Does hypometabolism constrain innate immune defense?
Abstract
Many animals routinely make energetic trade-offs to adjust to environmental demands and these trade-offs often have significant implications for survival. For example, environmental hypoxia is commonly experienced by many organisms and is an energetically challenging condition because reduced oxygen availability constrains aerobic energy production, which can be lethal. Many hypoxia-tolerant species downregulate metabolic demands when oxygen is limited; however, certain physiological functions are obligatory and must be maintained despite the need to conserve energy in hypoxia. Of particular interest is immunity (including both constitutive and induced immune functions) because mounting an immune response is among the most energetically expensive physiological processes but maintaining immune function is critical for survival in most environments. Intriguingly, physiological responses to hypoxia and pathogens share key molecular regulators such as hypoxia-inducible factor-1α, through which hypoxia can directly activate an immune response. This raises an interesting question: do hypoxia-tolerant species mount an immune response during periods of hypoxia-induced hypometabolism? Unfortunately, surprisingly few studies have examined interactions between immunity and hypometabolism in such species. Therefore, in this review, we consider mechanistic interactions between metabolism and immunity, as well as energetic trade-offs between these two systems, in hypoxia-tolerant animals but also in other models of hypometabolism, including neonates and hibernators. Specifically, we explore the hypothesis that such species have blunted immune responses in hypometabolic conditions and/or use alternative immune pathways when in a hypometabolic state. Evidence to date suggests that hypoxia-tolerant animals do maintain immunity in low oxygen conditions, but that the sensitivity of immune responses may be blunted.
1 INTRODUCTION TO HYPOMETABOLISM AND IMMUNITY
Various animals become hypometabolic in response to challenging environmental conditions (e.g., hypoxia, reduced food availability) or during species-specific seasonal behaviors (e.g., hibernation). While in a hypometabolic state, animals inherently have a reduced energy budget. Therefore, trade-offs must occur to allow an animal to maintain physiological and cellular functions that are energetically expensive but presumably obligatory, even when energy availability is limited during systemic hypometabolism. Of particular interest is the regulation of immunity in hypoxic environments (including constitutive immune processes that maintain immune function and induced immune responses that protect the host against nonself molecules). This is because immune competency is a crucial but energetically costly physiological mechanism for survival; but, many species employ metabolic rate suppression to thrive in naturally hypoxic environments where they are nonetheless exposed to bacteria and other pathogens. Intriguingly, key molecular signaling intermediates that regulate cellular and physiological responses to hypoxia can also regulate certain immune pathways, indicating that there is likely considerable overlap between the regulation of hypoxic hypometabolism and immunity.
In considering how immunity and hypometabolism interact in species adapted to live in low oxygen, we initially hypothesized that adaptive energetic trade-offs must occur in hypoxia-tolerant animals to circumvent low energy supply. However, given the scarcity of research in hypoxia-tolerant species, we expanded our hypothesis to include hypoxia-tolerant neonates from species that are hypoxia-intolerant as adults, and hibernators, as other animal models that regularly undergo periods of hypometabolism in hypoxia and/or due to other environmental challenges. We predicted that blunted immunity or the utilization of alternative immune pathways may be common in such species during periods of hypometabolism. In this review, we address this prediction by first discussing how immune system and hypometabolic signaling pathways interact at the cellular and molecular levels. We then critically evaluate the existing literature pertaining to the intersection of immunity and hypometabolism in model species and during developmental stages that are hypoxia-tolerant and/or regularly undergo periods of hypometabolism in nature (i.e., in hypoxia-tolerant adult animals, hypoxia-tolerant neonates from species that are otherwise hypoxia-intolerant as adults, and hibernators), in an attempt to understand how animals balance the need to reduce energy demand in hypometabolic conditions with the cost of maintaining presumably obligatory immune function.
2 MOUNTING AN IMMUNE RESPONSE IS ENERGETICALLY EXPENSIVE
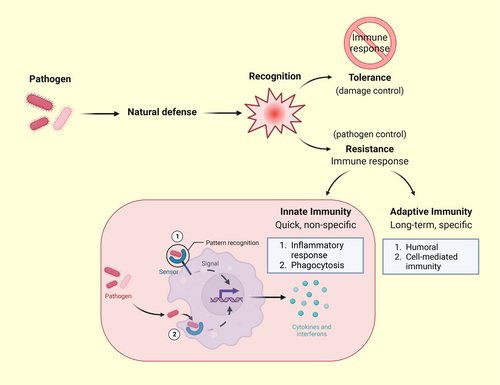
Before discussing specific animal examples, it is important to understand the cost of mounting immune responses, particularly in environments that place limits on aerobic energy generation.1 Mounting an immune response is energetically costly because activating the immune system requires the coordination of many cell types and cellular signaling systems (see below),2 and immune system activation is thus usually accompanied by a significant increase in metabolic rate.3-6 Indeed, it has been hypothesized that mounting an immune response may have a similar energetic cost to reproduction and growth.2 Therefore, it is likely that trade-offs with other biological functions occur when an immune response is activated. More specifically, energetic trade-offs with other important life-history traits may occur during the acute immune response as this is the most energetically costly phase in the activation of the immune system7-9 (As an aside it is important to note that hosts may also employ resistance or tolerance as protective strategies against pathogens. Resistance reduces the pathogen burden, whereas tolerance limits the damage caused by said pathogen and constrains the resulting immune response; Figure 1.10 This topic is beyond the scope of the present review but interested readers can find more information here11, 12).
Several studies have measured the energetic cost of mounting an immune response in various species. In general, hypoxia-intolerant mammals and birds appear to have moderate changes in resting metabolic rate following activation of the acute phase response by lipopolysaccharide (LPS: a commonly used substance to mimic a bacterial immune challenge). For example, zebra finches (Taeniopygia guttata, 1 mg LPS/kg),13 house sparrows (Passer domesticus, 1 mg LPS/kg14 and 5 mg LPS/kg15), and brown rats (Rattus norvegicus, 0.05 mg LPS/kg)16 exhibit ∼10%–30% increases in resting metabolic rate following LPS treatment (but see also Martin et al.14 and Sköld-Chiriac et al.17). In addition to LPS, other immune stimulants induce similar or larger increases in metabolic rate in mammals. For example, sepsis, injury, typhoid vaccination, and sickle cell disease increase metabolic rate ∼15%–57% in humans,18-22 interleukin (IL)-1 infusion, and inflammation increases metabolic rate ∼18%–28% in rats,23, 24 hemocyanin injection increases metabolic rate ∼30% in mice,5 and endotoxin increases metabolic rate ∼10%–49% in sheep.25, 26 The interspecies variability in these responses may be due to differing stimulation of the immune system by varied LPS doses, divergent immune sensitivity to different stimuli, and/or to concomitant adjustment of other systems that are being up- or down-regulated (see below).
Furthermore, it is important to clarify that mounting an immune response and maintaining constitutive immune function are two distinct processes with different energetic demands. Indeed, the maintenance of immunity seems to incur a low energetic cost compared with the initial activation of the immune system. However, although several studies have measured increases in whole-animal metabolic rate during immune activation, it is difficult to truly determine how much energy is required to mount a competent immune response in vertebrates,8, 27 because an increase in energy demand by the immune system is often accompanied by a shift in metabolic priorities to favor immunity and tissue repair9; this shift may mask the true energetic cost of immune system activation. For example, tree sparrows (Passer montanus) challenged with bacterial infection (phytohemagglutinin, PHA: an adaptive and innate immune challenge) or LPS have reduced egg production and daily locomotor activity compared with sham-injected conspecifics, suggesting that trade-offs are made to favor immune activation over reproductive or behavioral activity.28 Conversely, similarly treated house sparrows (Passer domesticus), which are an invasive species, do not exhibit changes in reproductive or behavioral activity following immune stimulation, leading the authors to suggest that invasive species may not invest in costly immune defenses, which could aid their invasion success but at the expense of immune protection.28 Together, these findings suggest that the energetic cost of mounting an immune response poses a clear conflict with other metabolic needs and species must therefore make trade-offs between immunity and other metabolic demands. Thus, an important caveat when evaluating results from studies that measure metabolic changes in response to an acute immune challenge is that it is difficult to determine the net metabolic cost of an immune response when other energetically demanding systems within the body are also being up- or down-regulated.
For these reasons, measures of metabolic rate changes in “normal” conditions may be inherently flawed. Instead, researchers may be better able to understand the true metabolic cost of immunity by measuring immunity-linked changes in metabolic rate in animals that are in a state of hypometabolism, in which non-obligatory metabolic processes are likely already downregulated, and thus less likely to mask the metabolic cost of immunity. This raises an interesting question: do hypoxia-tolerant animals (and other animals that have highly plastic metabolic rates, such as hibernators and neonates), which have evolved numerous elegant ways to conserve energy in a hypometabolic state, prioritize immunity over metabolic suppression? In other words, do species in a hypometabolic state maintain immune competency at the expense of other energetically demanding processes? Despite the importance of this question, few studies have considered trade-offs between immunity and other energy-consuming processes in a hypometabolic state, or how species adapted for hypometabolism with highly plastic metabolic systems manage these seemingly conflicting survival priorities.
3 PHYSIOLOGICAL RESPONSES TO HYPOXIA
Maintaining energetic homeostasis is crucial for the survival of vertebrate species. The primary source of cellular ATP generation is aerobic cellular respiration, which is inherently dependent on oxygen availability. Hypoxia (i.e., decreased oxygen availability) thus impairs aerobic cellular respiration in the electron transport system.29 Without a concomitant decrease in metabolic rate, the energy demands of an organism quickly outpace its energy supply,30 which can result in cellular and eventually whole animal death. Nonetheless, environmental hypoxia occurs in many ecosystems around the world and despite the challenges of living in hypoxia, many species thrive in these niches. For example, some fish tolerate aquatic hypoxia in the ocean31 or in intertidal pools,32, 33 animals living at high altitudes are exposed to lifelong hypobaric hypoxia,34, 35 and animals that reside in underground niches may experience intermittently hypoxic and hypercapnic conditions.36, 37 Given the energetic challenge presented by hypoxia, it is not surprising that oxygen availability is a powerful driver of evolutionary adaptations for species that inhabit hypoxic niches38; animals that dwell in these niches have developed numerous strategies to ameliorate the energetic challenge posed by hypoxia.36, 37, 39-44 In many of the most hypoxia-tolerant species, entrance into a systemic state of hypometabolism serves to rebalance energy supply and demand in hypoxia and is often crucial to survival when environmental oxygen supplies are limited. Interestingly, and relative to hypoxia-intolerant species, greater increases in resting metabolic rate during the acute activation phase of immunity have been reported for several putatively (but understudied) hypoxia-tolerant species. For example, resting metabolic rate increases by ∼40% following immune stimulation in Pekins ducks (Anas platyrhynchos, 0.1 mg LPS/kg),45 by ∼60% in Pallas's long-tongued bat (Glossophaga soricina, 2.84 ± 0.04 mg LPS/kg),46 and by ∼185% in Myotis (Myotis vivesi, 1.75 ± 0.06 mg LPS/kg).6, 47 Unfortunately, none of these studies incorporated hypoxia.
Animals employ a wide variety of strategies to mitigate the deleterious effects of environmental hypoxia (Figure 2). Where possible, animals first employ behavioral means to avoid or escape hypoxia.32 Such behavioral strategies (i.e., anapyrexia48) include seeking colder environments or reduced huddling in small rodents, to lower their body temperature and reduce systemic energy expenditure. Alternatively, organisms that remain active in short-term hypoxia or cannot escape hypoxia may increase their oxygen supply by enhancing oxygen extraction, or increasing ventilation and/or cardiac work (i.e., heart rate or stroke volume), or altering hemoglobin-oxygen-carrying capacity.36, 49
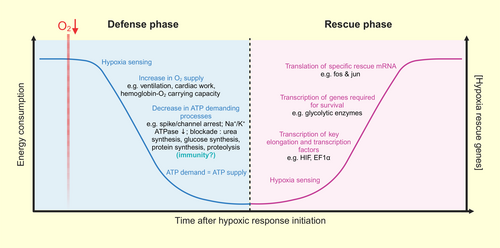
However, physiological mechanisms that increase oxygen supply may be energetically expensive and so are not an ideal strategy in animals that experience hypoxia regularly. Instead, decreasing oxygen demand at the cellular level offers a more efficient long-term strategy. For example, many hypoxia-tolerant species reduce energy demand by lowering body temperature and/or metabolic rate during prolonged or intermittent hypoxia exposure.36, 37, 50 Such a hypometabolic state during hypoxia has been demonstrated in a wide range of tolerant species and is achieved by numerous adjustments at the cellular level that downregulate ATP-depleting processes (e.g., gene and protein biosynthesis, ion-pumping, etc.) to balance ATP demand and supply.40, 51-56 Importantly, such a hypometabolic state may also occur outside of hypoxia; for example, some species enter torpor,57 which is characterized by a regulated and striking depression in metabolic rate and body temperature, when energy is scarce.58-60 Hibernation is also characterized by hypometabolism and likely requires similar trade-offs to balance energetic priorities as in hypoxia (see below). However, the regulation of energetically expensive immune processes in hypoxia is less well understood.
4 INNATE IMMUNITY AND HYPOXIA ARE LINKED IN HYPOXIA-INTOLERANT SPECIES
The innate immune system is the first line of defense against microbial pathogens.61 When damage-associated molecular pattern molecules (DAMPs) or pathogen-associated molecular patterns (PAMPs) are detected, an innate immune response is triggered. DAMPs can be activated by wounds, disease progression, or cell stress, whereas PAMPs can be activated by several microbial pathogens (i.e., bacteria, viruses, fungi), and are typically stimulated in experimental settings by LPS.61-64 Briefly, triggering an innate immune response activates macrophages, which are myeloid cells found in almost all tissues and that typically maintain tissue homeostasis throughout development and adulthood.65, 66 For example, microglia, the primary macrophages in the brain, monitor and sense microenvironmental changes and rapidly initiate an immune response to infections, injuries, and hypoxia, among other stimuli.67-69 Following stimulation of innate immunity, activated macrophages can then produce pro-inflammatory cytokines through a signaling pathway.64, 70, 71 Sickness behavior results from this cytokine production, which activates the neuroimmune and neuroendocrine systems.64, 71 For example, acute exposure to LPS can result in depression symptoms (i.e., anorexia, anhedonia),70, 72, 73 fever, hypersomnia, and reduced exploration and social interactions, among other effects.70
The inflammasome is another important component of the innate immune system that contributes to the activation of immune cells in response to a variety of cellular stressors. There are five receptors that can form inflammasomes and each detects distinct cellular perturbations.74 For instance, both nucleotide-binding domain leucine-rich repeat (NLR) containing caspase activation and recruitment domain (CARD) 4 (NLRC4) and pyrin respond to the presence of bacterial cell membrane components and bacterial toxins, whereas absent in melanoma 2 (AIM2) specifically recognizes RNA and DNA, whether their origin be bacterial, viral, or self. Cytosolic AIM2 can detect damage to the nuclear membrane or mitochondria DNA and can translocate to the nucleus in response to UV-mediated damage to nuclear DNA. Finally, NLR proteins 1 and 3 (NLRP1, NLRP3) sense a range of stimuli including bacterial toxins, particulate matter (protein or lipid crystals/aggregates), extracellular ATP, disruptions to lysosomal and mitochondrial membranes, changes in ion homeostasis, reactive oxygen species (ROS), etc. The NLRP3 inflammasome is the most studied inflammasome and is the major site of IL-1β and IL-18 activation. Once released from the cell, these perform a variety of functions, including, for example, recruiting phagocytic immune cells, activating T-helper cells, and inducing prostaglandin synthesis.
Activating and maintaining these pathways requires energy. Indeed, the inflammatory response involves significant metabolic costs, which may include an increase in ROS and reactive nitrogen species production, consumption of nutrients, and increased oxygen demand.2 However, physiological responses to hypoxia typically include systemic changes that regulate several energy-consuming cellular activities, often with the general outcome of reducing energy demand.75 It is therefore important to consider how the innate immune response is regulated in hypoxia-tolerant organisms because endogenous DAMPs can form in response to hypoxia or reoxygenation stress, and because hypoxia may alter an organism's ability to mount an immune response against pathogens. Indeed, hypoxic stress can induce cell damage and/or death, which leads to the release of DAMPs as cells are dismantled or degraded during pyroptosis (following inflammasome activation, see above), or apoptosis and necrosis (during hypoxia).
Interestingly, the hypoxic response and activation of innate immunity are linked on a molecular level through hypoxia-inducible factor-1α (HIF-1α; a key regulator in the hypoxic response; Figure 3). In addition to mediating cellular responses to hypoxia, HIF-1α activation encourages the phagocytic property of innate immune cells towards bacterial pathogens.76 While the mechanisms underlying this pathway are still not completely unraveled, it is well-established that hypoxia can induce an immune response in hypoxia-intolerant species. For example, hypoxia can induce inflammation and inflammation can also activate the HIF signaling pathway through activation of nuclear factor-κB (NF-κB; a mediator of innate immune responses).77-81 NF-κB is a family of proteins that regulate inflammation, immune responses, proliferation, apoptosis, and angiogenesis.82-86 The inhibitor for κB kinases (IKKs) plays an important role through NF-κB activation. For example, in IKKβ deficient cells, Hifa mRNA basal levels are significantly downregulate, and transcriptional activation of genes arising from HIF-1α upregulation is not induced in mouse macrophages following bacterial infection.81 Furthermore, NF-κB has binding sites in the early promoter region of the HIF-1α gene and is important for the constitutive expression of HIF-1α mRNA.61, 87 For example, NF-κB upregulates Hifa expression in macrophages and the hypoxia-induced increase of HIF-1α within the nucleus involves NF-κB-mediated transcriptional control.81 As a result, the expression of hypoxic genes can be upregulated by bacterial infections. For instance, LPS-mediated inflammation in murine macrophages upregulates pro-inflammatory genes (i.e., tumor necrosis factor (TNF)-α, IL-6, IL-10, IL-1β), which further upregulates HIF-1α and NF-κB p65.88-90 Therefore, crosstalk between these two pathways makes NF-κB an important regulator of the hypoxic response and demonstrates the involvement of HIF-1α following bacterial infection.
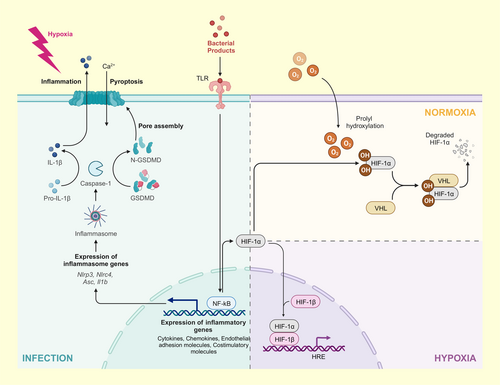
Similarly, inflammasomes are sensitive to endogenous danger signals and may be activated during hypoxic exposure. For instance, the activation of toll-like receptor (TLR) signaling cascades often leads to NF-κB activation and upregulation of NLRP3. As an example, cerebral ischemia followed by 24 h of reperfusion results in increased HIF-1α, NLRP3, IL-1β, and caspase-1 mRNA in rats.91 Conversely, inhibiting HIF-1α decreases NLRP3 and downstream gene transcript and protein levels, suggesting HIF-1α transcriptionally regulates inflammasome components.
Hypoxia/reoxygenation stress can also regulate the role of thioredoxin interacting protein (TXNIP), an inhibitor of the antioxidant protein thioredoxin. When dissociated from thioredoxin, TXNIP binds and activates the soluble inflammasome receptor NLRP3,92 which can increase innate immune signaling via the activation of caspase-1 and downstream cytokines. For instance, TXNIP is overexpressed in the hearts of mice exposed to 10 h of high-altitude conditions (426 mmHg at ∼4570 m) relative to mice held at normal atmospheric pressure (760 mmHg).93 Oxidative stress prevents TXNIP from binding thioredoxin, thereby promoting thioredoxin antioxidant activity. Indeed, thioredoxin levels and HIF-1α activity increase in hypoxic carcinomas.94, 95
In mice, exposure to hypobaric hypoxia (369.4 mmHg at ∼6000 m) does not affect the levels of serum IL-1β and TNF-α after a 6 h exposure but slightly downregulates IL-6, and these cytokines remained unchanged in the brain compared to normoxic controls, with no change in microglial density. However, after a 24 h exposure, IL-6 increases in the serum, and gene expression of IL-1β, IL-6, and TNF-α increase in the brain.96 Thus, hypoxia induces an immune response in mice; however, LPS induces a stronger immune response, characterized by an increase in cytokine expression levels and microglial density.96 Surprisingly, when these animals are exposed to hypoxia along with LPS, hypoxia amplifies the LPS-induced immune response. Specifically, cytokine expression is higher than with LPS alone, and vascular endothelial growth factor (regulated through the HIF signaling pathway) is upregulated and microglial density is augmented compared to hypoxia alone, but with no additive effect compared with the LPS treatment alone.96
Given that hypoxia-intolerant organisms mount an immune response to hypoxia, it is important to understand how oxygen availability impacts the outcome of bacterial infection. Pretreatment of mice in chronic hypoxia prior to bacterial infection reprograms the innate immune response by suppressing HIF-1α and reducing leukocyte glucose utilization.97 Without pretreatment, hypoxic mice have generally fewer immune molecules compared with normoxic mice. However, infection continuing past 24 h is fatal in hypoxia-treated mice.97 Nonetheless, hypoxic preconditioning improves mouse survival after infection.
Taken together, there is obviously a strong link between the hypoxic response and innate immunity. However, there is a general paucity of research exploring this interaction in hypoxia-tolerant animals. This is a glaring gap in the literature because there is a clear conflict between decreasing energy demands to tolerate hypoxia and the increased energy demand required to activate innate immune pathways, especially in environments where oxygen, and consequently energy, is limited. Therefore, studying hypoxia-tolerant animals will give us a better insight into possible energetic trade-offs between these two evolutionarily ancient pathways with opposing impacts on energetic homeostasis.
5 IMMUNE RESPONSE IN HYPOXIA-TOLERANT SPECIES
Given the obvious conflict between the cost of mounting an immune response and the need to conserve energy in hypoxia, we hypothesize that the immune systems of hypoxia-tolerant species may exhibit adaptations that minimize trade-offs with other physiological processes. For example, immunity may be blunted in hypoxia to delay activation and/or reduce the magnitude of immune recruitment when in hypoxia. Alternatively, hypoxia-tolerant species may employ different pathways or mechanisms to moderate immune responses that require less energy than those used by hypoxia-intolerant animals. Unfortunately, and as summarized in a recent review,98 inflammasomes have not been studied widely in many species outside of mice, rats, and humans, which are all hypoxia-intolerant. However, several studies have explored immunity in isolation in hypoxia-tolerant mammals, although most of these studies do not explicitly incorporate hypoxia into their experimental design.
Perhaps the best-characterized hypoxia-tolerant species in this context are naked mole-rats (Heterocephalus glaber), which putatively experience intermittent hypoxia in their underground burrows.99, 100 Presumably because of such intermittent exposure to environmental hypoxia, naked mole-rats are one of the most hypoxia-tolerant mammals identified. In laboratory setting, naked mole-rats can tolerate minutes in anoxia,101 hours in 3% O2,102 and weeks in 8% O2.103 Recent studies have elucidated a wide variety of strategies and adaptations that support hypoxia tolerance in this species. For example, naked mole-rats significantly decrease metabolic rate in an oxygen-dependent fashion through reductions in physical activity, body temperature, heart rate, breathing frequency, and numerous cellular mechanisms.100, 102, 104-107
Interestingly, several species of hypoxia-tolerant mole-rats either maintain or increase HIF-1α protein levels and DNA-binding capacity in brain during hypoxia108; HIF-1α levels are correlated with NLRP3 inflammasome component levels in hypoxic and reperfusion conditions (see above). Furthermore, baseline HIF-1α expression in naked mole-rats is significantly higher than in mice.109 This is presumably because naked mole-rats have a mutation within their von Hippel–Lindau-binding domain, which indicates they would have a higher baseline level of HIF-1α in normoxia due to decreased ubiquitin-dependant degradation of HIF-1α.38, 110 Furthermore, NF-κB signaling may be increased as part of the protective response to oxidative stress in this species.111 In support of this, hypoxia exposure of naked mole-rats is associated with increases in miR-155 and miR-365, two miRNAs whose expression correlates with NF-κB activation.112 Thus, it is possible that inflammasome activation is part of the molecular response to hypoxia in such hypoxia-tolerant organisms; however, studies are needed to explicitly investigate this hypothesis.
Conversely, it is possible that hypometabolic animals inhibit the formation of inflammasomes because mounting an innate immune response is too energetically demanding, or as a protective response to limit local tissue inflammation. Autophagy is a negative regulator of NLRP3 inflammasome activation since it degrades many of the possible triggers for NLRP3 activation, including protein aggregates and damaged cell components. Neonatal naked mole-rats may have higher levels of autophagy than neonatal mice, suggesting hypoxia-tolerant species may rely on autophagy to promote tissue homeostasis.113 Indeed, other studies report that hypoxia promotes autophagy, which leads to prolonged cell survival, and that LPS induces autophagy under hypoxia.114-116 Further exploration of the effect of hypoxia on the regulation of autophagy and its effect on the inflammasome in hypoxia-tolerant species is warranted.
An in vitro comparative study of the immune responses of naked mole-rats and mice found that the phagocytosis rate of macrophages is ∼11% higher in naked mole-rats, but the apoptosis rate of macrophages is ∼21% lower following LPS injection.117 Furthermore, mice macrophages increase TLR4 (LPS receptor) mRNA and protein expression, compared to naked mole-rats. However, naked mole-rats have a higher expression of NF-κB protein and downstream cytokines compared to mice. Stimulation with polyI:C to mimic viral infection induces similar changes.117 These results suggest that a smaller amount of TLR activation is required to trigger the NF-κB pathway in naked mole-rat macrophages and produce downstream cytokines to enhance immune response toward bacterial and viral infection. Although this research lacks data in hypoxic conditions, it nonetheless highlights a superior immune response.
As an aside, it is worth mentioning that, curiously, PHA-injected subterranean tucos-tucos (Ctenomys talarum) maintain their metabolic rate with no evidence of energetic trade-offs between immunity and reproduction.118 However, this does not mean that trade-offs do not exist with immunity in the form of other currencies that have yet to be measured. Indeed, hypometabolism leads to energetic trade-offs between immunity and homeothermy which promotes disease tolerance. As such, hypoxia-tolerant animals (and hibernators) may favor tissue tolerance rather than resistance to pathogens.10, 119
6 NEONATES AND THE INNATE IMMUNE RESPONSE
Neonates often exhibit physiological responses to hypoxia that are different from their adult counterparts. Some of these physiological differences resemble adaptations found in hypoxia-tolerant animals, which may contribute to neonatal hypoxia tolerance.120 Interestingly, many of the strategies that allow neonates to tolerate hypoxia are similar to those used by fossorial and diving mammals: neonates reduce metabolic demand, lower body temperature, and reduce breathing and heart rates when exposed to low oxygen conditions.120 Although few studies have explored interactions between immunity and hypoxia in neonates, neuronal injury from hypoxia-ischemia (HI) stress can result in long-term brain damage and developmental deficits, and considerable research has explored the molecular response of neonates to HI. This research provides some insight into interactions between immunity and low oxygen stress in this relatively hypoxia-tolerant developmental stage.
Neurodevelopmental issues from hypoxia are believed to stem from oxidative stress, inflammation, excitotoxicity, and cell death.121 The role of inflammasomes and pyroptosis in the neonatal response to hypoxia is still not fully understood, but inflammation exacerbates injury from HI in neonatal mice, such that larger infarcts are observed in mouse pups treated with pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α.122 Furthermore, IL-1β, a cytokine activated following inflammasome formation and pro-caspase-1 cleavage, is particularly harmful in the context of neuronal hypoxia in neonatal rodents.123, 124 Indeed, the pro-inflammatory cytokine IL-1β is upregulated in neonatal rat brains following HI and LPS/HI injury.125 Consistent with this, neonatal mice whose carotid arteries are ligated before exposure to 10% O2 for 1 h have increased expression of NLRP3 in astrocytes and microglia.126 However, NLRP3 and apoptosis-associated speck-like protein containing a CARD (ASC) neonatal knockout mice exposed to HI have an opposing phenotype.126 Specifically, NLRP3 deficiency is associated with increased infarct size and activated microglia, whereas ASC deficiency reduces infarct sizes and microglial activation.
In humans, the NLRP3 inflammasome is involved in neonatal encephalopathy (NE), a condition that manifests in children due to neuroinflammation (often from hypoxic insult), and which leads to brain damage and consequently disturbed neurological function after 35 weeks gestation.127 Briefly, neonates and school-aged children who were also previously diagnosed with NE have dysregulated plasma IL-1β and IL-18 levels compared to age-matched controls. Curiously, neonates have higher levels of the IL-1 signaling inhibitor, IL-1Ra, compared with age-matched controls but there is no change in IL-1Ra levels in school-aged children.127 This is intriguing because it suggests that neonatal humans, like hypoxia-tolerant mammals, may upregulate protective and anti-inflammatory molecules to reduce damage from hypoxia. This study also found that NLRP3 and ASC transcript levels are generally unchanged in NE neonates relative to control neonates but are significantly higher in NE children whose blood samples are stimulated with 10 ng/mL LPS relative to control children.127 Together, these data suggest that neonates exposed to hypoxia may beneficially downregulate inflammasome components (e.g., through modulation of IL-1 signaling via IL-1Ra) but neonatal hypoxia may nonetheless have long-lasting deleterious effects on innate immune activity in later stages of development.
Intriguingly, preconditioning with immune activation may also have protective effects. For example, LPS preconditioning prior to ischemic injury results in reduced inflammation (e.g., suppression of neutrophil infiltration and microglia/macrophage activation128) and brain injury in adults,129, 130 suggesting that LPS preconditioning may be neuroprotective against ischemic injury in adulthood. Some studies in neonates suggest that this is true in early development as well131; however, low doses of LPS also dramatically increase the vulnerability of neonatal brain to HI,132-134 suggesting that this interaction is complicated. Furthermore, repeated but brief episodes of HI increase brain injury in neonates, especially with frequent exposure.135 However, one needs to consider that in the neonatal stage, the brain is still not fully developed; LPS and/or HI cause different neuroinflammatory responses depending on the stage of brain maturation.125 For example, anti-inflammatory cytokines in the brain are unchanged (IL-6, IL-10) or downregulated (IL-1Ra, transforming growth factor-β1) following LPS, HI, or LPS + HI in rats at postnatal day 1. However, at postnatal day 12, anti-inflammatory cytokines are upregulated in HI or LPS + HI groups, but not in neonatal rats exposed to LPS alone. Also, intracerebral systemic immune cells are only recruited at postnatal day 12 under HI and LPS + HI treatments. A stronger neuroinflammatory response has been observed in term-like (postnatal day 12) rat brains compared to preterm-like brains (postnatal day 1). Neonates do demonstrate immune activation following HI and/or LPS exposure, however, this study demonstrates that immune system maturation occurs alongside brain maturation. Indeed, constitutive innate immune function develops in neonates and into adulthood.136-139 Further studies are needed to better understand the underlying mechanisms of innate immunity activation in neonates, but studies conducted to date suggest that hypoxia may moderate the LPS response in neonates and also turn on immunity without prior stimuli.140 Interestingly, hypoxia-tolerant animals share similar immune responses as neonates, such as downregulating inflammasome components.
7 HIBERNATORS AND THE INNATE IMMUNE RESPONSE
Although most hibernating mammals are fossorial and hibernate in burrows, hibernators do not necessarily encounter hypoxia. Nonetheless, as for many animals in hypoxia, successful hibernation requires metabolic plasticity such that major energy savings are achieved by entering a state of torpor. Various physiological systems are arrested or downregulated in torpor, including respiration, cardiac function, cell division, brain and kidney metabolism, etc.141-147 Hibernators use similar protective metabolic strategies as do hypoxia-tolerant species to drastically reduce oxygen requirements and maintain tissue homeostasis, but unlike in hypoxia, physiological processes in hibernators are mostly impaired by low body temperatures or nutrient limitations, rather than by limited oxygen availability. However, like hypoxia-tolerant nonhibernating species, hibernators are capable of extreme metabolic plasticity, and given the lack of research in hypoxia-tolerant species, hibernators offer alternative models in which to consider energetic trade-offs when mounting an immune response during hypometabolism.
Most evidence to date suggests that hibernators downregulate immunity when in a hypometabolic state. For example, when small hibernating rodents enter torpor, they reduce the levels of circulating immune cells and platelets.148-150 Among other changes, hibernators have decreased plasma leukocyte concentrations.149, 151-155 It is not well understood to where these blood cells disappear, but it is likely they are sequestered into the liver, lung, and lymphoid tissues, but not the spleen.150, 156, 157 Leukocyte sequestration could reduce the risk of triggering the inflammatory cascade, which may be damaging and could lead to cerebral ischemia.58
Components of innate immunity are also downregulated during torpor. The inflammatory cascade following bacterial infection typically results in the release of pyrogenic cytokines (e.g., TNF-α, IL-6, IL-1β).158-161 However, LPS injection in hibernating golden-mantled ground squirrels (Spermophilus lateralis) does not induce fever nor provoke arousal, compared to normothermic squirrels, which sustain a fever for more than 8 h. Upon arousal, the previously LPS-treated squirrels eventually develop a fever, and their arousal period is also significantly longer.162 It is possible that the LPS cascade leading to cytokine release is disabled (or at least parts of it) at low core body temperatures but turns back on when normothermia is re-established. Therefore, arousal events may occur to combat pathogens acquired prior to or during hibernation. In support of this hypothesis, TNF production and phagocytosis in peritoneal macrophages decrease during prehibernation and torpor (4°C) in arctic ground squirrels (Citellus undulatus). However, during hibernation at 27°C and 35°C, there is an increase in TNF release that is similar to that of active arctic squirrels, but the neutrophil count in the blood remains lower compared to during arousal.155 Further supporting this finding, an in vitro LPS challenge to leukocytes from hibernating spotted sousliks (Spermophilus suslicus) decreases interferons.163 Yet, macrophages sampled from summer and winter euthermic hibernating golden-mantled ground squirrels are equally able to recognize and bind LPS at various assay temperatures (5°C, 27°C, and 37°C).164 This is consistent with a study that showed the complement system remained active in torpid animals.165 Together, these results suggest a disconnect between sensing and triggering an inflammatory response during hypometabolism in hibernators.
Hibernators can dramatically reduce or even cease their food intake during winter months.166 This brings about an interesting question of how caloric restriction affects the ability to mount an immune response in these animals. It is logical to assume that energetic trade-offs would need to occur in such situations, like in hypoxia. However, studies have demonstrated that immunity is maintained during dietary restriction and that such caloric restriction can actually enhance specific host-defense programs.167 Fat-storing hibernators starve for 6–9 months during winter hibernation. Thus, the energy resources they carry in their white adipose tissue must be used parsimoniously to sustain the most important biological processes to ensure survival until spring arousal. Undernutrition and low pathogen exposure (expected in cold, winter burrows) are conditions that promote the use of innate immunity instead of adaptive immunity in humans.168 Considering 13-lined ground squirrels (Ictidomys tridecemlineatus) reside in solitary burrows and may not be exposed to bacterial/viral/parasitic pathogens during the winter, it is logical to investigate the regulation of inflammasomes that respond to endogenous cell stress in this species, as opposed to those that primarily detect PAMPs such as LPS. Interestingly, the inflammasome appears to be more activated in the early arousal period from a torpor-bout in the brown adipose tissue of hibernating 13-lined ground squirrels.169 Early arousal in the hibernating ground squirrel involves a rapid reoxygenation event, where breathing and heart rates increase before settling back down to resting rates.170 There is potential for the rewarming event to produce ROS, just as an ischemia-reoxygenation event might, leading to damage of proteins and lipids. Indeed, there is evidence that brown adipose tissue may accumulate oxidized macromolecules in the arousal period.171 Thus, ROS, an important activator of the NLRP3 inflammasome, may trigger an innate immune response in brown fat upon arousal from torpor. By contrast, white fat serves as a storage site for triglycerides and is known to upregulate several antioxidant enzymes such as thioredoxin, and superoxide dismutase 1 and 2.172 A decrease in caspase-1 activity in white adipose during late torpor and early arousal could suggest fewer endogenous damage signals such as ROS exist in this tissue during torpor or arousal.169 However, this is the first study probing the activation of inflammasomes in a species capable of metabolic rate suppression. Further studies are required to characterize the innate immune response in other hibernator tissues, as well as in other species that use torpor to survive inhospitable environmental conditions.
Importantly, dark, cold, and damp environments are ideal for both hibernators and the growth of microorganisms, such as fungi, which can be recognized by TLRs and NLRP3 inflammasome.173, 174 Hibernating bats are vulnerable to white-nose syndrome, a disease caused by a fungal infection. European greater mouse-eared bats (Myotis myotis) injected with the fungal antigen zymosan also have higher levels of haptoglobin compared to controls.175 Haptoglobin plays a role in the acute phase response of innate immunity through bacteriostatic and immunomodulatory effects. Haptoglobin is a liver-made protein that forms a complex with hemoglobin and is then cleared from the body, thus reducing oxidative damage.176 Increased levels of haptoglobin are found in bears in the late phase of hibernation.177 Free hemoglobin undergoes autoxidation, and the produced superoxide dismutates into hydrogen peroxide, which in turn can be used for various oxidative reactions.178 Hypoxia further favors autoxidation,178 and so increased levels of haptoglobin would be observed in both immune and hypoxic challenges. Haptoglobin seems to have significant immunological functions in hibernators and may therefore have an adaptation that permits immune competence during hypometabolism.179 Indeed, the release of haptoglobin would be energetically beneficial compared to more systematic immune responses. We hypothesize that hypoxia-tolerant mammals may also express higher levels of haptoglobin due to their similar hypometabolic state, but this hypothesis remains to be tested.
8 CONCLUSIONS
Although there is evidence of a blunted immune response toward acute hypoxia and inflammation in neonates, it is critical to note that their immune system is not mature at that stage, which may be confounding. Hibernators may therefore offer a better model for comparison as many hibernators survive months using very little oxygen to fuel metabolism and adults have a more developed immune system. Hibernators seem to have a disconnect between sensing and triggering an immune response when in hypometabolism, which suggests the utilization of alternative immune pathway regulators and supports our hypothesis. Taken together, these results suggest that hypoxia-tolerant animals may mount an immune response toward hypoxia and maintain immunity during hypoxia when pathogens arise but that the sensitivity of these systems in hypoxia may be blunted or delayed until reoxygenation. A key caveat of our review is that the immune response during chronic hypoxic exposure was not discussed due to a lack of research. It would be interesting to see if animals exhibit differing immune responses depending on the severity or duration of hypoxic exposure.
AUTHOR CONTRIBUTIONS
Matthew E. Pamenter: Conceptualization; writing – original draft; writing – review and editing; supervision. Karen L. Kadamani: Conceptualization; writing – original draft; writing – review and editing. Samantha M. Logan: Writing – review and editing.
ACKNOWLEDGMENTS
All figures are made with BioRender©.
CONFLICT OF INTEREST STATEMENT
The authors have declared that no conflicts of interest exist.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




