L-type Ca2+ channel current characteristics are preserved in rat tail artery myocytes after one-day storage
Abstract
Aim
To develop a cheap and simple method of storing for 24-h vascular tissue and single myocytes while preserving therein the biophysical and pharmacological characteristics of L-type Ca2+ channels and contractile activity.
Methods
Rings or vascular smooth muscle cells obtained from the rat tail main artery were used either freshly (R0h and VSMC0h) or stored for 24 h (R24h and VSMC24h) at 4 °C, to record whole-cell L-type Ca2+ currents (ICa(L)) or measure contractile responses.
Results
R0h/VSMC0h and R24h/VSMC24h comparably contracted when stimulated with phenylephrine, high KCl or ATP. In both VSMC0h and VSMC24h, ICa(L) was identified and characterized as a stable inward current for at least 35 min; ICa(L) was comparably inhibited by the Ca2+ antagonists nifedipine, verapamil and diltiazem and increased by the Ca2+ channel agonist (S)-(-)-Bay K 8644; current density and current–voltage relationships were similar; at more hyperpolarized holding potentials, ICa(L) intensity increased comparably; nifedipine shifted the steady-state inactivation curve towards more negative potentials, while verapamil blocked ICa(L) in a frequency-dependent manner and slowed down the rate of recovery from inactivation in a comparable way.
Conclusion
Findings show that smooth muscle contractile activity and the biophysical and pharmacological features of L-type Ca2+ channels are similar in VSMC24h and VSMC0h. The fact that reproducible results were obtained in vascular myocytes up to 24 h after dissociation may facilitate vascular smooth muscle cell investigation by increasing throughput and reducing the number of animals required.
In excitable cells, voltage-dependent Ca2+ channels are essential mediators of Ca2+ entry that triggers a variety of cellular responses including neurotransmitter release, gene transcription, enzyme activation and muscle contraction (Reid et al. 2003, Catterall et al. 2005, Gomez-Ospina et al. 2006, Wamhoff et al. 2006). Higher organisms, such as vertebrates, express different subtypes of Ca2+ channels (L, T, N, P/Q and R), which differ in their biophysical and pharmacological properties and cover diverse physiological roles (for a review, see Catterall et al. 2005). In vascular smooth muscle cells, the widely represented voltage-gated L-type Ca2+ channels play a key role in the regulation of cell contractility (Moosmang et al. 2003) and can be studied in different cellular models. Single smooth muscle cells, freshly isolated by enzymatic digestion, have been extensively used to investigate the role of these channels in the regulation of arterial tone (Robertson & Nelson 1994). In fact, these cells represent the native contractile phenotype of the tissue and possess all the membrane properties associated with muscle cell contraction in vivo. In a few words, the main advantage inherent to their use lies in the preservation of the physiological mechanisms of signal transduction. The process of enzymatic breakdown, however, produces cells of variable quality and may determine a proteolytic damage to membrane proteins, including voltage-gated L-type Ca2+ channels. Alternatively, smooth muscle cell cultures, representing a stable and reproducible preparation (Hall & Kotlikoff 1995), have been used to study cell proliferation and many other biological processes such as long-term effects of endogenous vasoactive agents and regulation of gene expression (Kleppisch et al. 1996). It should be stressed, however, that culture conditions can markedly alter cell structure and functions. In fact, cells in culture may differ markedly from freshly isolated myocytes in terms of both morphological and biochemical features, and it is well known that they dedifferentiate into a proliferative but non-contractile phenotype, thus hampering research on excitation–contraction coupling mechanisms (Hall & Kotlikoff 1995).
Expression of recombinant voltage-gated L-type Ca2+ channels (cloned from mammals, including humans) in suitable cell systems permits the study of their properties without the necessity of pharmacological intervention needed to ablate influences of the other channels. Such mutants are suitable to investigate in detail the structure–function relationship and/or map binding sites of pharmacological agents.
It is increasingly evident that, in many cell types, the phenotypic state may influence the properties of ion currents (Snetkov et al. 1996). So far, few studies have analysed the problems related to changes in the properties of ion channels of smooth muscle cells maintained in culture under different conditions, even if such changes may have important effects on cell functions (Yuan et al. 1993, Sobue et al. 1999). Ultrastructural changes as well as morphological alterations at molecular level have been evidenced when vascular smooth muscle cell primary cultures of 1–3 (Toro et al. 1986, Owens 1995) or 3–7 days (Neylon et al. 1994, Sobue et al. 1999), respectively, were compared. However, whether functional modifications of specific ion channels occur in cells cultured for short periods of time ought to be investigated in a systematic way. In particular, it would be interesting to assess whether both the channel expression and function change during a storage period of one or more days because this would allow the study of its role in long-term processes such as cell growth and proliferation, thus impacting both economically and ethically vascular smooth muscle cell experimentation.
Many attempts have been made in order to use animal or human vascular tissues on days subsequent to tissue harvesting. Besides the standard 0.9% NaCl solution, many other buffered, enriched and/or fortified physiological solutions have been developed to safeguard vessel tone development as well as endothelium-dependent and endothelium-independent relaxation in cold-stored arteries (Wilbring et al. 2013). However, the function of L-type voltage-gated Ca2+ channels, which plays a key role in the regulation of smooth muscle contractility, has not been thoroughly investigated in cold-stored tissue specimens until now (see as an example Neveu et al. 1994).
The aim of this work was to verify whether L-type Ca2+ channels of rat tail main artery myocytes, stored for 24 h at 4 °C in physiological buffered solution under normal atmosphere, maintained the same characteristics and functions of the channel protein of freshly isolated cells. The present data demonstrate that contractility of myocytes as well as the electrophysiological and pharmacological properties of L-type Ca2+ channels did not change 24 h after dissociation and storage at 4 °C, as compared to freshly isolated preparations.
Methods
Ethical approval
The study conforms to Good Publishing Practice in Physiology (Persson 2013) and was approved by the Animal Care and Ethics Committee of the Università di Siena, Italy (08-02-2012).
Animals
Male Wistar rats (8–11 weeks old – 300–400 g; Charles River Italia, Calco, Italy) were anaesthetized (i.p.) with a mixture of Ketavet® (30 mg kg−1 ketamine; Intervet, Aprilia, Italy) and Xilor® (8 mg kg−1 xylazine; Bio 98, San Lazzaro, Italy), decapitated and exsanguinated. The tail was cut immediately, cleaned of skin and placed in physiological solution (namely external solution, containing in mm: 130 NaCl, 5.6 KCl, 10 Hepes, 20 glucose, 1.2 MgCl2·6 H2O and 5 Na-pyruvate; pH 7.4). The tail main artery was dissected free of its connective tissue.
Cell isolation and ring preparation procedure
Smooth muscle cells were freshly isolated from the tail main artery under the following conditions: a 5-mm-long piece of artery was incubated at 37 °C for 40–45 min in 2 mL of 0.1 mm Ca2+ external solution containing 20 mm taurine (prepared by replacing NaCl with equimolar taurine), 1.35 mg mL−1 collagenase (type XI), 1 mg mL−1 soybean trypsin inhibitor and 1 mg mL−1 bovine serum albumin, which was gently bubbled with a 95% O2–5% CO2 gas mixture to gently stir the enzyme solution, as previously described (Fusi et al. 2001). Cells isolated without bubbling of the gas mixture did not show any difference (in terms of morphology, response to pharmacological stimulation, biophysical and pharmacological characteristics of ICa(L)) from those carboxygenated (F. Fusi and P. Mugnai, unpublished observation).
Two-mm-long rings were obtained from the tail main artery, deprived of the endothelium and mounted in a home-made Plexiglass support for tension recording as previously described (Bova et al. 1996) with slight modifications.
After isolation, cell suspension or rings were stored in 0.05 mm Ca2+ external solution containing 20 mm taurine and 0.5 mg mL−1 bovine serum albumin, at 4 °C under normal atmosphere.
Functional experiments
The Hepes buffer system present in the storage medium (i.e. external solution) may alter the response of vascular smooth muscle to vasoconstricting agents such as adrenaline and angiotensin II (Altura et al. 1980). Therefore, in functional experiments, rings or cells were continuously bathed/superfused at 37 °C with a modified Krebs-Henseleit, Hepes-free solution containing (in mm): 118 NaCl, 4.75 KCl, 2.5 CaCl2, 1.19 MgSO4·7H2O, 1.19 KH2PO4, 25 NaHCO3 and 11.5 glucose, bubbled with a 95% O2–5% CO2 gas mixture to create a pH of 7.4. Randomly selected, single-cell shortening was quantified using ImageJ by means of the Analyze/Measure plugin with the straight line tool (ver 1.46r; NIH, http://imagej.nih.gov/ij/download.html).
Rings were immersed in a double-chambered 20-mL organ bath filled with a modified Krebs-Henseleit, Hepes-free solution, and contractile tension was recorded using a digital PowerLab data acquisition system (PowerLab 8/30; ADInstruments, Castle Hill, NSW, Australia) and analysed by a LabChart Pro version 7.3.7 for Windows software (ADInstruments). After an equilibration period of 60 min, rings were contracted three consecutive times with both 90 mm K+ and 10 μm phenylephrine until a reproducible response was obtained.
Solution containing high K+ concentration was prepared by replacing NaCl with equimolar KCl.
Whole-cell patch-clamp recordings
Cells were continuously superfused with external solution containing 0.1 mm Ca2+ and 30 mm TEA using a peristaltic pump (LKB 2132, Bromma, Sweden), at a flow rate of 400 μL min−1. The conventional whole-cell patch-clamp method (Hamill et al. 1981) was employed to voltage-clamp smooth muscle cells. Recording electrodes were pulled from borosilicate glass capillaries (WPI, Berlin, Germany) and fire-polished to obtain a pipette resistance of 2–5 MΩ when filled with internal solution [containing, in mm: 100 CsCl, 10 Hepes, 11 EGTA, 1 CaCl2 (pCa 8.4), 2 MgCl2·6 H2O, 5 Na-pyruvate, 5 succinic acid, 5 oxaloacetic acid, 3 Na2-ATP and 5 phosphocreatine; pH was adjusted to 7.4 with CsOH]. Ca2+ concentration was calculated using the computer program EqCal (BioSoft, Cambridge, UK) by taking into account pH and Mg2+ concentration, as described by Fabiato and Fabiato (1979).
An Axopatch 200B patch-clamp amplifier (Molecular Devices Corporation, Sunnyvale, CA, USA) was used to generate and apply voltage pulses to the clamped cells and record the corresponding membrane currents. At the beginning of each experiment, the junction potential between the pipette and bath solution was electronically adjusted to zero. Current signals, after compensation for whole-cell capacitance and series resistance (between 70 and 80%), were low-pass-filtered at 1 kHz and digitized at 3 kHz prior to being stored on the computer hard disk. Electrophysiological responses were tested at room temperature (20–22 °C).
ICa(L) was always recorded in external solution containing 30 mm TEA as well as 5 mm Ca2+. Current was elicited with 250-ms clamp pulses (0.067 Hz, a frequency allowing full recovery of rat tail artery myocytes Ca2+ channels from inactivation; see below) to 0 or 10 mV from a Vh of −50 or −80 mV. Cells wherein experiments at a Vh of −80 mV were performed displayed L-type, but not T-type Ca2+ currents (see Petkov et al. 2001). Data were collected once the current amplitude had been stabilized (usually 7–10 min after the whole-cell configuration had been obtained). At this point, the various experimental protocols were performed as detailed below. Under these conditions, ICa(L) did not run down during the following 40 min (Fusi et al. 2012).
Activation curves were derived from the current–voltage relationships. Conductance (G) was calculated from the equation  , where ICa(L) is the peak current elicited by depolarizing test pulses in the range of −50 to 20 mV from Vh of −50 mV, Em is the membrane potential, and Erev is the reversal potential (181 mV, as estimated with the Nernst equation). Gmax is the maximal Ca2+ conductance (calculated at potentials ≥5 mV). The G/Gmax ratio was plotted against the membrane potential and fitted with the Boltzmann equation (Karmažínová & Lacinová 2010).
, where ICa(L) is the peak current elicited by depolarizing test pulses in the range of −50 to 20 mV from Vh of −50 mV, Em is the membrane potential, and Erev is the reversal potential (181 mV, as estimated with the Nernst equation). Gmax is the maximal Ca2+ conductance (calculated at potentials ≥5 mV). The G/Gmax ratio was plotted against the membrane potential and fitted with the Boltzmann equation (Karmažínová & Lacinová 2010).
Steady-state inactivation curves were obtained using a double-pulse protocol. Once various levels of the conditioning potential had been applied for 5 s, followed by a short (5-ms) return to the Vh, a test pulse (250 ms) to 10 mV was delivered to evoke the current. The delay between the conditioning potential and the test pulse allowed the full or near-complete deactivation of the channels simultaneously avoiding partial recovery from inactivation.
The frequency dependence of drug-induced effects on ICa(L) was assessed applying twenty depolarizing pulses of 50-ms duration to 10 mV from Vh of −80 mV at decreasing pulse intervals, ranging from 30, to 3 and 0.3 s (namely 0.033, 0.33 and 3.3 Hz) under control conditions. At the end of the protocols, verapamil was added to the bath solution and, after a 4-min interval without stimulation, the same protocols were repeated.
A two-pulse protocol was applied to measure the time course of recovery from inactivation: 2-s clamp pulses to 10 mV from a Vh of −80 mV were followed by a return to the Vh of variable duration to allow some channels to recover from inactivation. During the first pulse, the currents became inactivated by >93%. A second pulse (250 ms) to 10 mV was delivered to determine how much recovery had occurred during the time interval. Forty-second intervals in between each train of two pulses were adopted in order that current intensity returned to the first pulse value.
K+ currents were blocked with 30 mm TEA in the external solution and Cs+ in the internal solution. Current values were corrected for leakage and residual outward currents using 10 μm nifedipine, which completely blocked ICa(L).
The osmolarity of the 30 mm TEA- and 5 mm Ca2+-containing external solution (320 mosmol) and that of the internal solution (290 mosmol; Stansfeld & Mathie 1993) were measured with an osmometer (Osmostat OM 6020; Menarini Diagnostics, Florence, Italy).
Materials
The materials used included: collagenase (type XI), trypsin inhibitor, bovine serum albumin, TEA chloride, EGTA, Hepes, taurine, phenylephrine, ATP, nifedipine, verapamil, diltiazem and (S)-(-)-Bay K 8644 (from Sigma Chimica, Milan, Italy). Verapamil, dissolved directly in DMSO, (S)-(-)-Bay K 8644 and nifedipine, dissolved directly in ethanol, were diluted at least 1000 times prior to use. Control experiments confirmed that no response was induced in cell preparations when DMSO and ethanol, at the final concentration used in the above dilutions (0.1%, v v−1), were added alone (data not shown). Diltiazem was dissolved in bidistilled water. Phenylephrine was dissolved in 0.1 m HCl. Final drug concentrations are stated in the text.
Statistical analysis
Acquisition and analysis of data were accomplished using pClamp 10.2.0.16 software (Molecular Devices Corporation) and graphpad prism version 5.04 (GraphPad Software, San Diego, CA, USA). Data are reported as mean ± SEM; n is the number of cells or rings analysed (indicated in parentheses), isolated from at least three animals. Statistical analyses and significance, as measured by Student's t-test for paired or unpaired samples (two-tailed) and ordinary anova followed by Dunnett's or Bonferroni's post-test, were obtained using graphpad instat version 3.06 (GraphPad Software). In all comparisons, P < 0.05 was considered significant. The pharmacological response to each drug, described in terms of pIC50 or pEC50, was calculated by nonlinear regression analysis.
Results
Contractile effect of phenylephrine, high KCl and ATP
Vascular smooth muscle cells stored for 24 h (VSMC24h) at 4 °C and those freshly dissociated (VSMC0h) exhibited an ellipsoid form (10–15 μm in width, 35–55 μm in length) (Fig. 1a). Contraction by 1 μm phenylephrine, 60 mm KCl and 2 mm ATP was evaluated by recording the shortening as well as the formation of membranous evaginations (see Ives et al. 1978) of randomly selected cells. As shown in Figure 1a,b, addition of either phenylephrine or ATP caused both shortening and formation of membranous evaginations, while stimulation with high KCl shortened the cells by only about 10% of the initial length. In KCl-contracted cells, addition of ATP caused further muscle contraction and formation of membranous evaginations (Fig. 1b).

Phenylephrine (1 μm), KCl (60 mm) and ATP (2 mm) induced comparable contractions in both freshly prepared (R0h) and 24-h cold-stored rat tail artery rings (R24h) (Fig. 1c). Contractions to all agents reached a peak within 1 min: those to ATP were transient, whereas those to phenylephrine or KCl were maintained during administration of the drug (data not shown). All the contractions reversed rapidly on washout of the drugs.
Time course of ICa(L)
This series of experiments was carried out to characterize ICa(L) in both VSMC0h and VSMC24h. In VSMC0h, current amplitude elicited with 250-ms clamp pulses to test potentials of 10 mV from a Vh of −50 mV stabilized in about 10 min after the whole-cell configuration had been obtained and did not run down during the following 25 min (Fig. 2). Similar results were obtained in VSMC24h as well as in myocytes stored for up to 7 days after dissociation. Thereafter, no ‘patchable’ cells were found because myocytes tend to aggregate (data not shown). ICa(L) was blocked by 10 μm nifedipine, a well-known L-type Ca2+ channel blocker (Benowitz 2009). Also, current density and cell membrane capacitance were similar in both VSMC0h and VSMC24h (−0.87 ± 0.06 pA pF−1 and 52.86 ± 1.58 pF, n = 62; −0.89 ± 0.07 pA pF−1 and 51.06 ± 2.44 pF, n = 35, respectively; P > 0.05).
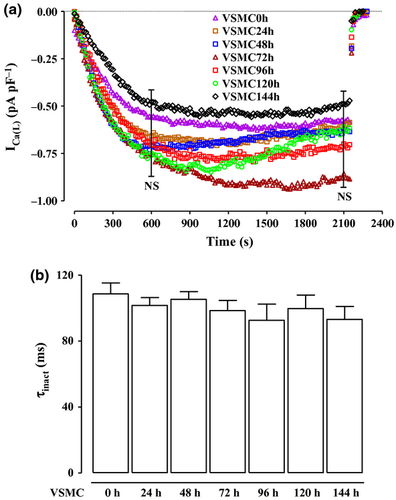
Under control conditions, the current evoked at 10 mV from a Vh of −50 mV inactivated with a time course that could be fitted by a monoexponential function. No differences were recorded in the τ of inactivation up to 7-day cold storage (P > 0.05 vs. VSMC0h; Figure 2b).
Current–voltage relationship
In a first series of experiments, families of inward currents elicited by 250-ms depolarizing steps to various potentials (ranging from −50 to 50 mV) from a Vh of −50 mV were recorded in VSMC0h and VSMC24h (Fig. 3a,b). Both current–voltage relationships showed detectable inward currents at approx. −30 mV (threshold) and maximum currents at about 15 mV (Fig. 3c). Similar results were obtained in myocytes stored for up to 7 days (data not shown).

The activation curves calculated from the current–voltage relationships of Figure 3c were fitted by the Boltzmann equation. Neither the 50% activation potential (−1.79 ± 1.09 mV, n = 10, VSMC0h, and 0.01 ± 0.70 mV, n = 12, VSMC24h; P > 0.05) nor the slope factor (10.07 ± 0.92 mV and 8.71 ± 0.59 mV, respectively; P > 0.05) was affected by the 24-h storage at 4 °C (Fig. 3d).
In the rat tail artery, inactivation of Ca2+ channels starts at very low membrane potentials (−80 mV) and a large number of Ca2+ channels are in the inactivated state already at −50 mV (Petkov et al. 2001). Therefore, to remove L-type Ca2+ channels inactivation, the current–voltage relationship was recorded, in the same cell, first at Vh of −50 mV and then at Vh of −80 mV. At the latter Vh, current density recorded at the maximum of 15 mV in both cell popula-tions (−0.78 ± 0.08 pA pF−1, n = 6, VSMC0h, and −0.73 ± 0.06 pA pF−1, n = 6, VSMC24h) was significantly higher than that recorded at Vh of −50 mV (−0.66 ± 0.7 pA pF−1, and −0.61 ± 0.07 pA pF−1, respectively; P < 0.05).
Effect of various Ca2+ channel modulators on ICa(L)
Three major classes of drugs are known to selectively affect L-type Ca2+ channels (i.e. dihydropyridines, phenylalkylamines and benzothiazepines). Therefore, the effects of (S)-(-)-Bay K 8644, nifedipine, verapamil and diltiazem on inward currents were investigated. Figure 4a (inset) shows typical recordings of ICa(L) elicited in VSMC24h with a clamp pulse to 0 mV from a Vh of −50 mV under control conditions and after the addition of various concentrations (100 pm–1 μm) of the L-type Ca2+ channel agonist (S)-(-)-Bay K 8644 (Hess et al. 1984). (S)-(-)-Bay K 8644 enhanced ICa(L) in a concentration-dependent manner (Fig. 4a) with a pEC50 value of 8.74 ± 0.10 (n = 8). This effect was reverted by the subsequent addition of 10 μm nifedipine (Fig. 4a, inset). Similar results were obtained in VSMC0h (pEC50 value of 8.73 ± 0.07, n = 7; P > 0.05; Fig. 4a).

The three well-known L-type Ca2+ channel blockers nifedipine, verapamil and diltiazem (Benowitz 2009) inhibited peak ICa(L), elicited with a clamp pulse to 10 mV from a Vh of −50 mV, in a concentration-dependent manner (Fig. 4b–d), with pIC50 values of 7.73 ± 0.09 (n = 5), 6.32 ± 0.14 (n = 7) and 5.96 ± 0.09 (n = 7) in VSMC0h and 7.67 ± 0.09 (n = 5), 6.25 ± 0.20 (n = 6) and 6.13 ± 0.16 (n = 6) in VSMC24h, respectively (P > 0.05).
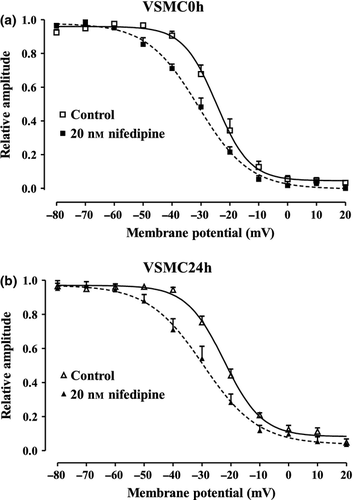
Effect of nifedipine on steady-state inactivation curve for ICa(L)
The voltage dependence of ICa(L) was assessed by determining the steady-state inactivation curve using a double-pulse protocol and nifedipine as the reference compound. Fifty per cent inactivation potential and slope values were similar in both VSMC0h and VSMC24h (Table 1). Nifedipine (20 nm) significantly shifted the steady-state inactivation curve to more negative potentials (Fig. 5) and modified its slope value in both cell populations (Table 1).
| Control | Nifedipine | |||
|---|---|---|---|---|
| V50 inact (mV) | Slopeinact (mV) | V50 inact (mV) | Slopeinact (mV) | |
| VSMC0h | −24.51 ± 1.63 | −5.76 ± 0.17 | −31.01 ± 1.72* | −8.58 ± 0.31*** |
| VSMC24h | −22.48 ± 1.00 | −6.55 ± 0.34 | −29.98 ± 2.25* | −8.83 ± 0.96* |
- Fifty per cent inactivation potential (V50 inact) and slope factor for inactivation (slopeinact) were obtained from steady-state inactivation curves, fitted by the Boltzmann equation. Data represent the mean ± SEM (n = 7, VSMC0h; n = 6, VSMC24h).
- *P < 0.05, ***P < 0.001 vs. control, Student's t-test for paired samples. P > 0.05, VSMC0h vs. VSMC24h, Student's t-test for unpaired samples.
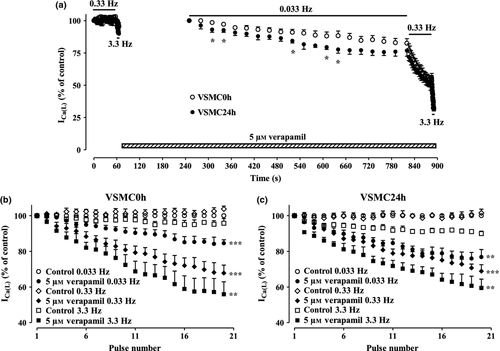
Frequency-dependent block of ICa(L) by verapamil
To assess whether verapamil block of ICa(L) was frequency dependent, its inhibition was recorded during twenty depolarizing pulses of 50-ms duration to 10 mV from a Vh of −80 mV applied at various stimulation frequencies (0.033, 0.33 and 3.3 Hz). Verapamil (5 μm) produced a comparable frequency-dependent block of ICa(L) in both VSMC0h and VSMC24h (Fig. 6a). This frequency dependence, calculated by normalizing the current amplitude evoked by the 20th applied stimulus against that induced by the first-step pulse at each frequency, was significantly different from that observed under control conditions (Fig. 6b,c).
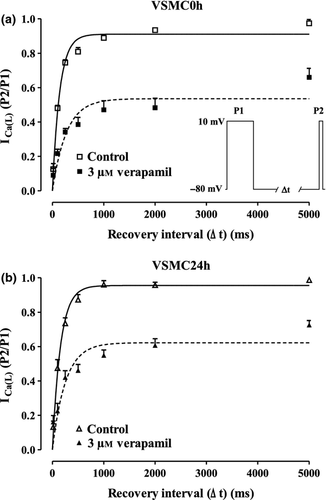
Effects of verapamil on recovery from inactivation
Many L-type Ca2+ channel blockers (e.g. verapamil) that inhibit ICa(L) in a frequency-dependent fashion also affect the recovery of the channel from inactivation. To assess this feature, conditioning pulses to 10 mV followed by a return to the Vh (−80 mV) of variable duration were delivered to both VSMC0h and VSMC24h. Verapamil significantly slowed down the rate of recovery from inactivation (Fig. 7). In fact, monoexponential fitting of plots gave τ values of 143.3 ± 6.5 ms (n = 5; VSMC0h) and 163.1 ± 21.3 ms (n = 7; VSMC24h) under control conditions and of 262.6 ± 47.2 and 279.8 ± 48.3 ms in the presence of 3 μm verapamil (P < 0.05, Student's t-test for paired samples), respectively.
Discussion
It is known that smooth muscle cells freshly isolated from the tissue and those maintained in culture for a long time differ substantially in their morphological and biochemical characteristics, such as the protein expression profile of contractile machinery and cytoskeleton (Neylon et al. 1994). In many cell types, culture age influences the properties and expression of ion channels, such as L- and T-type Ca2+ channels (Kuga et al. 1996, Gollasch et al. 1998, Guo et al. 1998), tetrodotoxin-sensitive Na+ channels (Quignard et al. 1997) as well as various types of K+ channels (Yuan et al. 1990, 1993, Kleppisch et al. 1996, Snetkov et al. 1996, 1998). However, to our knowledge, no data are available on the properties of L-type Ca2+ channels in vascular smooth muscle cells 24 h after dissociation. This study shows for the first time that neither biophysical nor pharmacological modifications took place in ICa(L) of cells stored for 24 h at 4 °C in a physiological buffered solution under normal atmosphere. In addition, a morphological analysis carried out by inspecting cell images revealed no differences between VSMC0h and VSMC24h, which appeared bright, had the same elongated shape and marked contours, thus indicating that the storing conditions did not apparently affect cell volume and cytoskeleton structure that may have influenced L-type Ca2+ channel expression. Finally, the storing conditions did not affect the contractile phenotype of both VSMC0h and VSMC24h, which responded with comparable contraction to stimulation of either α1 adrenergic or purinergic receptors as well as to membrane depolarization. Similar results were obtained in tail artery rings, in agreement with what previously observed by others as well as in this laboratory (Evans & Kennedy 1994, Bova et al. 1996). As compared to phenylephrine, which gave rise to intermediate responses, high K+-induced contraction was lower in single myocytes than in rings. This was probably due to the loss or sudden change of focal contact tension on disruption of the extracellular matrix preceding isolation of single smooth muscle cells that may considerably alter cell signalling systems leading to contraction (Ratz et al. 2005). On the contrary, ATP- induced contraction was smaller in rings than in single myocytes, possibly owing to the greater breakdown of the agonist in the intact tissue compared with the single-cell system (Evans & Kennedy 1994). These observations suggest that neither changes in the contractile apparatus, α1 adrenergic and purinergic receptors, and modification of downstream mechanisms leading to the release of Ca2+ from intracellular stores, nor functional alterations of L-type Ca2+ channels occurred within the 24-h storage of both tissue and cells at 4 °C.
The direct measurement of mRNA levels and channel proteins is exceptionally challenging in vascular smooth muscle cells isolated from the rat tail main artery, due to their very low yield (Tang & Wang 2001). However, the fact that ICa(L) amplitude, normalized to the cell surface, showed no statistically significant differences in the two populations and in cells stored for up to 7 days suggests that the number of L-type Ca2+ channels did not undergo modifications during a 144-h storage period, thus giving rise to an unchanged macroscopic current. Obviously, the possibility that the number of channels may be decreased along with an increase in their opening time cannot be ruled out, because this phenomenon would generate a macroscopic current of the same density; only single-channel experiments will clarify this point.
The biophysical analysis of ICa(L) has highlighted the strong similarities between VSMC0h and VSMC24h. In fact, the current–voltage relationships recorded at Vh of either −50 or −80 mV overlapped. This supports once more the hypothesis that the channel density as well as their open probability was unchanged. In fact, it is well established that the current flowing through an ion channel at specific membrane potentials is proportional to both driving force and open probability of the channel (Karmažínová & Lacinová 2010).
It has been shown that the Ca2+ current threshold in rat tail artery myocytes is set to about −30 mV when only L-type Ca2+ channels are expressed or to values more negative than −40 mV when both L- and T-type Ca2+ channels are present (Wang et al. 1989, Petkov et al. 2001). Therefore, the current recorded here exhibited characteristics amenable to only L-type Ca2+ channels.
In agreement with data previously published by this laboratory (Petkov et al. 2001), maximal ICa density recorded here was ≤1 pA/pF−1. Comparable currents were observed in rat tail artery primary cell culture, even in high Ba2+-containing media (Wang et al. 1989). This low conductivity of Ca2+ channels might explain why Toro et al. (1986) did not detect any ICa in these cells, even with the use of up to 20 mm Ca2+ in the bath solution. The small ICa might be the consequence of the low density of Ca2+ channels in the membrane. In fact, a study on arterial smooth muscle cells has estimated that about 5000 Ca2+ channels are present in a single cell membrane (Rubart et al. 1996); moreover, the small ICa cannot be ascribed to the incomplete recovery of channels from inactivation, because the frequency of stimulation applied was adequately low to warrant full recovery.
The steady-state inactivation protocol is commonly used to unravel drugs that bind preferentially channels in the inactivated state (Lacinová 2005). The inactivation curves here recorded at Vh of −50 mV indeed allowed an accurate analysis of the channel sensitivity to the reference ligand nifedipine. In fact, this Ca2+ antagonist altered the proportion of channels in the inactivated state (as demonstrated by the displacement of V50 towards more hyperpolarized potential) and the voltage sensitivity of the channel (i.e. the slope of the curve) comparably in both VSMC0h and VSMC24h. This is in agreement with what has been previously reported for nicardipine in the rabbit small intestine (Kuriyama et al. 1995). Moreover, the voltage dependence of activation and inactivation showed similar values in both cell populations. Therefore, it can be concluded that the mechanisms underlying L-type Ca2+ channel opening and closing did not undergo modifications during the 24-h storage at 4 °C.
L-type Ca2+ channel protein integrity was further investigated by analysing its sensitivity to either agonists or blockers of ICa(L). In fact, it is well known that even small changes in structure (e.g. a change in the amino acid sequence) of the recognition/binding site affect the activity of these drugs (Mitterdorfer et al. 1998, Striessnig et al. 1998). The present results reveal an unmodified pharmacological sensitivity of both cell populations to ICa(L) modulators. Furthermore, comparison of the kinetics of the current decay showed that L-type Ca2+ channels did not undergo changes in cells stored for up to 7 days. Alternative splicing of the L-type Ca2+ channel α1 subunit has been shown to modify the ion current phenotype (e.g. sensitivity to blockade by antagonists, current density and activation and inactivation characteristics; for a review, see Cheng et al. 2009). The findings here presented provide evidence that L-type Ca2+ channels in VSMC24h showed biophysical features and drug susceptibility unchanged compared to VSMC0h. Furthermore, the VSMC24h channels exhibited a stable current density, identical to that of freshly isolated myocytes; this holds true even for cells stored for up to 7 days. In spite of the lack of major changes, further experiments are needed to rule out the expression of different splice variants before concluding that VSMC24h and VSMC0h have an identical L-type Ca2+ channel.
In conclusion, this study demonstrates that rat tail artery myocytes can be used for up to 24 h with the certainty of obtaining comparable results. This observation, especially when confirmed on other vascular beds or even on other tissues, may have important economic and ethical impact on vascular smooth muscle cells experimentation by increasing throughput and reducing the number of animals required. Furthermore, these findings may have implications in cardiac as well as transplant surgery, where a critical graft situation could force to store not utilized, explanted vessel segments for the case of early graft failure. Until now, in fact, tissue banking for long-term storage of explanted vessels has been limited by methods yet inadequate to preserve vessel function (Wilbring et al. 2013). The solution here employed might therefore represent a worthwhile, additional option to reduce or even prevent the loss of function during cold storage, thus improving the availability of vessel grafts for allotransplantation.
Conflict of interest
None.
We wish to thank Dr. Beatrice Marenghi for the assistance in some preliminary experiments. This work was funded by a grant from the Ministero degli Affari Esteri (Rome, Italy) as stipulated by Law 212 (26-2-1992). P. Mugnai and M. Durante received a personal Ph.D. scholarship from the University of Siena.




