MP Biomedicals). The V4 region of the 16S rRNA gene was amplified with primers 515F and 806R61 in duplicate PCR reactions in 25 μL volumes containing 1× 5PRIME HotMasterMix (5PRIME), 200 nM of each primer, 0.4 mg/mL BSA, and 5% dimethylsulfoxide. The PCR program consisted in an initial denaturation for 3 min at 94°C; followed by 25 cycles of denaturation for 45 s at 94°C, annealing for 60 s at 52°C and elongation for 90 s at 72°C; and a final elongation step for 10 min at 72°C. Duplicates were combined, purified with the NucleoSpin Gel and PCR Clean-Up kit (Macherey-Nagel), and quantified using the Quant-iT PicoGreen dsDNA kit (Invitrogen). Purified amplicons were diluted to 10 ng/μL, pooled in equal amounts and purified again using Ampure magnetic purification beads (Agencourt) to remove short products. Negative controls were run for each sample and the absence of detectable PCR products was confirmed with gel electrophoresis.
Amplicons were sequenced in a MiSeq instrument (RTA version 1.17.28, bundled with MCS version 2.5; Illumina) with the V2 kit (2 × 250 bp paired-end reads; Illumina). Illumina reads were merged using Usearch v.11 64-bit62 allowing for up to 30 mismatches63 in the alignment of the paired-end reads while discarding reads with a merged length greater than 270 bp and fewer than 230 bp. The merged reads were quality filtered based on expected errors removing reads above the threshold of 1.64 The merged reads were turned into Zero-radius operational taxonomic units (Zotus)65 by compiling the sequences into sets of unique reads and performing error-correction using the UNOISE3 algorithm66 discarding sequences with fewer than 4 reads. The Zotus were assigned taxonomy using DADA2's67 assignTaxonomy (minBoot = 50) and assignSpecies, using formatted version of the Silva v.138 database.68 A phylogenetic tree of the sequences was created with the help of the MAFFT software v.7.40769 and the FastTree software v.2.1.10.70 The Zotu-table was filtered based on proportion where Zotus with less than 21 reads in all samples (0.0001% of total reads) were removed, this filtering procedure retained 17 816 004 (99.6%) of the reads and 1008 (39.3%) Zotus. The average read depth for the 284 samples included in this study after the final filtering was 62 732 reads with a SEM of ±951.
5.5 Statistical analysis
5.5.1 Statistical analyses were performed in R and GraphPad Prism 8
Alpha diversity was calculated using phyloseq::estimate_richness()71 function and beta diversity ordination was performed using phyloseq::ordinate(). Evaluation of beta diversity was calculated by phyloseq::distance() and evaluated using the vegan::adonis()72 with 9999 permutations. Comparison of alpha diversity between days and beta diversity between days were performed using Wilcoxon rank sum test using the wilcox. test() included in R-base. Testing for differences in proportion was performed using DESeq273 requiring the taxa to exist in at least 30% of the samples tested. Spearman's rank correlation in terms of proportion of species between human samples, inoculum and mice samples were calculated using cor.test() from base R. All multiple comparisons were adjusted for false discovery rate < 5% using Benjamini-Hochberg.
Organ weights at the end of the experiment were compared using unpaired Student's t-test. Relative weight gain at days 14 and 16 and food consumption were compared using Wilcoxon rank sum test.
AUTHOR CONTRIBUTIONS
Rima Chakaroun: Visualization; writing – review and editing. Marc Schoeler: Conceptualization; investigation; methodology; formal analysis; data curation; visualization; writing – original draft; writing – review and editing; project administration. Harald Brolin: Investigation; visualization; formal analysis; data curation; writing – review and editing. Ingrid Larsson: Writing – review and editing. Rosie Perkins: Writing – review and editing. Hanns-Ulrich Marschall: Methodology; writing – review and editing. Robert Caesar: Conceptualization; writing – review and editing. Fredrik Bäckhed: Conceptualization; methodology; supervision; funding acquisition; resources; project administration; writing – review and editing.
ACKNOWLEDGMENTS
We thank Jeffrey Gordon, Michael Barratt, and Valentina Tremaroli for vital feedback on study design and data interpretation, Anna Hallén, Louise Helldén, Carina Arvidsson, Oskar Persson, Manuela Krämer, and Robert Jakubowicz for technical assistance as well as Rikard Landberg and Rikard Fristed for help with diet processing.
FUNDING INFORMATION
This study was supported by the Swedish Research Council (2019–01599), JPI (A healthy diet for a healthy life; 2017-01996_3), Transatlantic Networks of Excellence Award from the Leducq Foundation (17CVD01), AFA insurances, Swedish Heart Lung Foundation (20180600), Knut and Alice Wallenberg Foundation (2017.0026), grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-718101). F.B. is Wallenberg Scholar and Torsten Söderberg Professor in Medicine.
CONFLICT OF INTEREST STATEMENT
F.B. is cofounder of Implexion Pharma AB and Roxbiosens Inc. He receives research funding from Biogaia AB and is on the scientific advisory board of Bactolife A/S. The other authors declare no competing interests.
INSTITUTIONAL REVIEW BOARD STATEMENT
The human diet intervention was conducted according to the guidelines of the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (Reference 2020-01453). All animal procedures were approved by the Gothenburg Animal Ethics Committee.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in this study.
Open Research
DATA AVAILABILITY STATEMENT
The data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB43025 (https://www.ebi.ac.uk/ena/browser/view/PRJEB43025).




