No impact of sex and age on beta-adrenoceptor-mediated inotropy in human right atrial trabeculae
Abstract
Aim
There is an increasing awareness of the impact of age and sex on cardiovascular diseases (CVDs). Differences in physiology are suspected. Beta-adrenoceptors (beta-ARs) are an important drug target in CVD and potential differences might have significant impact on the treatment of many patients. To investigate whether age and sex affects beta-AR function, we analysed a large data set on beta-AR-induced inotropy in human atrial trabeculae.
Methods
We performed multivariable analysis of individual atrial contractility data from trabeculae obtained during heart surgery of patients in sinus rhythm (535 trabeculae from 165 patients). Noradrenaline or adrenaline were used in the presence of the beta2-selective antagonist (ICI 118 551, 50 nmol/L) or the beta1-selective antagonist (CGP 20712A, 300 nmol/L) to stimulate beta1-AR or beta2-AR respectively. Agonist concentration required to achieve half-maximum inotropic effects (EC50) was taken as a measure of beta-AR sensitivity.
Results
Impact of clinical variables was modelled using multivariable mixed model regression. As previously reported, chronic treatment with beta-blockers sensitized beta-AR. However, there was no significant interaction between basal force, maximum force and beta-AR sensitivity when age and sex were modelled continuously. In addition, there was no statistically significant effect of body mass index or diabetes on atrial contractility.
Conclusion
Our large, multivariable analysis shows that neither age nor sex affects beta-AR-mediated inotropy or catecholamine sensitivity in human atrial trabeculae. These findings may have important clinical implications because beta-ARs, as a common drug target in CVD and heart failure, do not behave differently in women and men across age decades.
1 INTRODUCTION
Beta-blockers have been central in the treatment of patients with several cardiovascular diseases (CVD), including heart failure, hypertension, coronary artery disease and atrial fibrillation for several decades. Especially in heart failure treatment, beta-blockers play a central role and have contributed to reduced mortality in those patients. Potential differences in the response to beta-blocker treatment by age and sex have been suspected. In vitro studies in human heart tissue indicated that the sensitivity of cardiac beta-adrenoceptors (beta-ARs) declines with age.1-4 However, the sample size in these studies was small. Furthermore, studies compared tissues obtained from very young adults (on average 20 years) to middle-aged people (50 years),2, 3 while patients with CVD are usually much older. Evidence for sex-related differences in beta-AR-regulated inotropy is derived from animal studies (mostly mice and rats) only. Whether results obtained in small animals are directly transferable to the situation in humans remains unclear. The question has clinical relevance because altered beta-AR function predicts altered outcome of beta-blocker treatment. In contrast, a recent meta-analysis showed similarly beneficial effects of beta-blocker treatment in heart failure across all ages and sexes.5
Surprisingly, limited data on potential differences in the mechanisms of beta-blocker actions with ageing and by sex are available in contrast to the broad prescription in clinical routine. We measured beta-AR-evoked inotropy in human right atrial tissue in several studies and therefore analysed the effects of sex and age on beta-AR-induced inotropy in human atrial tissue.
2 RESULTS
2.1 Clinical characteristics
We analysed data from 535 trabeculae obtained from 165 patients. Samples were obtained from patients undergoing isolated coronary bypass grafting (CABG; n = 89, 53.9%), valvular surgery (n = 54, 31.7%) or combined CABG and valve procedures (n = 22, 13.4%). The mean patient age was 67 ± 11 years, and 26% of patients were women. Mean body mass index (BMI) was 27.6 kg/m2. One third of patients had diabetes mellitus. The main indication for cardiac surgery was coronary artery disease (67%), followed by heart valve disease. Left ventricular end-diastolic diameter (LVEDD; 51.4 mm) and left ventricular ejection fraction (LVEF; 53.4%) were preserved, and left ventricular end-diastolic pressure (16.8 mmHg) and left atrial size (43.2 mm) only slightly increased. The majority of patients were treated with ACE-inhibitors or AT1-blockers. One third of patients were on beta-blockers. Details regarding clinical characteristics are given in Table 1.
| n = 165 | Male (n = 122) | Female (n = 43) | |
|---|---|---|---|
| Patient characteristics | |||
| Age (y) | 67.2 ± 10.8 | 66.2 ± 11.4 | 69.8 ± 8.5 |
| Sex, male n (%) | 122 (73.9) | ||
| Body mass index kg/m2 | 27.7 ± 4.6 | 27.4 ± 4.6 | 28.4 ± 4.5 |
| Hypertension n (%) | 134 (81.2) | 99 (81.1%) | 35 (81.4%) |
| Diabetes n (%) | 56 (34.1) | 39 (32.2%) | 17 (39.5%) |
| Hyperlipidaemia n (%) | 97 (71.3) | 68 (70.1%) | 29 (74.4%) |
| Coronary disease n (%) | 89 (53.9) | 72 (59.0%) | 17 (39.5%) |
| Valve disease n (%) | 54 (32.7) | 37 (30.3%) | 17 (39.5%) |
| Left ventricular ejection fraction (%) | 53.4 ± 15.1 | 52.1 ± 15.5 | 56.8 ± 1 3.4 |
| Left ventricular end-diastolic diameter (mm) | 51.4 ± 7.3 | 52.1 ± 7.4 | 49.5 ± 6.9 |
| Left ventricular end-diastolic pressure (mm Hg) | 16.8 ± 8.3 | 17.0 ± 8.9 | 16.4 ± 6.3 |
| Left atrial diameter (mm) | 43.2 ± 5.7 | 42.9 ± 5.4 | 43.9 ± 6.4 |
| Medication | |||
| Beta-blockers n (%) | 116 (70.3) | 86 (70.5%) | 28 (65.1%) |
| Carvedilol n (%) | 11 (6.7) | 6 (4.9%) | 5 (11.6%) |
| Ca2+ channel-blockers n (%) | 19 (11.5) | 12 (9.8%) | 7 (16.3%) |
| ACE-inhibitors n (%) | 117 (70.9) | 87 (71.3%) | 30 (69.8%) |
| AT1-blockers n (%) | 16 (9.7) | 12 (9.8%) | 4 (9.3%) |
| Diuretics n (%) | 64 (38.8) | 43 (35.2%) | 21 (48.8%) |
| Statins n (%) | 63 (38.2) | 48 (39.3%) | 15 (34.9%) |
| Nitrates n (%) | 25 (15.1) | 20 (16.4%) | 5 (11.6%) |
Note
- Data are in mean ± standard deviation or numbers (%).
2.2 Univariable analysis
First, a univariate analysis including all 535 trabeculae from 165 patients was performed. There was no effect of age or sex on beta-AR sensitivity, basal force and maximum force. The same holds true for BMI and diabetes (Table 2). Hyperlipidaemia was associated with higher sensitivity of beta-ARs. Impairment of left ventricular function was associated with a decreased beta-AR sensitivity (lesser sensitivity [=higher EC50 value of beta-AR agonists] with larger LVEDD and with smaller LVEF). The presence of valve disease was associated with a lower basal force, but without effects on maximum force. Pretreatment with diuretics or carvedilol was associated with reduced beta-AR sensitivity, without effect on force values. All other variables did not have a significant influence on beta-AR sensitivity or force measurements in the univariate analysis (Figure 1).
| (n = 535/165) | LogEC50 (M) | Log Basal Force (mN) | Log Max Force (mN) | |||
|---|---|---|---|---|---|---|
| beta (95% CI) | P-value | beta (95% CI) | P-value | beta (95% CI) | P-value | |
| Patient characteristics | ||||||
| Age | 0.014 (−0.021 to 0.049) | .43 | 0.014 (−0.011 to 0.039) | .27 | 0.003 (−0.012 to 0.019) | .65 |
| Sex (male) | 0.033 (−0.140 to 0.206) | .71 | 0.009 (−0.115 to 0.133) | .88 | −0.025 (−0.100 to 0.050) | .51 |
| BMI | −0.004 (−0.020 to 0.013) | .67 | 0.001 (−0.011 to 0.013) | .83 | 0.000 (−0.007 to 0.007) | .97 |
| Hypertension | 0.109 (−0.085 to 0.303) | .27 | 0.026 (−0.112 to 0.165) | .71 | 0.042 (−0.042 to 0.125) | .33 |
| Diabetes | 0.002 (−0.156 to 0.161) | .98 | −0.011 (−0.124 to 0.102) | .85 | −0.024 (−0.092 to 0.045) | .5 |
| Hyperlipidaemia | 0.213 (0.032 to 0.394) | .021 | 0.028 (−0.101 to 0.157) | .68 | 0.013 (−0.063 to 0.090) | .73 |
| Coronary artery disease | 0.129 (−0.030 to 0.289) | .11 | 0.111 (−0.002 to 0.224) | .055 | 0.003 (−0.066 to 0.073) | .92 |
| Valve disease | −0.136 (−0.286 to 0.014) | .08 | −0.138 (−0.244 to −0.032) | .01 | −0.031 (−0.096 to 0.034) | .35 |
| LVEF (%) | 0.005 (0.000 to 0.010) | .04 | 0.002 (−0.002 to 0.005) | .37 | 0.001 (−0.001 to 0.003) | .29 |
| LVEDD (mm) | −0.122 (−0.240 to 0.004) | .042 | −0.045 (−0.100 to 0.009) | .1 | −0.012 (−0.063 to 0.039) | .64 |
| LVEDP (mm Hg) | −0.007 (−0.020 to 0.006) | .28 | −0.008 (−0.016 to 0.000) | .05 | −0.004 (−0.009 to 0.000) | .07 |
| LA diameter (mm) | −0.030 (−0.108 to 0.048) | .45 | −0.045 (−0.100 to 0.009) | .1 | −0.009 (−0.042 to 0.024) | .6 |
| Medication | ||||||
| Beta-Blockers | 0.179 (0.006 to 0.353) | .043 | 0.045 (−0.080 to 0.169) | .48 | −0.003 (−0.078 to 0.073) | .95 |
| Carvedilol | −0.939 (−1.191 to 0.687) | <.001 | −0.077 (−0.284 to 0.131) | .46 | −0.005 (−0.131 to 0.120) | .93 |
| Ca2+ channel-blockers | −0.157 (−0.389 to 0.075) | .18 | −0.048 (−0.215 to 0.119) | .57 | −0.013 (−0.114 to 0.088) | .81 |
| ACE-inhibitor | −0.074 (−0.241 to 0.092) | .38 | −0.032 (−0.152 to 0.087) | .59 | −0.020 (−0.092 to 0.052) | .59 |
| AT1-blockers | 0.164 (−0.091 to 0.418) | .21 | 0.173 (−0.013 to 0.358) | .068 | 0.045 (−0.068 to 0.157) | .44 |
| Diuretics | −0.167 (−0.321 to 0.014) | .032 | −0.010 (−0.121 to 0.102) | .87 | 0.026 (−0.041 to 0.093) | .45 |
| Statins | 0.026 (−0.129 to 0.181) | .75 | 0.080 (−0.030 to 0.190) | .16 | 0.048 (−0.019 to 0.114) | .16 |
| Nitrates | 0.177 (−0.031 to 0.386) | .096 | 0.077 (−0.073 to 0.227) | .31 | 0.056 (−0.034 to 0.146) | .22 |
- Significant P values are given in bold/italic.
- Abbreviations: BMI, body mass index; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEDP, Left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction.
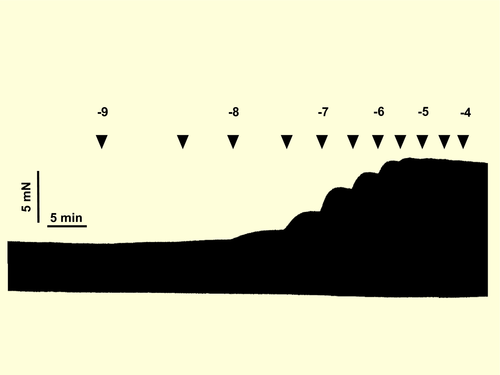
2.3 Multivariable analysis
For multivariable analysis, all patients with missing clinical variables had to be excluded. About 221 muscles from 65 patients could be used for multivariable analysis. There was no effect of age and sex neither on beta-AR sensitivity nor on basal or stimulated force (Table 3, Figures 2-4). In contrast to the univariate analysis, now a clear association between beta-blocker treatment and beta-AR sensitivity was observed (Table 3, Figure 3). Beta-blocker treatment (exception carvedilol) was associated with a higher beta-AR sensitivity. The beta-blocker treatment-associated increase in beta-AR sensitivity was higher for beta2-AR (activated with adrenaline) than for beta1-AR (activated with noradrenaline, Figure 2), but was independent of age and sex (Figure 3). In line with the findings from the univariate analysis, carvedilol was associated with a marked decrease of the apparent beta-AR sensitivity with larger effects on beta2-AR than on beta1-AR (Figure S1). Effects of hyperlipidaemia, LVEDD, LVEF and diuretics on beta-AR-mediated sensitivity that were seen in the univariate analysis could not be confirmed in multivariable modelling (Figure S2).
| (n = 221/67) | Log EC50 (M) | Log basal force (mN) | Log max force (mN) | |||
|---|---|---|---|---|---|---|
| beta (95% CI) | P-value | beta (95% CI) | P-value | beta (95% CI) | P-value | |
| Patient characteristics | ||||||
| Age | 0.023 (−0.035 to 0.081) | .44 | −0.011 (−0.056 to 0.033) | .62 | −0.016 (−0.043 to 0.011) | .26 |
| Sex (male) | −0.053 (−0.308 to 0.203) | .69 | −0.081 (−0.276 to 0.115) | .42 | −0.084 (−0.203 to 0.035) | .17 |
| Hyperlipidaemia | 0.057 (−0.201 to 0.315) | .67 | −0.146 (−0.342 to 0.051) | .15 | −0.120 (−0.239 to 0.001) | .047 |
| Valve disease | −0.089 (−0.363 to 0.185) | .52 | −0.122 (−0.332 to 0.088) | .26 | 0.000 (−0.127 to 0.127) | .99 |
| LVEF (%) | 0.002 (−0.006 to 0.011) | .6 | −0.003 (−0.010 to 0.003) | .34 | −0.003 (−0.007 to 0.002) | .22 |
| LVEDD (mm) | 0.003 (−0.014 to 0.020) | .69 | 0.005 (−0.008 to 0.018) | .41 | 0.001 (−0.007 to 0.009) | .81 |
| LVEDP (mm Hg) | 0.000 (−0.014 to 0.014) | .98 | −0.006 (−0.017 to 0.005) | .26 | −0.004 (−0.010 to 0.002) | .21 |
| LA diameter (mm) | −0.002 (−0.024 to 0.019) | .85 | −0.011 (−0.028 to 0.005) | .19 | −0.005 (−0.015 to 0.005) | .34 |
| Medication | ||||||
| Beta-Blockers | 0.476 (0.232 to 0.721) | <.001 | 0.174 (−0.014 to 0.362) | .07 | 0.063 (−0.051 to 0.177) | .28 |
| Carvedilol | −0.635 (−1.065 to −0.205) | .004 | −0.099 (−0.425 to 0.227) | .55 | −0.065 (−0.262 to 0.133) | .52 |
| Ca2+ channel-blockers | −0.042 (−0.320 to 0.236) | .77 | 0.103 (−0.110 to 0.316) | .34 | 0.046 (−0.084 to 0.175) | .49 |
| ACE-inhibitor | −0.098 (−0.384 to 0.189) | .5 | −0.062 (−0.284 to 0.159) | .58 | 0.006 (−0.129 to 0.140) | .94 |
| AT1-blockers | −0.044 (−0.430 to 0.342) | .82 | 0.059 (−0.241 to 0.359) | .7 | 0.024 (−0.159 to 0.206) | .8 |
| Diuretics | −0.193 (−0.443 to 0.057) | .13 | −0.065 (−0.255 to 0.125) | .5 | 0.027 (−0.089 to 0.142) | .65 |
| Statins | −0.059 (−0.292 to 0.175) | .62 | 0.101 (−0.078 to 0.280) | .27 | 0.072 (−0.037 to 0.181) | .19 |
| Nitrates | 0.099 (−0.242 to 0.439) | .57 | 0.170 (−0.089 to 0.430) | .2 | 0.160 (0.003 to 0.317) | .046 |
- Significant P values are given in bold/italic.
- Abbreviations: LA, Left atrium; LVEDD, Left ventricular end-diastolic diameter; LVEDP, Left ventricular end-diastolic pressure; LVEF, Left ventricular ejection fraction.
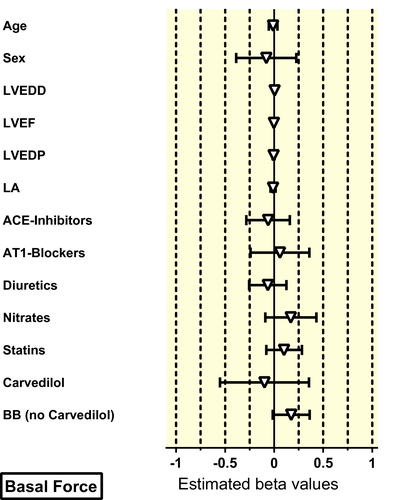
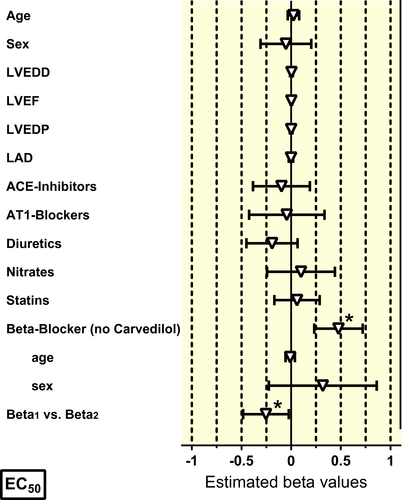
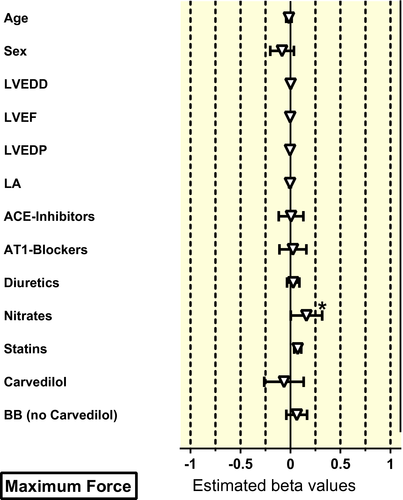
3 DISCUSSION
3.1 Principal findings
In our in vitro study of human atrial heart muscle samples, we did not find evidence for age- or sex-related effects on beta-AR sensitivity. Patients in our study had common cardiovascular pathologies such as coronary artery disease, valvular heart disease, hypertension, obesity and diabetes. Furthermore, patients with heart failure and reduced left ventricular ejection fraction were included. Therefore, our cohort should be a representative of contemporary patients with CVDs.
3.2 Methodological context: application of multivariable models
Using a multivariable analysis, we measured the impact of clinical variables, including age and sex, on functional properties of human heart tissue under in vitro conditions. Multivariable analysis using mixed models represents state of the art when the impact of clinical characteristics is measured in epidemiological studies. Mixed models have remained uncommon in the analysis of in vitro studies. In this context, it should be noted that experimental evidence for an age-dependent decline in cardiac beta-AR sensitivity comes from smaller size studies,1, 7 not allowing application of multivariable analysis. Sex-dependent differences in beta-AR inotropy are expected from animal studies with even less individuals.
3.3 Methodological context: relevance of findings in atrial tissue
Our results obtained in right atrial tissue cannot be translated one-to-one to the situation in left or right ventricles. In atrial tissue, sensitivity of β-AR to produce positive inotropic effects is around 0.7 log units higher than in ventricles in the hearts of mice and rat.6, 7 Reason for that difference is unclear. The same hold true for human tissue.8, 9 However, results are hard to interpret since β-AR remodelling in ventricular vs atrial tissue may differ between individual patients. It is almost impossible to get right atrial trabeculae and ventricular tissue from the same patient. To best of our knowledge, there is only one study where such a head-to-head comparison was done. Authors found heart failure-associated decrease in β-AR density in ventricles nicely paralleled in left and right atrial tissues.10 This finding makes us confident that atrial tissue can be useful to investigate basic mechanisms of β-AR physiology. Further work is needed to verify if age does not affect β-AR sensitivity in human ventricular tissue.
3.4 Methodological context: heart rate response as a measure for β-AR sensitivity
There are some doubts about taking heart rate responses upon stress/exercise as a measure for β-AR sensitivity. Heart rate increase in aged people is decreased and often taken as an indication for reduced β-AR sensitivity. However, activity of major contributors of pacemaking in mice heart (L-Type and T-type Ca2+ channels as well as funny channels) is decreased in old mice, while response of the respective channels to β-AR stimulations is nicely preserved.11 These findings indicate that intrinsic electrophysiological properties of sinus node cells rather than desensitized β-AR are involved in blunted heart rate response. Situation is less clear for diabetes. In sinus node cells of diabetic rats, not only the expression for gene coding for L-Type and T-type Ca2+ and funny channels but also for NCX is reduced,12 and data on β-AR responses of the channels are not yet available. In humans, the rate-lowering effect of the HCN blocker ivabradine was correlated with the intrinsic heart rate13 and exercise training reduces heart rate via downregulation of HCN4.14 Collectively, these results may explain the apparent discrepancy between our results and clinical reports about decreased heart rate response in elderly people, patients with diabetes or restricted exercise capacitance.15-17
3.5 Clinical context: age
Physical working capacity declines with age. Smaller contribution of sympathetic nervous system activity to adaptation was suspected from a smaller propranolol-sensitive component of exercise-induced increase in cardiac output in old vs young healthy individuals. Since catecholamine levels were found to increase with age,18 it seemed reasonable to suspect desensitization of beta-AR with age. In line with this assumption, heart rate increase upon stimulation by exogenous agonists was consistently smaller in older healthy people. However, increase in heart rate and cardiac output upon activation of beta-AR may be affected by several autonomous reflex mechanisms, impeding interpretation of these findings.
Measurements in isolated cardiomyocytes seem ideal to elaborate changes in intrinsic beta-AR function. Cell shortening response upon isoprenaline was smaller in cardiomyocytes isolated from unused donor hearts obtained from older individuals.1 The reduction of isoprenaline response was restricted to cells from the left ventricle. There were no age-dependent changes in myocytes isolated from right ventricles. The age-dependent reduction in isoprenaline effects was later confirmed in intact isometrically contracting right ventricular trabeculae, again from unused donor hearts. Interestingly, the 50% reduction in maximum isoprenaline effect came along with a 10-fold higher concentration of isoprenaline to evoke half-maximum effects (EC50) in trabeculae obtained from older humans.2 In the same year, the 10-fold higher EC50 for isoprenaline to evoke positive inotropy was confirmed by a retrospective study using atrial instead of ventricular human tissue. In this study, no data about maximum effects of isoprenaline have been reported.3 However, it should be noted that later work using cell shortening in isolated ventricular cardiomyocytes failed to reproduce the higher EC50 for isoprenaline in cells from older healthy donors.19
In our study, we neither saw an increase in EC50 for noradrenaline and adrenaline to evoke positive inotropy nor a decrease in maximum effects of either compounds. Discrepancies to the previously published studies are difficult to explain. Methodological differences should be of minor importance, since the two studies reporting higher EC50 values in tissue from older donors2, 3 used an almost identical experimental approach: isometrically contracting muscle strips. However, the two above-mentioned studies used rather small sample sizes from a heterogeneous patient population. Younger donors/patients including children were used as tissue donors, in this study. The authors stated: ‘The changes we observed in this study may not have been due to ageing process but instead may have occurred during the maturation process. We cannot rule out this possibility, and it will be rather impossible to obtain access to tissues from non-failing human hearts with a more appropriate age distribution’.3 This was an almost prophetic statement, since up to now there is no follow-up study that has solved the issue.
In our present study, we used a different approach. Because of restricted access to healthy human tissue, we collected a large database from patients in sinus rhythm requiring open heart surgery and used a multivariable statistical model, commonly used in epidemiological studies, to adjust for potential bias due to heterogeneous baseline patient characteristics. As a result, we here provide clear evidence that age does not reduce sensitivity of beta-AR in human hearts, neither in men nor in women.
3.6 Clinical context: sex
Most of our knowledge about sex effects on beta-AR function in cardiomyocytes comes from small animal studies. Mouse, rat, guinea pig and kitten were used for that purpose and gave conflicting results even within the same species. Basal force was reported lower in ventricular preparations from females compared to males in some studies, while others found no difference. The density of beta-AR was reported to be lower in rat ventricular cardiomyocytes from females than males, with lower increase of cAMP and calcium currents upon high concentrations of isoprenaline.20 Later studies found only a trend towards lower beta-AR density in ventricular myocytes from females (same species: rat).21 The sensitivity to isoprenaline to evoke positive inotropy in intact muscle preparations from rats was not different in the majority of studies.22-25 Only one study reported markedly lower sensitivity for isoprenaline in female vs male rat ventricular tissue.26 No difference in beta-AR density and in maximum inotropy with isoprenaline stimulation was seen between donor heart tissues obtained from male vs female humans (no data on beta-AR sensitivity from this study).27
3.7 Clinical context: obesity and diabetes mellitus
The mean BMI of patients in our study was 27.7 kg/m2. One third of patients had diabetes mellitus. Despite relevant prevalence of obesity and diabetes mellitus, we could not detect any impact of either entity on basal force or beta-AR-evoked inotropy. This finding may be surprising since many studies report a negative impact of both obesity and diabetes mellitus on cardiac performance.28, 29 Our results should be interpreted with caution, since clinical and basic science reports focus on ventricular function and our study was done on atrial tissues. It remains unclear whether obesity/diabetes-related pathophysiological changes affect ventricles and atria to a similar extent. On the other hand, our findings may indicate that functional impairment in obesity and diabetes may not relate to remodelling of the myocardium but rather to functional effects of secretory products released from adipocytes.30-32
3.8 Clinical context: beta-blocker treatment
Beta-blockers are a mainstay in the treatment of heart failure. In patients with advanced heart failure, beta-blocker treatment was associated with increased myocardial mRNA expression for SERCA and α-myosin heavy chain. Chronic treatment with metoprolol has been shown to reverse beta-AR downregulation in heart failure patients.32, 33 However, it is known that beta-blocker treatment sensitizes beta-AR, as described almost 30 years ago.34, 35 Both studies reported large effects of beta-blocker treatment on beta2, but not on beta1-AR, coming to the possibly wrong conclusion that beta-blocker-induced sensitization is selective for beta2-AR. From our larger study, we can conclude that both beta1- and beta2-AR are sensitized with a significantly larger effect size on beta2-AR. Theoretically, sensitization of beta-AR by beta-blocker treatment could help to overcome the desensitization of beta-AR in heart failure patients. However, the amount of sensitization of beta-AR we and others found34, 35 (about half a log unit) is in the same order of magnitude of the expected rightward shift of the concentration-response curve by metoprolol at typical plasma concentrations,9 indicating no net effect at rest. This may change under exercise, but the question whether beta-AR sensitization contributes to the beneficial effect of chronic beta-blocker treatment in heart failure remains open. In any case, our results clearly indicate that neither age nor sex affect chronic effects of beta-blockers on beta-ARs. Regarding carvedilol, we can confirm our earlier findings that beta-AR blockage by carvedilol persists under in vitro condition with larger effect on beta2-AR than on beta1-AR36 qualifying carvedilol as a beta-blocker that preferentially inhibits beta2-AR. Carvedilol but not metoprolol reduces beta-adrenergic responsiveness after complete elimination from plasma in vivo,37 suggesting that carvedilol accumulates in sarcolemma or sticks on β-AR.36 The data are also well in line with earlier studies showing a persistent antagonism by carvedilol.37, 38
In conclusion, our contemporary data in a large cohort suggest that no clinically relevant age or sex differences exist in the treatment with beta-blockers. Clinicians should be encouraged to provide guideline-recommended beta-blocker therapy across age groups and in both genders.
4 LIMITATIONS
The mean age of our cohort indicates that the majority of female patients have reached menopause. As a result, putative oestrogen-mediated effects on beta-AR function may have been lower than in women before menopause. In addition, there is drop in testosterone levels in elderly man. As a result, in an elderly population, influence of sex hormones is expected to be reduced in both sexes. Replacement of myocardial cells by fibrotic tissue should depress contractility. Here we have not measured fibrosis. Two rather large studies report consistently an increase in fibrosis with age in human right atrial appendages from 6.3% (age <45 years) to 24.7% (age >60 years).39, 40 Seemingly, such an increase in fibrosis does not depress marked contractility. Only few patients had enlarged left ventricles and reduced ejection fraction, probably hampering to detect an association between heart failure and beta-AR dysfunction, as previously described.
5 MATERIALS AND METHODS
Data were retrospectively analysed from 165 patients undergoing cardiac surgery. All patients were in sinus rhythm at the time of surgery. Patients with atrial fibrillation were excluded. After excision, tissue was immediately placed at room temperature into a non-oxygenated cardioplegic solution (in mmol/L): NaCl 100, taurine 50, glucose 20, KCl 10, MgS04 5, MOPS (3-(N-morpholino)propanesulfonic acid) 5, KH2PO4 1.2, containing 30 mmol/L of the myosin ATPase inhibitor BDM (2,3-butanedione monoxime) and transferred to the laboratory in less than 10 minutes.
The study followed the declaration of Helsinki and all patients gave written informed consent. All investigations were approved by the Medical Faculty Ethics Committee of the Technical University Dresden, approval number EK 114082202, and by University Ethics Committee at University Medical Center Eppendorf, Hamburg, approval number OB-088/04 and PV3759. Material is conform to good publishing practice in physiology.41 Small intact trabeculae (1- to 2-mm thick, about 10-mm long) were prepared from right atrial appendages in oxygenated, modified Tyrode's solution at room temperature containing (mmol/L): NaCl 126.7, KCl 5.4, CaCl21.8, MgCl2 1.05, NaHCO3 22.0, NaH2PO4 0.42, EDTA 0.04, ascorbic acid 0.2 and glucose 5.0. The solution was maintained at pH 7.4 by bubbling a mixture of 5% CO2 and 95% O2. Trabeculae were mounted in pairs, attached to Swema 4–45 strain gauge transducers in an apparatus containing the above solution at 37°Celsius, paced at 1 Hz and prestretched to 50% of the resting tension associated with maximum developed force.
All tissues were incubated with 5 μmol/L phenoxybenzamine for 90 minutes to block alpha-AR and neuronal and extraneuronal uptake of catecholamines and either 300 nmol/L CGP 20712A to block beta1-AR or 50 nmol/L ICI 118 551 to block beta2-AR. Contractile force was recorded through PowerLab amplifiers on a Chart for Windows, version 5.0, recording programme (ADInstruments Pty Ltd.).
Force data were not normalized.42 Inotropic effects were given in absolute values.
A single cumulative concentration-effect curve was established for selective beta1-AR stimulation by applying (−)-noradrenaline in the presence of 50 nmol/L ICI 118 551, for selective beta2-AR stimulation with (−)-adrenaline in the presence of 300 nmol/L CGP 20712A.
5.1 Drugs
CGP 20712A was from Novartis (Basel, Switzerland). ICI 118 551 was from Tocris (Bristol, UK) and (−)-adrenaline and (−)-noradrenaline were from Sigma Chemie. Phenoxybenzamine was from Röhm Pharma.
5.2 Statistics


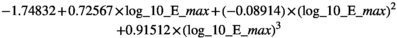

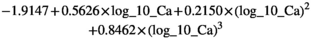
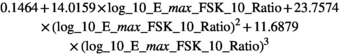
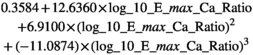
The beta-coefficients obtained in the mixed model regressions with the above transformed values represent the change of transformed measurement value per unit change in patient characteristic. Here we reported beta-coefficients which represent an increase of untransformed measurement value per unit increase of patient characteristic, by dividing the mixed model beta-coefficients by slope of the polynomial at the average measurement value as a first order approximation.
CONFLICT OF INTEREST
None declared.




